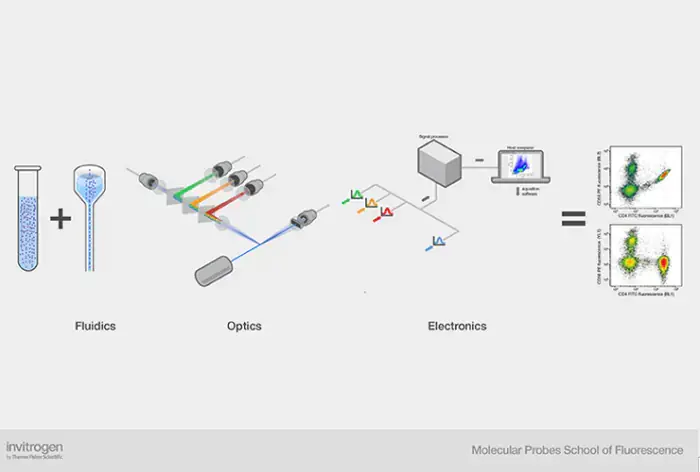
What is Flow Cytometry?
- Flow cytometry is a powerful technology that utilizes lasers to detect and measure various physical and chemical characteristics of cells or particles in a complex fluid mixture. This technique has gained significant popularity in both research and clinical settings due to its ability to rapidly analyze multiple attributes of cells, providing qualitative and quantitative data.
- The process of flow cytometry involves suspending a sample containing cells or particles in a fluid medium, which is then injected into a flow cytometer instrument. The sample is focused to flow through a laser beam, where the interaction of light with the cells provides valuable information about their properties. By using laser scattering, the flow cytometer can measure the size, internal complexity or granularity, and fluorescence intensity of the particles.
- Although flow cytometry is commonly associated with the analysis of cells, it can also be applied to chromosomes, molecules, or other suspended particles in a fluid. The technique offers versatility and has numerous applications in different fields, including basic research, clinical practice, and clinical trials.
- Some of the common applications of flow cytometry include cell counting, cell sorting, determining cell characteristics and function, detecting microorganisms, biomarker detection, protein engineering detection, diagnosis of health disorders such as blood cancers, and measuring genome size. Flow cytometry analyzers provide quantifiable data from samples, while cell sorters can physically separate and purify cells of interest based on their optical properties.
- During flow cytometry analysis, each particle is examined for visible light scatter and fluorescence characteristics. Visible light scatter is assessed in two directions: forward scatter (FSC), which indicates the relative size of the cell, and side scatter (SSC), which reflects the internal complexity or granularity of the cell. Fluorescence, on the other hand, is independent of light scattering and is used to measure specific characteristics of the particles. This is achieved by labeling cells with fluorescent markers, such as fluorescent proteins or fluorescently labeled antibodies.
- Over the years, the field of flow cytometry has witnessed significant advancements in terms of instrumentation and reagent availability. Multiple laser systems and specialized equipment have become commonplace, enabling researchers to analyze samples more efficiently. The availability of a wide range of fluorochromes for conjugating antibodies and the expansion of fluorescent protein options for transfection have also expanded the capabilities of flow cytometry experiments. These developments have facilitated the use of a large number of parameters in flow cytometry research, allowing for more comprehensive analysis.
- In addition to experimental advancements, data analysis techniques in flow cytometry have also evolved. While traditional histogram gating and analysis with two parameters are still widely used, novel cluster data analysis algorithms such as principal component analysis (PCA), spanning-tree progression analysis of density-normalized events (SPADE), and t-distributed stochastic neighbor embedding (tSNE) have been implemented to extract meaningful information from high-dimensional flow cytometry data.
- In conclusion, flow cytometry is a versatile and valuable technique for analyzing cells or particles in a fluid mixture. Its ability to rapidly measure various characteristics of particles, coupled with advancements in instrumentation and data analysis, has made it an indispensable tool in fields such as immunology, virology, molecular biology, cancer biology, and infectious disease monitoring. The continuous development and refinement of flow cytometry methods will further enhance our understanding of complex biological systems and facilitate advancements in various areas of research and clinical practice.
Definition of Flow cytometry
Flow cytometry is a laser-based technology used to analyze and measure physical and chemical characteristics of cells or particles in a fluid mixture. It provides rapid and quantitative information about cell size, complexity, and fluorescence intensity, enabling various applications in research and clinical settings.
Purpose of flow cytometry
Flow cytometry is used to identify and measure cells in solution, providing useful information about their properties and functions. It is generally used to assess peripheral blood, bone marrow, and other body fluids, making it an important tool in clinical and research contexts.
Flow cytometry may measure a variety of cell-related characteristics, including:
- Cell size: Flow cytometry can determine cell size, providing information on their morphology and potential problems.
- Cell granularity: This approach evaluates the internal complexity or granularity of cells, which can indicate their differentiation or activation state.
- Total DNA: Flow cytometry can detect ploidy variations and abnormalities in DNA content by measuring the total amount of DNA in cells.
- Newly generated DNA: Flow cytometry can be used to assess the rate of DNA synthesis in cells, providing information regarding cell cycle dynamics and proliferation.
- Gene expression: Flow cytometry can quantify gene expression levels in individual cells, allowing particular cell groups to be identified based on their molecular profiles.
- Surface receptors: The approach detects and quantifies surface receptors on cells, providing information about their functional features and interactions.
- Intracellular proteins: Flow cytometry can also be used to study intracellular proteins, allowing researchers to explore signaling cascades, protein expression, and cellular responses.
- Transient signals: Flow cytometry can capture and evaluate transitory signaling events within cells, providing insights into dynamic biological processes.
One of flow cytometry’s primary advantages is its capacity to rapidly measure numerous parameters in a single sample, cell by cell. Flow cytometers can quantify three to six characteristics or components in about 10,000 cells in minutes, enabling for efficient and high-throughput analysis.
Flow cytometry’s overall goal is to give researchers and doctors with a strong tool for researching cellular features, functions, and disorders. Its speed, adaptability, and quantitative nature make it a vital tool in a variety of applications, including immunology, cancer research, hematological problem detection, and therapy response monitoring.
Flow Cytometry Principle
Flow cytometry works based on the principles of light scattering and fluorescence. It involves passing particles or cells in a fluid stream through a laser beam and measuring the light scattered by these particles and the fluorescence emitted when they interact with specific markers.
Light scattering is used to analyze the physical properties of particles. Forward-scattered light (FSC) measures the size or cell-surface area of the particles, detecting diffracted light dispersed in the forward direction. Side-scattered light (SSC) provides information about the internal complexity or granularity of the cells, detecting refracted and reflected light that occurs at interfaces within the cell where there is a change in the refractive index. FSC and SSC measurements are utilized to differentiate cell types within a heterogeneous cell population.
Fluorescence is employed to detect specific molecules or markers within the cells. Fluorescent markers, known as fluorochromes, are used to label cellular molecules such as proteins or nucleic acids. When excited by the laser light, the fluorescent compound absorbs energy and an electron within the compound is raised to a higher energy level. The excited electron then decays back to its ground state, emitting excess energy in the form of fluorescence. Detectors collect the emitted fluorescence, which is specific to the fluorescent compound used. By using different fluorochromes, separate subpopulations of cells can be distinguished based on their fluorescence patterns. Combined with FSC and SSC data, the fluorescence patterns enable the identification of different cell types and the determination of their relative percentages in a sample.
The light signals detected by the instruments are converted into electronic signals by the electronics system. These electronic signals are then processed by a computer, which analyzes and interprets the data, providing quantitative measurements and visual representations of the analyzed particles or cells.

How does flow cytometry work?
The first step in beginning flow cytometry is to prepare the sample for analysis. It is necessary to suspend cells obtained from cell culture, blood, or disaggregated tissues. This cell suspension is divided among several tubes for staining, but a portion of unstained cells is retained as a control. Antibodies tagged with fluorescent probes or cellular component-staining dyes are utilised to stain the remaining samples. Before examining intracellular proteins, cells must be frozen (in a formalin buffer) and permeabilized (with a permeabilizing agent) so that antibodies and dyes can enter the cell. After antibody or dye incubation, the cells are washed and resuspended in a saline-based buffer for analysis. The sample is subsequently delivered to the flow cytometer after preparation.
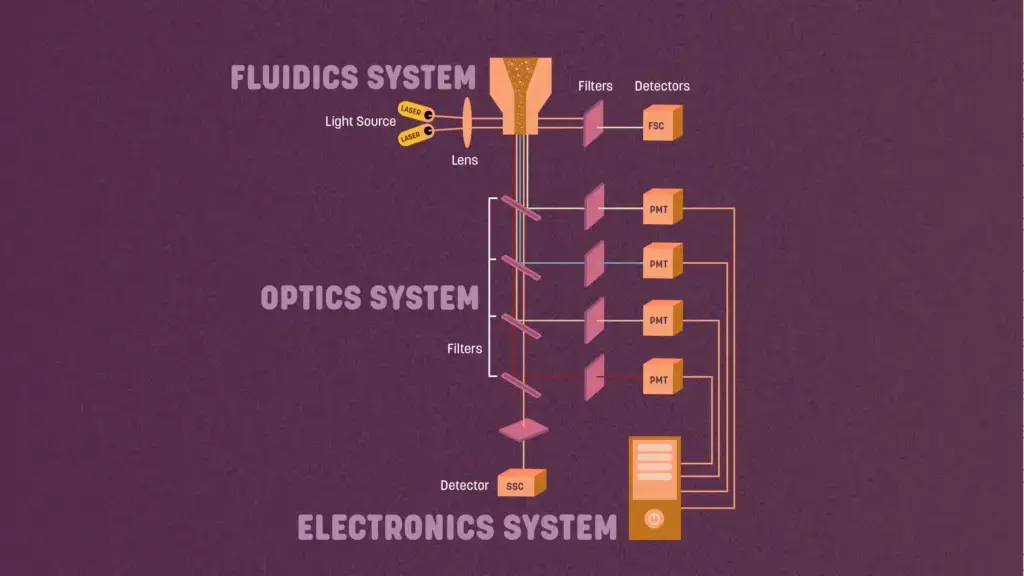
Fluidics, optics, and electronics are the three primary components of a flow cytometer. In the flow cell, the centre portion of the cytometer, the sample material interacts with the excitation light and scatters light, which is then collected by the detection systems.
- The fluidics system contains sheath fluid (a saline-based buffer or water) that is forced through the machine to direct the cell sample past the laser for individual measurement of each cell.
- The optics are comprised of lasers, which emit light to the samples, and photomultiplier tubes (PMTs), which gather the signal scattered by the sample.
- The electronics of the cytometer translate the observed signal into digital parameters that may be evaluated by software.
Forward scatter, side scatter and fluorescent signals
Forward scatter, side scatter, and fluorescence signals are the three principal output metrics of flow cytometry. Visible laser light reflects off each cell, revealing the cell’s general size and form.
Forward scatter
- Forward scatter (FSC) is the scattering that comes from the forward direction and reflects the size of the cells.
- The scatter is measured along the laser’s path, and each cell causes diffraction by bending light around its sides.
- Consequently, the intensity of FSC reflects the cell’s diameter.

Side scatter
- The side scatter (SSC) is the scatter measured at 90 degrees to the laser beam and is indicative of the granularity or complexity of the cell.
- Specific cellular features, such as granules and the nucleus, affect the direction of light waves that enter the cell, causing the light to be refracted by these structures.
- The more intense the SSC, the greater the refraction, and the greater the predicted granularity of the cell.

Fluorescent signals
- fluorescent signal is the third output measurement. This is the light released when fluorophores are excited by a laser.
- Upon excitation, fluorophores are molecules that can absorb and emit light. Photons from the laser light source are absorbed by the electrons of the fluorophore, elevating their energy level.
- In order to return to their ground state, these electrons emit energy as photons. During this process, the amount of energy lost to molecule interactions influences the wavelength of these photons.
- Each fluorophore has its own spectrum of emission, which may partially overlap with those of other fluorophores.
- Fluorophores can be used to mark antibodies, which can then bind to and detect specific antigens on or within a cell, or as a dye to stain cells directly, for example by binding to DNA.

Instrumentation/Parts of Flow Cytometry
Fluidics System:
- Transports particles in a fluid stream to the laser beam.
- Sample is injected into a stream of sheath fluid.
- Flow chamber design focuses the sample core in the center of the sheath fluid.
- Focusing is achieved by the flow of sheath fluid, which restricts particles to the center of the sample core.
Optics System:
- Consists of excitation optics and collection optics.
- Excitation optics shape and focus the laser beam to interact with the sample.
- Collection optics collect the emitted light after the particle-laser interaction.
- Optical detectors, such as photomultiplier tubes (PMTs) and a photodiode, capture the scattered and fluorescence signals.
- Filters are used to select specific wavelengths for each detector.
Electronics System:
- Converts signals from the detectors into digital signals for analysis.
- Photomultiplier tubes convert light signals into electrical currents, which are then amplified and converted into voltage pulses.
- The Analog-to-Digital Converter (ADC) converts these pulses into digital numbers.
- Digital signals are further processed and analyzed by a computer.
Developments in Flow Cytometry Instrumentation:
- Mass cytometry (cyTOF):
- Uses heavy metal ion tags instead of fluorophore-labeled antibodies.
- Over 100 metal probes with limited signal overlap are available.
- Enables high-dimensional analysis and in-depth phenotyping.
- Vaporizes, atomizes, and ionizes droplets containing cells for analysis.
- Mass spectrometer detects the ions produced and analyzes their time-of-flight.
- Spectral flow cytometry:
- Measures the entire spectrum of each fluorophore instead of assessing peak emission.
- Uses a series of detectors and algorithms to separate the spectra.
- Allows for the use of fluorophores with overlapping spectra, increasing the number of parameters analyzed.
- Imaging cytometry:
- Combines traditional flow cytometry with fluorescence microscopy.
- Enables assessment of cell morphology and visualization of protein co-expression, cell binding, nuclear translocation, and immune cell synapses.
Cell Sorting:
- Fluorescence-activated cell sorter (FACS) is used for sorting cells based on specific characteristics.
- Cells are selected and guided into collection vessels to purify the sample.
- Sorting can be performed using traditional flow cytometry or spectral-based techniques.
- Cells are separated based on positive or negative parameters using high-frequency oscillation of the sample liquid stream and charge-based sorting.
- Sorting is performed in sterile conditions and with gentle procedures to maintain cell viability.
Note: Some of the developments in flow cytometry instrumentation, such as mass cytometry, may not be compatible with traditional cell sorting due to the destructive nature of the analysis process.
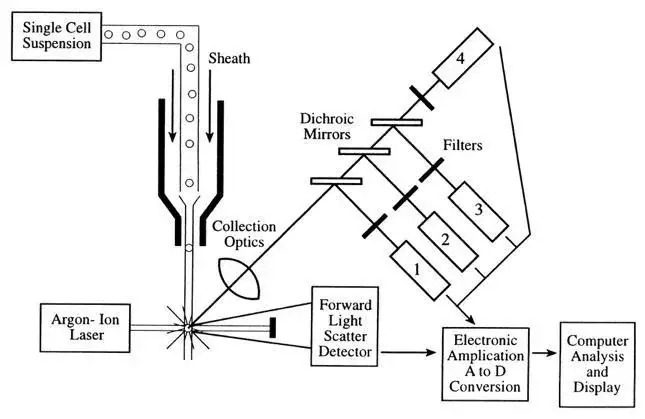
- Traditional Flow Cytometers:
- Fluidics System:
- Sheath fluid delivers and focuses the sample to the laser interception point.
- Pressurized buffered saline solution is commonly used.
- Optical System:
- Excitation optics (lasers) generate visible and fluorescent light signals.
- Collection optics (photomultiplier tubes or PMTs, photodiodes) detect and analyze the emitted light.
- Dichroic filters steer fluorescent light to specific detectors.
- Bandpass filters determine the wavelengths of light to be read for each fluorochrome.
- Electronic System:
- Converts signals from detectors into digital signals for computer analysis.
- Photomultiplier tubes convert light signals into electrical currents, amplified and converted to voltage pulses.
- Analog-to-Digital Converter (ADC) converts voltage pulses to digital numbers.
- Acoustic Focusing Cytometers:
- Utilize ultrasonic waves to focus cells for laser interrogation.
- Allows for higher sample input and reduces sample clogging.
- Can have up to 4 lasers and 14 fluorescence channels.
- Cell Sorters:
- Purify and collect samples for further analysis.
- Selects cells or particles positive/negative for desired parameters and directs them into collection vessels.
- Separates cells by oscillating the sample stream to generate drops with positive/negative charges.
- Drops are directed to specific collection vessels based on their charge.
- Two types: quartz cuvette and “jet-in-air” cell sorters.
- Imaging Cytometers:
- Combine flow cytometry with fluorescence microscopy.
- Enable rapid analysis of sample morphology and multi-parameter fluorescence at single cell and population levels.
- Useful for various applications like cell signaling, co-localization studies, and cell-to-cell interactions.
- Mass Cytometers:
- Combine time-of-flight mass spectrometry and flow cytometry.
- Use heavy metal ion-tagged antibodies for detection.
- Do not have FSC or SSC light detection.
- No cellular autofluorescence signals and no need for compensation.
- Sample is destroyed during analysis, so cell sorting is not possible.
- Lower acquisition rate compared to standard flow cytometry.
- Cytometers for Bead Array Analysis:
- Analyze multiplex bead arrays for analyzing multiple analytes.
- Utilize capture beads with known fluorescence and reporter molecules for quantification.
- Small flow cytometers with 2 lasers and 96-well loaders are used.
- Spectral Analyzers:
- Address spectral overlap between fluorochromes.
- Measure entire fluorescent emission spectra to create a spectral fingerprint.
- Unmix the spectra during analysis for pure signals of each fluorochrome.
- New Detector Technologies:
- Photomultiplier tubes (PMTs) are standard detectors.
- Solid-state detectors like avalanche photodiodes (APDs) and silicon photodiodes (SiPDs) are emerging alternatives.
- APDs are sensitive, inexpensive, and spectrally responsive in the long red region.
- SiPDs show promise as solid-state detectors.
Note: Some cytometers mentioned, such as imaging cytometers and mass cytometers, represent advancements and variations in traditional flow cytometry technology.
Protocol/Procedure/Process/Steps of Flow Cytometry
The flow cytometric process involves several steps, including sample preparation, antibody staining, and running the samples. Here’s an overview of the flow cytometric process:
- Sample Preparation: The cells under analysis need to be in a single-cell suspension. Clumped cells or cells from solid organs are dissociated into a single-cell suspension through enzymatic digestion or mechanical dissociation. Mechanical filtration may be employed to remove debris and obtain high-quality flow data.
- Antibody Staining: The cells in the sample are incubated with fluorochrome-conjugated antibodies specific to surface markers or intracellular antigens of interest. Antibodies can be directly conjugated to fluorophores or indirectly detected using fluorophore-conjugated secondary antibodies. Intracellular staining requires permeabilization of cells before antibody incubation.
- Running Samples: Control samples are run initially to adjust detector voltages and optimize instrument settings. The flow rates in the cytometer are set, and the prepared sample is introduced into the flow cytometer.
- Fluid Stream and Droplet Formation: The cell suspension is entrained in a swiftly moving liquid stream. The flow is organized to ensure sufficient distance between cells. A vibrating mechanism causes the stream to fragment into separate droplets, with minimal possibility of multiple cells per droplet. Before breaking into droplets, the stream passes through a fluorescence measuring station where the fluorescent properties of each cell are detected.
- Droplet Charging and Sorting: At the point of droplet formation, a charging ring deposits a charge based on the preceding fluorescence measurement. The opposite charge is captured by the droplet as it breaks away from the stream. An electrostatic deflection mechanism segregates the charged droplets into containers according to their charge. In some systems, the charge is directly delivered to the stream, and the resulting droplet retains the same charge.
- Fluorescence Measurement: As the droplets pass through a laser beam, the individual cells within them are analyzed for their fluorescence properties. A positive or negative charge is assigned to droplets containing a single cell based on whether the cell exhibits fluorescence due to the presence of the fluorescently tagged antibody.
- Collection and Analysis: Droplets carrying a single cell, identified by their charge, are directed into distinct collecting tubes or containers. This allows for the separation and collection of cells labeled with the fluorescent antibody. The collected cells can be further analyzed or sorted based on their characteristics.
Throughout the process, the flow cytometer’s electronics system converts the detected light signals into electronic signals, which are processed by a computer for data analysis and visualization. The results provide valuable insights into the cellular characteristics, composition, and functional properties of the analyzed sample.
Multicolor flow cytometry
- Multicolor flow cytometry is an effective method for analyzing complicated cell populations seen in blood, tissues, and other biological samples. Researchers can identify and describe different cell types and functional markers within a sample by utilizing multiple fluorescent dyes or markers at the same time.
- To mark specific cell populations, fluorescent dyes such as fluorophores or propidium iodide are routinely used. When activated by lasers, these dyes emit light at specific wavelengths, allowing for the detection and sorting of cells based on their fluorescence features.
- Multiple lasers and detectors on instruments allow for the measurement of twelve or more colors, allowing for the examination of multiple fluorescent markers in a single experiment. To distinguish itself from the other fluorescent markers in the panel, each fluorescent marker is stimulated at a different wavelength of light.
- Extending a staining panel from 4 to 6 to more than 12 colors necessitates a methodical approach and careful optimization. A good panel is designed by taking into account criteria such as the stain index, which determines the brightness of a fluorochrome, and suitably matching fluorochromes to get ideal results.
- A thorough pre-use investigation is required to determine the optimal settings for the staining panel. Researchers can maximize the effectiveness of multicolor flow cytometry by adhering to basic panel design guidelines. This entails careful planning, using the stain index, and ensuring correct compensation to reduce spectrum overlap between fluorochromes.
- Multicolor flow cytometry has transformed the investigation of complicated cell populations by giving researchers a new tool for investigating several markers at the same time. It has greatly increased our understanding of cellular variety, functional properties, and disease states in heterogeneous samples.
Types of Flow Cytometer
1. Traditional Flow Cytometers
- Traditional flow cytometers consist of fluidics, optical, and electronics technologies.
- The fluidics system comprises of sheath fluid (often a buffered saline solution) that is pressured to transport and focus the sample at the laser intercept or interrogation point, where it is examined.
- The optical system is comprised of excitation optics (lasers) and collecting optics (photomultiplier tubes or PMTs and photodiodes) that generate the visible and fluorescent light signals needed for sample analysis.
- A series of dichroic filters direct fluorescent light to specific detectors, while bandpass filters define the wavelengths of light that are measured, allowing for the detection and measurement of each distinct fluorochrome.
- Dichroic filters are filters that pass through light with shorter or longer wavelengths while reflecting the remaining light at an angle. A 450 Dichroic Long Pass (DLP) filter, for instance, allows light with wavelengths longer than 450 nm to pass through while reflecting shorter wavelengths at an angle to be directed to another detector.
- Bandpass filters detect a narrow band of light with a certain wavelength. A 450/50 bandpass filter, for instance, allows fluorescent light with a wavelength of 450 nm +/- 25 nm to pass through the filter and be read by the detector.
- The electronic system turns the signals from the detectors into computer-readable digital signals.
- Multiple laser systems are prevalent, with instruments frequently containing twenty parameters (FSC, SSC and 18 fluorescent detectors).
- New instrument systems with five or more lasers and 30–50 parameters are being introduced, however they are uncommon. Traditional flow cytometers often employ lasers with wavelengths of 488 nm (blue), 405 nm (violet), 532 nm (green), 552 nm (green), 561 nm (green-yellow), 640 nm (red), and 355 nm (ultraviolet).
- There are available additional laser wavelengths for specialised purposes. In an effort to increase sensitivity, certain devices have replaced PMTs with avalanche photodiodes (APD) for fluorescence detection.
2. Acoustic Focusing Cytometers
- This cytometer utilises ultrasonic waves to more precisely concentrate cells for laser examination.
- This sort of acoustic focusing permits greater sample input and reduces sample clogging.
- This cytometer is equipped with up to four lasers and fourteen fluorescence channels.
3. Cell Sorters
- The cell sorter is a form of conventional flow cytometer that may purify and collect samples for further examination.
- A cell sorter enables the operator to choose (gate) a population of cells or particles that are positive (or negative) for the specified parameters and then guide those cells to a collection vessel.
- By oscillating the sample stream of liquid at a high frequency to generate drops, the cell sorter separates cells.
- The drops are then given a positive or negative charge and sent to a certain collection vessel based on their charge by passing through metal deflection plates.
- Tubes, slides, and plates are acceptable collection vessels (96-well or 384-well are common).
- There are two types of cell sorters distinguished by the location of the laser interrogation point: quartz cuvette and “jet-in-air.”
- The quartz cuvette cell sorters are easy to arrange for a sort because their laser alignment is fixed.
- The “jet in air” cell sorters require daily laser alignment and are more complex to set up, but they are more versatile for detecting minute particles.
4. Imaging Cytometers
- Combining conventional flow cytometry with fluorescent microscopy, imaging flow cytometers (IFC) combine flow cytometry and microscopy.
- This enables quick single-cell and population-level morphology and multi-parameter fluorescence analysis of a sample.
- IFC can detect protein distributions within individual cells, similar to a confocal or fluorescence microscope, and can also process huge numbers of cells, similar to a flow cytometer.
- They are particularly effective in numerous applications, including cell signalling, co-localization investigations, cell-to-cell contacts, DNA damage and repair, and any application that requires the coordination of cellular location and fluorescence expression on large populations of cells.
5. Mass Cytometers
- Mass cytometers are instruments that combine time-of-flight mass spectrometry and flow cytometry.
- Instead of fluorescently-tagged antibodies, cells are labelled with heavy metal ion-tagged antibodies (often from the lanthanide series) and detected using time-of-flight mass spectrometry.
- Mass cytometers lack FSC and SSC light detection, making the standard method of detecting cell aggregates impossible. Nevertheless, other techniques, such as cell barcoding, can be used for this purpose.
- Also, mass cytometry lacks autofluorescence signals from cells, and reagents lack the emission spectrum overlap associated with fluorescent labelling, therefore correction is unnecessary.
- However, because the sample is destroyed during analysis, cell sorting is not possible, and the acquisition rate is significantly lower than that of a typical flow cytometer (1,000 cells/second as opposed to 10,000 cells/second).
- The number of commercially available reagents for 40 channels will rise with the introduction of other metal ions, such as platinum, for conjugation with antibodies.
6. Cytometers for Bead Array Analysis
- Multiplex bead arrays for assessing huge quantities of analytes in tiny sample volumes have gained popularity.
- Briefly, these assays use capture beads with a defined quantity of fluorescence in a given channel and a reporter molecule detected by a separate laser to quantify the amount of analyte caught by a single bead.
- It is roughly similar to one hundred ELISA tests.
- To assess these tests, compact flow cytometers with typically two lasers and 96-well loaders have been created.
- These instruments have tiny footprints and optical bench designs that are tuned for detecting and distinguishing beads with varying fluorescence levels along two channels.
- There have been created instruments that can identify 100–500 distinct bead combinations.
7. Spectral Analyzers
- Compensating (or removing spectral overlap) between flurochromes is one of the difficulties of multiparameter flow cytometry. The spectral analyzer is a novel type of flow cytometer built expressly to overcome this issue.
- A spectral analyzer examines the complete emission spectrum of each fluorochrome in a multicolor sample to produce a spectral fingerprint.
- Then, each spectrum is separated during analysis to provide a pure signal for each fluorochrome.
- As a detection method for high-dimensional flow cytometry, spectral analysis is gradually replacing standard PMTs.
8. New Detector Technologies
- Photomultiplier tubes (PMTs) continue to be the detector technology of choice for flow cytometry. They are useful for fluorescence technology due to their great sensitivity and low background.
- However, certain cytometers are beginning to use solid-state detectors. Avalanche photodiodes (APDs) are affordable, sensitive, and extremely linear, and their spectrum responsiveness is enhanced in the long red region.
- Silicon photodiodes (SiPDs) are another potential solid state detector technology.
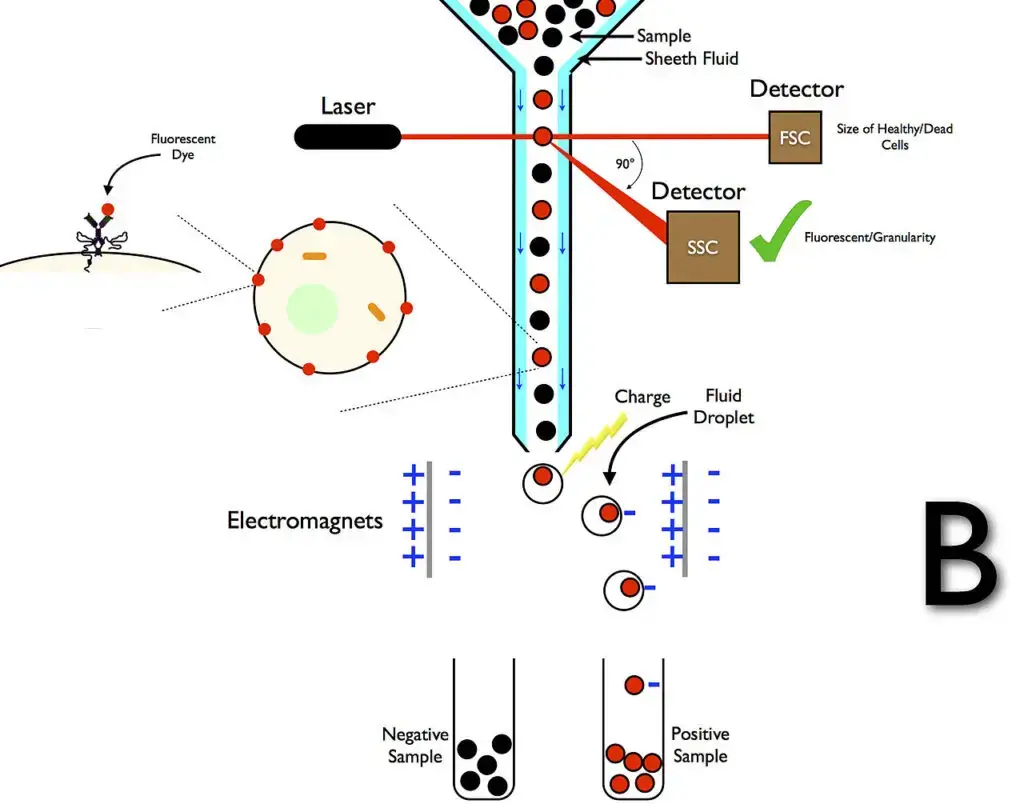
Cell sorting by flow cytometry
- Flow cytometry cell sorting is a strong technique for purifying certain cell populations based on their physical properties. Flow cytometers with sorting capabilities can identify and isolate cells of interest for further analysis or experimental application by combining the concepts of flow cytometry and droplet technology.
- Cell sorting was invented in 1965 by physicist Mack J. Fulwyler at the Los Alamos National Laboratory, who built the first prototype sorter. Fulwyler created a system capable of identifying and classifying cells based on certain properties by combining a Coulter volume sensor with a newly designed ink jet printer. Len Herzenberg later pioneered the creation of live cell sorters, also known as fluorescence-activated cell sorters (FACS), earning him the prestigious Kyoto Prize in 2006.
- The process in flow cytometry cell sorters begins with the introduction of a sample into a stream of sheath fluid flowing through a flow cell. The laser detects the cells in the stream, which are subsequently conveyed through a vibrating nozzle. This nozzle produces droplets, each of which normally contains one or no cells. An electrical charging ring is placed right before the droplets separate from the stream to sort the cells. Prior to droplet separation, the ring charges the cell based on its measured fluorescence intensity. As the droplet separates, it carries an opposing charge, which allows for electrostatic deflection.
- The charged droplets are diverted into specified containers based on their charge using an electrostatic deflection device. In other systems, the charge is applied directly to the stream, and the droplet keeps the same sign as the stream. Following the droplet separation procedure, the stream is neutralized. After sorting, the cells are collected and ready for further cultivation, manipulation, or analysis.
- Cell sorting using flow cytometry has various advantages in cellular investigation and research. It allows researchers to isolate specific cell populations from heterogeneous materials, allowing them to investigate and evaluate cells with specific traits or markers of interest. This method has numerous applications in domains such as immunology, cancer research, stem cell research, and drug discovery.
- In summary, flow cytometry cell sorting uses the principles of flow cytometry with droplet technology to extract cells based on their physical features. This technology has transformed cellular analysis by providing researchers with a strong tool for purifying and studying certain cell populations in order to gain a deeper grasp of their biology and possible applications in a variety of scientific and medical areas.
Measurable parameters
Measurable parameters in cellular analysis and flow cytometry encompass a wide range of characteristics and markers that provide valuable insights into cell biology and function. These parameters can be quantified and measured using flow cytometry techniques, enabling researchers to study and analyze different aspects of cells. Here are some notable measurable parameters commonly investigated:
- Apoptosis: Flow cytometry allows the quantification of apoptosis by assessing DNA degradation, mitochondrial membrane potential, permeability changes, and caspase activity. These measurements provide valuable information about programmed cell death.
- Cell adherence: Flow cytometry can be employed to study the adherence of cells, such as pathogen-host cell adherence. By using specific markers or fluorescent tags, researchers can quantify and analyze cell adhesion events.
- Cell pigments: Certain cells contain pigments like chlorophyll or phycoerythrin. Flow cytometry can measure the presence and levels of these pigments, providing information about cellular metabolism and physiological states.
- Cell surface antigens: Flow cytometry is widely used to identify and quantify cell surface antigens, commonly known as Cluster of Differentiation (CD) markers. By labeling cells with specific antibodies, researchers can characterize cell populations based on their surface antigen expression.
- Cell viability: Flow cytometry enables the assessment of cell viability by using fluorescent dyes or markers that distinguish live cells from dead cells. This parameter is crucial in determining cell health and response to experimental conditions or treatments.
- Circulating tumor cells: Flow cytometry plays a crucial role in the isolation and purification of circulating tumor cells (CTCs) from blood samples. By targeting specific markers or properties, CTCs can be identified and analyzed, aiding in cancer diagnosis, prognosis, and treatment monitoring.
- Characterizing multidrug resistance (MDR): Flow cytometry allows the characterization of MDR in cancer cells by measuring the efflux of fluorescent dyes or specific markers associated with drug resistance mechanisms. This information helps in understanding and combating drug resistance in cancer treatment.
- Chromosome analysis and sorting: Flow cytometry can assist in chromosome analysis and sorting, enabling the construction of libraries and identification of specific chromosomes or chromosomal abnormalities.
- DNA copy number variation: Flow cytometry techniques like Flow-FISH or BACs-on-Beads technology enable the measurement of DNA copy number variation. These methods provide insights into genomic alterations associated with diseases, including cancer.
- Protein expression and modifications: Flow cytometry facilitates the measurement of protein expression levels and modifications, such as phosphorylation, by using fluorescently labeled antibodies or probes. This parameter aids in understanding protein function and signaling pathways.
- Membrane fluidity: Flow cytometry can assess membrane fluidity by using fluorescent probes sensitive to membrane properties. This parameter provides insights into cell membrane dynamics and function.
- Total DNA and RNA content: Flow cytometry enables the quantification of total DNA and RNA content in cells. This information is valuable for cell cycle analysis, proliferation studies, and assessing changes in gene expression.
- Monitoring intracellular parameters: Flow cytometry allows the measurement of intracellular parameters such as pH, intracellular ionized calcium and magnesium levels, membrane potential, glutathione levels, and oxidative burst. These measurements provide insights into cellular metabolism, signaling, and oxidative stress.
- Scattering of light: Flow cytometry utilizes forward scatter (FSC) and side scatter (SSC) measurements to evaluate cell volume and morphological complexity, respectively. These parameters provide information about cell size, granularity, and morphology.
- Transgenic products: Flow cytometry is instrumental in analyzing transgenic products in vivo, particularly fluorescent proteins like green fluorescent protein (GFP) or related variants. This allows researchers to study gene expression, protein localization, and cellular dynamics.
- Various combinations: Flow cytometry offers the flexibility to combine different measurable parameters, such as DNA/surface antigens, to gain a comprehensive understanding of cellular characteristics and functions.
Reagents Used in Flow cytometry
Flow cytometry utilizes a wide range of reagents to facilitate the detection and analysis of cells and particles. These reagents play a crucial role in labeling specific targets and generating measurable signals. Here are some of the commonly used reagents in flow cytometry:
- Small Organic Molecules: Small organic molecules such as fluoroscein, Alexa Fluor dyes, Texas Red, Pacific Blue, Cy5, and others are frequently used for antibody conjugation. They have consistent emission spectra but a small Stokes shift. These dyes are stable and relatively easy to conjugate to antibodies.
- Phycobiliproteins: Derived from cyanobacteria, dinoflagellates, and algae, phycobiliproteins are large protein molecules. Examples include phycoerythrin (PE), allophycocyanin (APC), and peridinin chlorophyll protein (PerCP). These proteins offer large Stokes shifts and are excellent for quantitative flow cytometry. However, they are prone to photobleaching and may not be suitable for long or repeated exposure to excitation sources.
- Quantum Dots: Quantum dots are semiconductor nanocrystals with fluorescence emission spectra associated with their size. They are optimally excited by UV or violet lasers but can be minimally excited by multiple lasers, which can complicate fluorescence compensation. While they were once popular, polymer dyes have largely replaced quantum dots in multi-parameter staining panels due to their ease of use.
- Polymer Dyes: Polymer dyes consist of polymer chains that can absorb and emit light at specific wavelengths based on their length and attached molecular subunits. These dyes offer stability and similar quantum efficiency to phycobiliproteins but with increased photostability. They can be precisely tuned to absorb light at specific wavelengths, making them suitable for multi-parameter experiments. Examples include Brilliant Violet (BV), Brilliant Ultraviolet (BUV), and Brilliant Blue (BB) reagents.
- Tandem Dyes: Tandem dyes combine phycobiliproteins or polymer dyes with small organic fluorochromes to create dyes that utilize fluorescence energy transfer (FRET). This allows for increased available fluorochromes that can be excited with a single laser source. Tandem dyes are bright with large Stokes shifts, making them useful for low antigen density situations. However, they can be less stable than the donor fluorochromes and may vary in their energy transfer efficiency between different lots.
- Metal Conjugates for Mass Cytometry: Antibodies for mass cytometry are conjugated to heavy metal ions from the lanthanide series. These non-fluorescent probes are specifically designed for mass cytometry and enable the detection of multiple parameters simultaneously. Different lanthanide series isotopes can be used for antibody conjugation.
- Fluorescent Proteins: Fluorescent proteins, such as green fluorescent protein (GFP), are commonly used as reporter systems for gene expression. They offer a wide range of excitation and emission spectra, and their use has expanded with the availability of multiple laser wavelengths in modern flow cytometers.
- Nucleic Acid Dyes: Nucleic acid dyes bind to DNA, RNA, or both, and they are used for various purposes, including cell cycle analysis, chromosome sorting, stem cell analysis, and cell viability assessment. Examples include Propidium Iodide, 7AAD, DyeCycle Violet, DAPI, Hoescht 33342, and Chromomycin A3.
- Proliferation Dyes: Proliferation dyes, such as CFSE (Carboxyfluorescein succinimidyl ester), are used to track multiple divisions of proliferating cells. These dyes are inherited by daughter cells and their dilution indicates cell division. They are suitable for long-term proliferation studies.
- Viability Dyes: Viability dyes are used to determine cell viability by assessing membrane integrity. Dyes like Propidium iodide, DAPI, Live/Dead reagents, and Zombie dyes can indicate if a cell membrane is intact. Some of these dyes can be fixed and used for infectious cells or cells that require staining for internal antigens.
- Calcium Indicator Dyes: Calcium indicator dyes undergo a color shift upon binding to calcium ions. They are used to indicate cell activation and signaling. Common examples include indo-1 and fluo-3, which provide information about intracellular calcium levels.
These reagents and dyes enable researchers to label and detect specific targets of interest, providing valuable insights into cellular characteristics, signaling pathways, and functional analysis in flow cytometry experiments.
Flow cytometry data analyzed
After the gating and initial analysis steps, more advanced techniques are employed to analyze high-dimensional flow cytometry data. Here are some commonly used methods:
- Principal Component Analysis (PCA): PCA is a dimensionality reduction technique that identifies the most significant patterns and relationships in complex data. It transforms the original data into a new set of variables called principal components, which are linear combinations of the original parameters. PCA helps visualize and understand the major sources of variation in the dataset.
- Spanning-Tree Progression Analysis of Density-Normalized Events (SPADE): SPADE is a clustering algorithm that constructs a minimum-spanning tree to identify phenotypically similar cell populations. It uses density-based downsampling to normalize cell numbers and create a hierarchical tree structure. SPADE allows visualization of cellular relationships and facilitates the identification of rare cell populations.
- t-Stochastic Neighbor Embedding (tSNE): tSNE is a nonlinear dimensionality reduction technique that maps high-dimensional data to a lower-dimensional space while preserving local similarities between cells. It is particularly useful for visualizing cell populations with distinct phenotypes and identifying cellular subsets that may be overlooked in traditional analysis.
- FlowSOM: FlowSOM is a self-organizing map (SOM)-based clustering algorithm that groups cells into clusters based on similarities in marker expression patterns. It creates a two-dimensional grid where each node represents a cluster, and cells are assigned to the most similar node. FlowSOM aids in the identification of cell subsets and can be combined with visualization techniques like heatmaps and histograms.
- Differential Expression Analysis: Differential expression analysis compares marker expression levels between different conditions or groups of cells. Statistical tests, such as t-tests or Wilcoxon rank-sum tests, are applied to identify markers that show significant differences in expression. This analysis helps identify markers associated with specific cellular states or disease conditions.
- Phenograph: Phenograph is a clustering algorithm that identifies phenotypically distinct cell populations based on shared marker expression patterns. It utilizes a graph-based approach to partition cells into clusters and is especially useful for the analysis of large datasets. Phenograph can be combined with other visualization techniques to explore and interpret complex flow cytometry data.
These analysis techniques enable researchers to gain insights into the heterogeneity of cell populations, identify rare cell subsets, explore cellular phenotypes, and uncover potential biomarkers or signaling pathways of interest. The choice of analysis method depends on the specific research question, dataset complexity, and desired visualization options.

FACS Sorting/facs flow cytometry
- Flow cytometry is a specialised kind of fluorescence-activated cell sorting (FACS).
- It provides a method for sorting a heterogeneous mixture of living cells into two or more containers based on the individual light scattering and fluorescence features of each cell.
- It differs from flow cytometry in that it gives distinctive characterisation rather than simply counting and sorting cells.
- It is usual for the two concepts to collaborate in a co-characterization process to provide a comprehensive qualitative and quantitative method for flow cytometric analysis.
Challenges and limitations of flow cytometry
Flow cytometry, while a powerful technique, does have certain challenges and limitations that need to be considered. Here are some key challenges associated with flow cytometry:
- Fluidics and Flow Interruptions: The fluidic system in flow cytometers requires careful maintenance to ensure consistent flow rates and prevent blockages. The presence of cells, debris, or contamination can cause blockades in the system, leading to interruptions in the flow. This can result in inaccurate or incomplete data acquisition and affect the overall analysis.
- Laser Alignment: Accurate laser alignment is crucial for obtaining reliable and reproducible results. Misalignment of lasers can lead to inconsistent excitation and detection of fluorochromes, affecting the accuracy of the measurements. Regular checks and adjustments are necessary to maintain optimal laser alignment.
- Cell Damage: Flow cytometry involves subjecting cells to hydrodynamic forces, pressure changes, and laser irradiation, which can potentially cause cellular damage. This damage may alter the characteristics of cells, affecting their analysis and subsequent culturing, particularly during cell sorting procedures. It is important to optimize instrument settings and operating parameters to minimize cell damage.
- Inability to Assess Tissue Characteristics: Flow cytometry primarily focuses on analyzing single cells in suspension and does not provide information about the spatial organization and tissue architecture found in intact tissues. This limitation restricts the ability to study cellular interactions within their native tissue context, which is crucial for understanding complex biological processes.
- Cost Considerations: Flow cytometers can be expensive to acquire and maintain, especially high-parameter instruments capable of analyzing multiple parameters simultaneously. The cost of reagents, antibodies, and maintenance can also be substantial. These factors may limit access to flow cytometry for some research groups or institutions with limited resources.
- Data Analysis Complexity: Flow cytometry generates large multidimensional datasets, which require advanced data analysis techniques and specialized software. Analyzing and interpreting high-dimensional data can be complex and time-consuming, necessitating expertise in bioinformatics and computational analysis.
- Specificity and Background Noise: Achieving high specificity in flow cytometry experiments can be challenging. Cross-reactivity of antibodies, autofluorescence, and nonspecific binding can contribute to background noise and affect the accuracy of the analysis. Proper controls, appropriate antibody selection, and thorough optimization are necessary to minimize these effects.
Despite these challenges, flow cytometry remains a valuable tool for cell biology and clinical applications. Researchers continue to develop strategies and improvements to overcome these limitations, such as advances in instrument design, reagent development, and data analysis techniques.
Applications of Flow cytometry
Flow cytometry has a wide range of applications across various fields. Here are some notable applications:
- Clinical Diagnostics: Flow cytometry plays a crucial role in clinical laboratories for the diagnosis and monitoring of diseases. It is used in the detection of malignancies, such as leukemia and lymphoma, by analyzing the abnormal cell populations present in bodily fluids or tissues. It helps in characterizing and quantifying different types of cells, identifying specific cell markers, and monitoring disease progression.
- Cell Sorting: Flow cytometers equipped with cell sorters allow the isolation and purification of specific cell populations. This is particularly valuable for researchers studying rare cell subsets or needing to separate cells for further analysis or experimentation. Cells of interest can be sorted based on their unique characteristics, such as cell surface markers or fluorescent protein expression, enabling downstream applications.
- DNA Analysis: Flow cytometry can be used to measure DNA content in cells. By labeling DNA with fluorescent markers, researchers can analyze the cell cycle phases and assess the proliferation status of cells. This information is valuable for studying cell division, cell cycle regulation, and identifying abnormal DNA content in diseases such as cancer.
- Cell Proliferation and Viability: Flow cytometry allows the measurement of cell proliferation and viability. Proliferation dyes, like carboxyfluorescein succinimidyl ester (CFSE), track the division of labeled cells over time, providing insights into cell proliferation rates and patterns. Viability dyes, such as propidium iodide or annexin V, can distinguish live cells from dead or apoptotic cells, facilitating studies on cell viability and programmed cell death.
- Immunophenotyping: Flow cytometry is extensively used in immunology research for immunophenotyping, which involves characterizing immune cell populations based on their surface markers. By labeling cells with specific antibodies conjugated to fluorophores, researchers can identify and quantify immune cell subsets, assess their activation status, and investigate immune responses in various contexts, including infectious diseases and autoimmune disorders.
- Microbiology and Virology: Flow cytometry has applications in microbiology and virology. It can be used to analyze and enumerate microbial populations in environmental samples, monitor microbial growth, and assess the viability of bacteria. Additionally, flow cytometry aids in the study of viral infections, including viral entry, replication, and the host immune response to viruses.
- Plant Biology: Flow cytometry is employed in plant biology to analyze plant cells and tissues. It facilitates the study of plant cell cycle dynamics, DNA ploidy levels, and cell differentiation. Flow cytometry is also used for plant breeding programs to assess genome size and ploidy variations, aiding in the selection and characterization of plant varieties.
- Marine Biology: Flow cytometry has found applications in marine biology for the analysis of marine microorganisms, including phytoplankton and bacteria. It enables the assessment of population dynamics, species composition, and physiological characteristics of marine organisms, contributing to ecological studies and environmental monitoring.
These are just a few examples of the broad range of applications of flow cytometry. The versatility and versatility of this technique make it an indispensable tool in many research fields, allowing for in-depth analysis of cells, biomolecules, and complex biological processes.
Applications of Flow cytometry in Different Fields
Flow cytometry is a powerful technique with diverse applications across various fields of study. Here are some of the key applications of flow cytometry in different disciplines:
- Immunology:
- Immunophenotyping: Flow cytometry allows the simultaneous analysis of multiple cell populations based on specific cell surface markers, such as CD markers. This technique is widely used to characterize immune cell populations, including T cells, B cells, monocytes, and natural killer cells.
- Antigen Specific Responses: Flow cytometry can measure immune responses to specific antigens by stimulating cells with the antigen of interest and analyzing cytokine production, proliferation, activation, memory, or antigen recognition using MHC multimers.
- Intracellular Cytokine Analysis: By treating cells with protein transport inhibitors, flow cytometry can detect intracellular cytokine production, providing insights into immune responses.
- Proliferation Analysis: Flow cytometry enables the assessment of cell proliferation using markers such as BrdU incorporation, CFSE dye dilution, or the expression of proliferation-related antigens like Ki67 or PCNA.
- Apoptosis Analysis: Flow cytometry can detect apoptotic cells by targeting various apoptotic events, including Annexin V staining, DNA fragmentation (TUNEL assay), caspase activation, mitochondrial membrane potential, and chromatin condensation.
- Molecular Biology:
- Fluorescent Protein Analysis: Flow cytometry can be used to analyze the expression of fluorescent proteins, which are often used as markers for protein expression. This technique is valuable for studying gene expression, protein-protein interactions, and tracking transplanted cells.
- Cell Cycle Analysis: Flow cytometry combined with DNA staining allows the analysis of cell cycle phases, providing insights into cell proliferation and DNA content.
- Signal Transduction Flow Cytometry: Antibodies against signaling molecules enable the study of signaling pathways in mixed populations of cells.
- RNA Flow Cytometry: Flow cytometry combined with fluorescent in situ hybridization (FISH) allows the detection of RNA expression along with protein expression.
- Cell Sorting: Flow cytometry with cell sorting capabilities can separate and purify cells or particles based on their fluorescent properties. This technique is used for various applications, including isolating transfected cells, stem cells, specific immune cell populations, and tumor cells.
- Other Applications:
- Absolute Cell Counting: Flow cytometry can determine the absolute number of cells per milliliter by comparing the gated cell population with fluorescent bead counts.
- Quantitative Flow Cytometry: Using standards, flow cytometry can quantitate the amount of fluorescence on cells, allowing measurement of antibody binding capacity or molecules of equivalent soluble fluorochrome.
- Multiplexed Bead Array Assays: Sets of fluorescently labeled beads coated with specific antibodies can be used to detect multiple soluble proteins or nucleic acids simultaneously.
- Phagocytosis Assays: Flow cytometry can detect and quantify phagocytosis by using fluorescently labeled bioparticles or bacteria.
- Small Particle Analysis and Sorting: Advanced flow cytometers can detect and sort sub-micron particles, such as exosomes and viruses, opening up new possibilities for studying these small entities.
These are just a few examples of the applications of flow cytometry in different fields. The versatility of flow cytometry makes it an invaluable tool for a wide range of research areas, including immunology, molecular biology, cell biology, and more.
Examples of Flow Cytometry Experiments
Immunophenotyping by Using Flow Cytometry
- Immunophenotyping is the most common flow cytometry application. It employs the unique capability of flow cytometry to examine mixed cell populations for many parameters concurrently.
- In its most basic form, an immunophenotyping assay consists of cells labelled with fluorochrome-conjugated antibodies that target cell surface antigens.
- Human Leukocyte Differentiation Workshops provide “cluster of differentiation” (CD) numbers to the majority of these antigens so that an uniform nomenclature may be used to identify monoclonal antibodies that target specific cellular antigens. CD3 is “cluster of differentiation number 3” and is used to designate the T cell co-receptor found on all T cells.
- The majority of immune cells have CD markers that distinguish them as a population of cells. These cell markers are referred to as lineage markers and are utilised to identify distinct cell types for further examination in every immunophenotyping assay.
- T cell markers (CD3, CD4, CD8), B cell markers (CD19, CD20), monocyte markers (CD14, CD11b), and NK cell markers (CD3, CD4, CD8) are some examples (CD56, CD161).
- In addition to lineage markers, other markers are employed to characterise each cell group. These may include activation markers (CD69, CD25, CD62L), memory markers (CD45RO, CD27), tissue-homing markers (α4/β7), and chemokine receptor markers (CCR7, CCR5, CXCR4, CCR6).
- Typically, immunophenotyping tests include intracellular markers such as FoxP3 (which defines Treg cells), cytokines (IFN-γ, TNF-α, IL-2 define TH1 cells), proliferation markers (Ki67, CFSE), and antigen-specific markers (major histocompatibility or MHC Tetramers).
- Although current instruments and reagents are capable of 28-color immunophenotyping tests, 12–15-color experiments are more typical.
Antigen Specific Responses by Using Flow Cytometry
- By stimulating cells with a specific antigen and then examining for cytokine production, proliferation, activation, memory, or antigen recognition through MHC multimers, it is possible to evaluate antigen-specific responses.
- MHC multimers are biotinylated MHC monomers (MHC-I or MHC-II) attached to a fluorescent streptavidin backbone in groups of four (tetramer), five (pentamer), or ten (octamer) (dextramer).
- These MHC multimers are “loaded” with the desired antigen and then utilised to bind to T cells that identify the antigen, so signalling the level of response to a particular antigen.
- This application is frequently employed in vaccine research.
Intracellular Cytokine Analysis by Using Flow Cytometry
- In order to do intracellular cytokine analysis, cells are treated for 2–12 hours with a protein transport inhibitor (Brefeldin A or Monensin) so that any cytokines produced by the cells can accumulate within the cell, allowing for improved detection.
- During this incubation, cells can be stimulated with various antigens, such as peptides from a vaccination, to test immunological response.
- Following treatment with a protein transport inhibitor, cells are stained for cell surface and viability markers, then frozen and permeabilized for intracellular staining with anti-cytokine antibodies.
Apoptosis Analysis by Using Flow Cytometry
- Apoptosis, or programmed cell death, is an often-studied process in immunology and other disciplines.
- It is utilised to preserve the immune system’s homeostasis by eliminating cells without provoking an inflammatory response (necrosis).
- It is the death process for clonally enlarged T cells following an immune response, self-targeting T cells, autoreactive B cells, and numerous other immune cells.
- Flow cytometry utilises numerous targets along the cascade of events associated with apoptosis to detect apoptosis.
- Annexin V staining targets the translocation of the plasma membrane, the TUNEL (TdT dUTP Nick End Labeling) assay targets the endonuclease digestion of DNA, the activation of Caspases can be targeted by antibodies and dyes, mitochondrial apoptosis is targeted by dyes that determine mitochondrial membrane potential, and chromatin condensation in the nucleus is detected by staining with Hoescht 33342.
- Annexin V is a phospholipid-binding protein that attaches to phosphatidylserine during apoptosis, when it is translocated to the outer layer of the cell membrane.
- Annexin V should be stained with a viability exclusion dye (such as propidium iodide) to confirm that the binding occurs on the outer surface of the cellular membrane.
- TUNEL is a method that uses the ability of terminal deoxynucleotidyl transferase (TdT) to identify DNA breaks associated with apoptosis with dUTP (deoxyuridine triphosphate) or BrdU.
- Before data collection, the dUTP or BrdU is labelled with a fluorophore for detection, and the cells are counterstained with a DNA dye.
- In most cases of apoptosis, the caspase signalling pathway is active. Utilizing intracellular labelling and antibodies specific to the active form of caspase 3, this is targeted.
- Additional tests employ fluorogenic substrates that, when exposed to caspase activity, are cleaved and emit light.
- Mitochondrial apoptosis does not always utilise the caspase route; hence, many detection approaches are utilised.
- The majority of these techniques examine mitochondrial membrane potential, including the use of the dye JC-1. However, there is an antibody targeting APO2.7 that is only expressed during apoptosis and is localised on the mitochondrial membrane.
Fluorescent Protein Analysis by Using Flow Cytometry
- Fluorescent proteins (GFP, mCherry, YFP, etc.) are employed as protein expression indicators.
- Typically, cells are transfected with a plasmid that encodes a gene of interest and a fluorescent protein, as well as a promotor sequence. The fluorescent protein’s expression serves as an indication of the gene of interest.
- In recent years, the development of a split bi- or tri-party fluorescence complementation connected to other proteins has made it possible to identify RNA–protein and protein–protein interactions.
- These techniques have improved the detection and separation of cells in which fluorescence is detectable only in response to a surrogate.
- This method has numerous uses, including in vivo tracking of transplanted cells, detection of bacterial or viral infections, and gene deletion in cells to further elucidate gene function.
Cell Cycle Analysis by Using Flow Cytometry
- Assays for cell cycle analysis involve the saturation labelling of DNA with a DNA-binding dye. In the majority of instances, the cells are fixed using a 70% ethanol solution, which permeates the cells, and then stained with the dye (PI, 7AAD, DAPI).
- Hoescht 33342 is one such dye that may enter living cells and stain DNA without harming the cells.
- In this sort of study, samples are obtained at a low flow rate using linear amplification, and the cell cycle phases are determined using ploidy modelling software.
Absolute Cell Counting by Using Flow Cytometry
- Every immunophenotyping investigation can use absolute cell counts. Fluorescent beads of a known concentration are acquired with the sample for the operation.
- The sample is evaluated, and the number of gated cells for the population of interest is compared to the number of beads obtained in the same sample to determine the number of cells per millilitre.
Multiplexed Bead Array Assays by Using Flow Cytometry
- Assays utilising multiplexed bead arrays consist of arrays of beads coated with antibodies against particular soluble proteins or nucleic acids.
- Each bead has a known amount of fluorescence and a distinct target, which determines its placement in the matrix.
- Up to 100 beads are incubated with the material of interest, treated with a fluorescence reporter, and then acquired on a flow cytometer equipped with at least two lasers to detect the two distinct fluorochromes.
- Utilizing fluorescence, specialised software is employed to calculate analyte concentrations.
Phagocytosis Assays by Using Flow Cytometry
- Using fluorescently tagged bioparticles or bacteria, flow cytometry can be used to detect phagocytosis.
- The bacteria are labelled with a pH-sensitive dye that fluoresces only when exposed to the acidic pH of a phagosome, suggesting that they have been phagocytosed.
Small Particle Analysis and Sorting by Using Flow Cytometry
- Enhanced sensitivity flow cytometers make it possible to detect and sort exosomes and other submicron particles.
- The analysis of exosomes, viruses, and other subcellular particles generates new applications in numerous domains, including cancer biology, cancer therapy, and vaccine creation.
- This application is still in its infancy, but techniques and instrumentation are advancing rapidly to make it more accessible in the near future.
FAQ
What is flow cytometry?
Flow cytometry is a technology used to evaluate cells for multiple reasons, including cell counting, phenotyping, cell cycle analysis, and viability. Light generated by lasers in a flow cytometer is scattered by cells in the sample, recorded by detectors, and converted into signals that can be examined and evaluated.
What are Multiplexed Bead Array Assays?
Assays utilising multiplexed bead arrays consist of arrays of beads coated with antibodies against particular soluble proteins or nucleic acids. Each bead has a known amount of fluorescence and a distinct target, which determines its placement in the matrix. Up to 100 beads are incubated with the material of interest, treated with a fluorescence reporter, and then acquired on a flow cytometer equipped with at least two lasers to detect the two distinct fluorochromes. Utilizing fluorescence, specialised software is employed to calculate analyte concentrations.
What is Quantitative Flow Cytometry?
Using a bead-based standard, quantitative flow cytometry generates a staining curve of known fluorescence amounts. The cells are then acquired using the same instrument parameters, and linear regression analysis is applied to determine the quantity of fluorescence on the cells. This can be expressed as Antibodies Bound per Cell (ABC), Antibody Binding Capacity (ABC), or Molecules of Equivalent Soluble Fluorochrome, depending on the bead system utilised (MESF). PE is the optimal fluorochrome for this application because, due to its size, it virtually always binds to an antibody with a ratio of 1:1 fluorochrome to protein. By constructing a standard curve and regression using MESF-bead data in every particular experiment, Molecular Equivalent of Soluble Fluorescence (MESF) standards can be utilised to convert arbitrary fluorescence intensity values to the number of fluorescent molecules on a cell.
How Cell sorting done in the flow cytometer?
To separate and purify cells or particles for further study, cell sorting employs a flow cytometer with cell sorting capabilities. Essentially, a cell sorter can separate any cell or particle that can be made fluorescent. It is possible to sort cells into 96 or 384 well plates, tubes, and slides. Transfected cells expressing a fluorescent protein, stem cells, tumor-infiltrating lymphocytes, tumour cells, and white blood cell populations are typical sample types. Scaling up the amount of antibody required to stain large quantities of cells is a crucial aspect of any cell sorting procedure.
What is Signal Transduction Flow Cytometry?
This application employs antibodies designed to bind to unphosphorylated and phosphorylated signalling molecules. The use of these reagents and specialized buffers in staining panels allows for the study of signaling pathways in mixed populations of cells.
What is RNA Flow Cytometry?
RNA flow cytometry combines flow cytometry with fluorescent in situ hybridization (FISH) in order to detect both RNA and protein expression. This method necessitates optimization of the staining panel, as not all fluorochrome-conjugated antibodies can resist successive 1-hour incubations at 40°C. When no antibodies are available for a target, RNA expression is a useful alternative.
References
- McKinnon KM. Flow Cytometry: An Overview. Curr Protoc Immunol. 2018 Feb 21;120:5.1.1-5.1.11. doi: 10.1002/cpim.40. PMID: 29512141; PMCID: PMC5939936.
- Brehm-Stecher, B. F. (2014). Flow Cytometry. Encyclopedia of Food Microbiology, 943–953. doi:10.1016/b978-0-12-384730-0.00127-0
- Chantzoura, E., & Kaji, K. (2017). Flow Cytometry. Basic Science Methods for Clinical Researchers, 173–189. doi:10.1016/b978-0-12-803077-6.00010-2
- Berny-Lang, M. A., Frelinger, A. L., Barnard, M. R., & Michelson, A. D. (2013). Flow Cytometry. Platelets, 581–602. doi:10.1016/b978-0-12-387837-3.00029-8
- Roederer, M., Parks, D. R., Herzenberg, L. A., & Herzenberg, L. A. (1998). Flow Cytometry. Encyclopedia of Immunology, 932–943. doi:10.1006/rwei.1999.0243
- https://www.bu.edu/flow-cytometry/files/2010/10/BD-Flow-Cytom-Learning-Guide.pdf
- https://www.slideshare.net/tashagarwal/flow-cytometry-46618943
- https://www.abcam.com/protocols/introduction-to-flow-cytometry
- https://www.bosterbio.com/protocol-and-troubleshooting/flow-cytometry-principle
- https://www.technologynetworks.com/cell-science/articles/what-is-flow-cytometry-343977
- https://www.thermofisher.com/in/en/home/life-science/cell-analysis/cell-analysis-learning-center/molecular-probes-school-of-fluorescence/flow-cytometry-basics/flow-cytometry-fundamentals/how-flow-cytometer-works.html
- https://nanocellect.com/blog/how-does-flow-cytometry-work/
- https://my.clevelandclinic.org/health/diagnostics/22086-flow-cytometry