Endospore staining is the special staining method used to differentiate the bacterial vegetative cell and its endospore. It is the process applied because some bacteria under harsh environmental conditions form a resistant structure known as the endospore.
It is a non-vegetative body and it is produced mainly by members of Bacillus spp and Clostridium spp when there is lack of nutrients, water, oxygen or when the temperature becomes unsuitable. These endospores have a tough outer covering which is made in such a way that it can resist high temperature, radiation and many chemicals, so the normal basic stains cannot enter inside.
It is the staining technique where a strong dye together with heat-steam is used so that the dye can penetrate into the spore coat. This is referred to as the endospore stain or spore stain. The major purpose is to detect and identify the presence of endospores and the vegetative forms within the same bacterial cell. In this process, the endospore appears as a distinct stained body while the surrounding cell takes the counterstain.
It is used in the laboratory to distinguish species because the shape and position of endospores (central, terminal or subterminal) is different in different bacteria.
Some of the important endospore-forming bacteria are Bacillus anthracis, Bacillus cereus, Clostridium tetani and Clostridium botulinum. These organisms are commonly found in soil but they cause diseases in humans and animals due to toxin production.
In routine work, two methods are mostly used for staining. These are Schaeffer–Fulton method where Malachite green dye and safranin are used, and Dorner method where Carbolfuchsin, acid alcohol and Nigrosin solution is used.
Principle of Endospore Staining
The principle of endospore staining is based on the ability to differentiate the highly resistant endospore from the vegetative cell. It is the process where the staining reagent must penetrate the thick spore wall which is made of a keratin-like protein. This structure is so tough that normal dyes cannot enter, so the method uses heat-steam to soften the spore coat and allow the primary stain to move inside. It is the principle used in the Schaeffer–Fulton method where malachite green is the primary dye. When the slide is heated, the dye moves through the spore coat, and once cooling occurs the coat becomes impermeable again trapping the stain within the spore body.
It is important that malachite green has very low affinity for the vegetative cell and because it is water soluble, the vegetative cell loses the stain when the slide is washed with water. This is the differential step since endospores retain the green colour but the surrounding cells are decolorized. The counterstain safranin is then taken up by the vegetative cell producing a pink-red colour. The difference in dye retention makes it possible to detect whether endospores are present or absent in the bacterial smear. Some procedures may increase dye concentration or heating time to improve penetration while modern phase-contrast microscopy is also used for better visualization of the endospore structure.
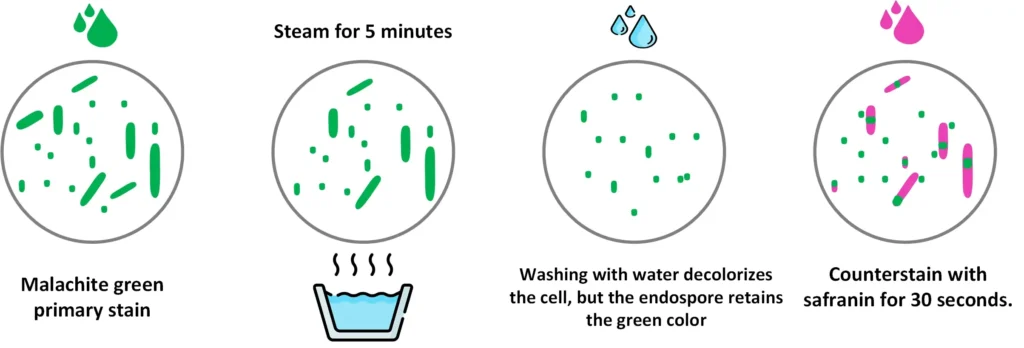
A. Schaeffer-Fulton Staining
The Schaeffer–Fulton stain is the commonly used endospore staining method in basic laboratories because it is simple and it is rapid in identifying the bacterial spore. It uses malachite green dye which is an alkaline, water-soluble reagent with pH around 11–11.2. In this method, the slide is exposed to steamed heat which softens the tough endospore covering, and this allows the malachite green to enter inside the spore.
The dye binds only mildly to the spore wall and if the heat is not applied the dye is easily washed away with water, therefore the heating step is important to permit the proper penetration of the stain.
Water is used as the decolorizing agent and it removes the malachite green from the vegetative cells while the endospores retain the green colour. After decolorization, the counterstain safranin is applied which stains the vegetative forms of the Firmicutes bacteria. The vegetative cells take up the pink-red colour of safranin while the spores appear green because they have retained malachite green within the spore coat.
It is the process that depends on the alkaline nature of malachite green which is positively charged, and the cytoplasm of bacterial cells being basophilic helps in attracting the dye so staining becomes easier. Under the microscope, the vegetative cells show a pink-red appearance whereas the endospores appear as green oval or spherical bodies within or outside the cell.
Principle
In this method, the heat helps the primary stain to enter the resistant layers of the endospore. Malachite green is the dye that is driven into the endospore by steaming because the spore coat is very tough and does not allow easy penetration.
During rinsing with water, the malachite green is removed from the vegetative cells but it is not washed out from the endospores. In this step the vegetative cells lose the green colour and later take the counterstain safranin.
The endospores remain green due to retention of the primary dye, while the vegetative cells appear pink-red. This difference in dye retention is the basis for differentiation between the two forms.
Requirement
- Malachite green dye (primary stain)
- Water used as the decolorizer
- Safranin solution (counterstain)
- Equipment: Glass slide, Inoculation loop, Bunsen burner
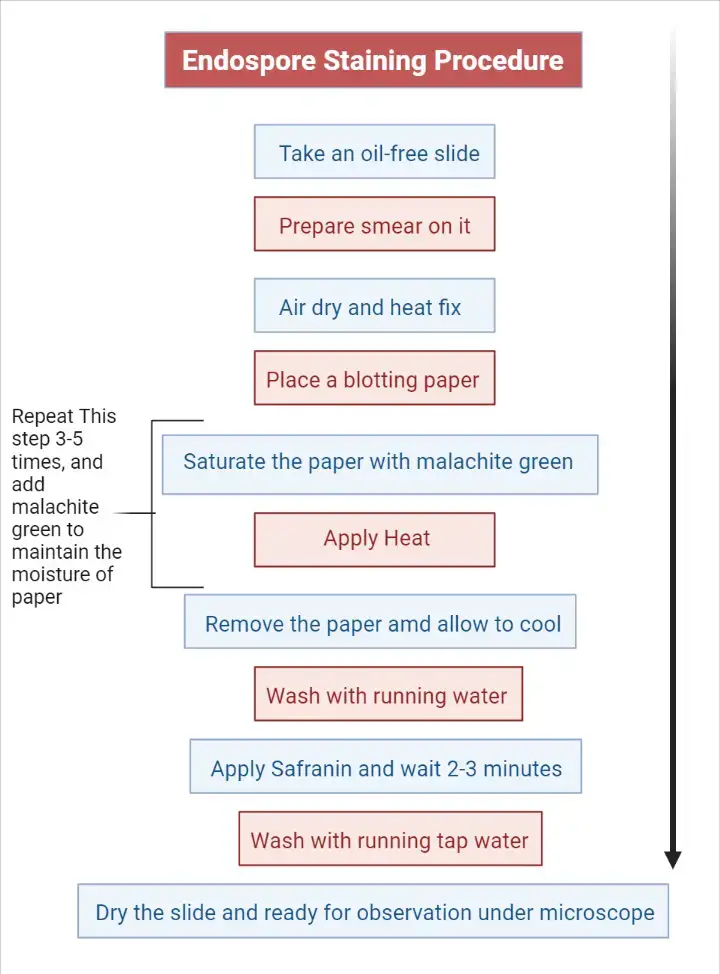
Procedure
- Clean the glass slide with alcohol so that any stains is removed.
- Put two small drops of water in each circle using a sterile inoculation loop.
- Open the culture tube aseptically, flame the mouth and collect a loopful of the bacterial culture.
- Flame the tube again and close it.
- Smear the bacterial culture properly in the drop of water on the slide.
- Allow the smear to air dry till it becomes completely dry.
- Heat-fix the slide by passing it over the blue flame 3–4 times with smear facing up.
- Let the slide cool and then staining is started.
Staining Steps
- Cover the smear with a piece of absorbent paper.
- Place the slide on a staining rack kept over steaming water.
- Flood the absorbent paper with malachite green and allow it to steam for 3–5 minutes.
- Remove the stained absorbent paper carefully and allow the slide to cool for 1–2 minutes.
- Rinse the slide gently with tap water by tilting it so that extra dye is removed from both sides.
- Add safranin as counterstain for 1 minute.
- Rinse the slide again with water to remove the safranin reagent.
- Dry the bottom part of the slide properly before placing it on the microscope stage.
- Observe the smear under oil immersion lens at 1000× magnification.
Endospore Staining Results
- Endospore: Will appear in green color.
- Vegetative Cells: Will appear in brownish-red to pink color.
It is observed that the vegetative cells stain pink or red while the endospores appear green. The vegetative forms take up the counterstain safranin and therefore the colour becomes pink-red.
The endospores retain the malachite green dye, so these structures is seen as green in the smear. This is because during the smearing and heat-fixing step, the malachite green is penetrated into the endospore with the help of steam. In the water rinse the dye is not washed away easily.
For the vegetative cells, the malachite green is removed due to their fragile outer covering, so they finally take the counterstain and appear pink-red in the stained preparation.
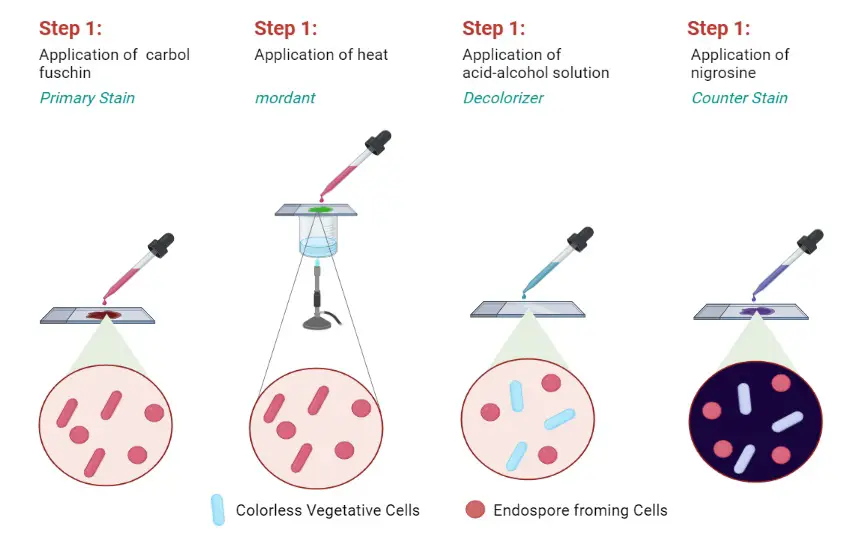
B. Endospore Staining by Dorner’s Method
The Dorner method is an older technique that is used for staining endospores. It is the process where resistant bacterial endospores is visualized separately from the vegetative cells. The method was first published by Dorner in 1922 and it remained an important differential staining procedure.
Dorner’s original technique was time consuming and the heating step was very lengthy. It is the reason why in 1933, Schaeffer and Fulton modified the method, making it more simple and faster. Because of this simplified procedure, the Schaeffer–Fulton method became more widely used in routine staining.
Principle
In this method, the heat or steam helps the primary dye to enter the endospore’s thick protective covering. The dye does not easily penetrate without heating. A special feature of the Dorner method is the use of Nigrosin as the negative stain. In this step the background becomes dark and the endospores appear bright due to the primary stain.
This is referred to as differentiation through contrast with the background rather than staining the vegetative cells strongly. Hence the endospore is clearly seen against the dark field appearance.
Requirement
- Carbolfuchsin stain – It is used as the primary stain. It can be prepared by mixing 0.3 g basic fuchsin with 10 ml ethanol (95% vol/vol), then adding 5 ml phenol (heat-melted crystals) and finally 95 ml distilled water.
- Decolorizing solvent (acid-alcohol) – This is used for removing excess stain. It is prepared by taking 97 ml ethanol (95% vol/vol) and adding 3 ml concentrated hydrochloric acid.
- Nigrosin solution – It is used as the counterstain for giving a dark background. The solution is prepared by dissolving 10 g nigrosin in 100 ml distilled water.
Procedure
Preparation of Slide
- Clean the glass slide with alcohol so any stains is removed.
- Put two small drops of water in each circle using a sterile inoculation loop.
- Open the culture tube aseptically, flame the mouth and collect a loopful of the bacterial culture.
- Flame the tube again and close it.
- Smear the bacterial culture in the drop of water on the slide.
- Air dry the smear till it becomes completely dry.
- Heat-fix the slide by passing it over the blue flame 3–4 times with smear facing up.
- Allow the slide to cool and staining is started.
Staining Steps
- Cover the smear with an absorbent paper.
- Saturate the paper with carbol-fuchsin and steam it over a boiling water bath for about 5–10 minutes, adding dye when required.
- Remove the absorbent paper and decolorize the smear with acid-alcohol for 1 minute.
- Rinse the slide with tap water and tap dry.
- Add a thin film of nigrosin reagent as the counterstain.
- Observe the preparation under the oil immersion lens (1000×) for the presence of endospores.
Result
It is observed that the vegetative cells appear colourless in the stained preparation. The endospores retain the carbol-fuchsin dye and therefore these structures is seen as red.
This contrast is produced because the background becomes dark due to nigrosin and the unstained vegetative cells remain clear, while the endospores hold the primary stain and show a bright red appearance.
- Endospore: Will appear in red color, against dark background.
- Vegetative Cells: Will appear as colorless.
Other Methods of endospore staining
Although the idea of endospore staining is consistent, there are differences in the main stain, counterstain, and use of decolorizer. Several are summarised below.
| Method | Primary Stain | Decolorizer | Counterstain | Interpretation |
| Modified Zeihl-Nelson’s method | Carbol Fuschin | 0.25-0.5% sulphuric acid | Leoffler’s methylene blue | Spores appear red, bacteria are blue |
| Dorner method | Carbol Fuschin | Acid-alcohol | Nigrosin | Spores red Bacteria colorless Background Black |
| Schaeffer-Fulton Stain | Malachite Green | Water | Safranin | Spores appear green vegetative cells appear pink/red |
| Bartholomew and mittwer method | Malachite Green | Water | Safranin | Spores appear green vegetative cells appear pink/red |
| Abbott’s method | Methylene Blue | Acid alcohol | Aniline fuschin | Spores appear blue bacteria are red |
| Moeller’s stain | Carbol fuschin | Acidified ethanol | Methylene blue | Spores appear red bacteria are Blue |
| Modified Moller’s stain | Kinyoun’s Carbol fuschin | 2%sulphuric acid and 80% ethanol | Loeffler methylene blue | Spores appear red bacteria are Blue |
Applications of the Endospore stain
- It is used to differentiate spore-forming bacteria from non-spore-forming ones.
- The stain helps to see the endospore separately from the vegetative cell in the same smear.
- It is used to identify the location of the endospore inside the cell, whether it is central, terminal or subterminal.
- The shape of the endospore like spherical or elliptical is also observed by this stain.
- It is used to study whether the sporangium is distended by the spore or not.
- It is important in clinical diagnosis because pathogenic genera like Bacillus and Clostridium produce endospores.
- The stain helps in early identification of diseases such as anthrax, tetanus, botulism and gas gangrene.
- It shows the resistant nature of endospores which explain why they survive heat, radiation, disinfectants and antibiotics.
- It is used in industrial microbiology for checking sterilization processes.
- In environmental studies it helps to understand survival of bacteria in unfavourable conditions.
Advantages of endospore staining
- It is used to differentiate spore-forming bacteria from non-spore-forming ones clearly.
- The method shows green spores and pink cells which help in early identification of spore-forming genera like Bacillus and Clostridium.
- It allows the observation of the endospore shape such as spherical or elliptical.
- The exact location of the endospore inside the vegetative cell is seen whether it is central, terminal or subterminal.
- It helps to distinguish true spores from other structures that may look similar.
- It is useful in presumptive diagnosis because the position and shape of the spores is included in microscopy reports.
- It is important in clinical samples for identifying pathogens that cause diseases like tetanus, anthrax, botulism and gas gangrene.
- The stain also shows why endospores are more resistant to heat, radiation, disinfectants and antibiotics.
- It is used in industrial microbiology to check the efficiency of sterilization procedures.
- The stain gives information about bacterial survival mechanisms under adverse conditions.
- The Schaeffer–Fulton method is simpler and faster compared to older techniques like the Dorner method.
Limitations of endospore staining
- The stain only shows the presence of endospores and does not indicate viability or activity.
- Some bacteria with resistant cell walls like Mycobacterium may retain malachite green and give misleading results.
- The same information about spore position and shape can also be seen by phase-contrast microscopy without staining.
- Endospores may also appear as clear outlines in a simple Gram stain, so the stain is not always necessary.
- The method depends heavily on steaming which makes the procedure slow and sometimes messy.
- If heating is not done properly the sample may get damaged.
- Poor technique can give false results because the stain penetration depends on correct timing and temperature.
- Insufficient steaming may fail to stain the spores and give a false negative result.
- If the smear is not rinsed well the vegetative cells may retain green dye and appear similar to spores.
- There is a safety concern when absorbent paper is used during steaming because it may burn or drip.
- The age of the culture is very important since young cultures may not show any endospores.
Endospore Staining Video
FAQ
What is endospore staining?
Endospore staining is a type of differential staining technique used in microbiology to identify and distinguish bacterial endospores from other types of bacteria.
How does endospore staining work?
Endospore staining involves the use of two dyes, one to stain the endospores and another to decolorize the surrounding bacterial cells. The endospores take up the stain and appear dark against a lighter background, making them easily visible under a microscope.
What is the purpose of endospore staining?
The purpose of endospore staining is to specifically detect and visualize endospores, which are highly resistant structures produced by some types of bacteria. The staining can also be used to differentiate between endospore-forming and non-endospore-forming bacteria.
What are the steps involved in endospore staining?
The steps involved in endospore staining include heat fixation, staining with a primary dye, decolorization, counterstaining, and observation under a microscope.
What are the dyes used in endospore staining?
The dyes used in endospore staining are typically malachite green for the primary stain and safranin for the counterstain.
Can endospore staining be used to determine the viability of bacterial populations?
Yes, endospore staining can be used to determine the viability of bacterial populations, as dead endospores will not take up the stain and will appear unstained or lighter in color.
How does endospore staining compare to other staining techniques?
Endospore staining is a specific and relatively simple staining technique that is highly effective in identifying endospores. It is less complex and less time-consuming compared to other staining techniques such as the Gram stain.
What are the advantages of endospore staining?
The advantages of endospore staining include specificity, ease of use, cost-effectiveness, and resistance to decolorization.
Can endospore staining be used in food and water quality control?
Yes, endospore staining can be used in food and water quality control to identify the presence of endospore-forming bacteria, which can indicate contamination or spoilage.
What are the applications of endospore staining in bacterial research?
The applications of endospore staining in bacterial research include the study of endospore formation, structure, and resistance, as well as the investigation of the mechanisms involved in endospore formation and germination.
- Ahern, H. (n.d.). Differential Staining Techniques. In Microbiology: A Laboratory Experience. Milne Publishing.
- Aryal, S. (2022, August 10). Endospore Staining- Principle, Reagents, Procedure and Result. Microbiology Info.
- Basta, M., & Annamaraju, P. (2023, January 30). Bacterial Spores. In StatPearls [Internet].
- StatPearls Publishing. Retrieved from NCBI Bookshelf.
- Bush, P., & Martin, K. (2024). Endospore Stain. In WPUNJ Microbiology Laboratory Manual. WPU Pressbooks.
- Endospore Staining: A Comprehensive Analysis of Structural Resistance and Differential Diagnostic Protocols. (n.d.).
- Endospore staining. (n.d.). In Wikipedia.
- GMP Plastic. (2025, April 2). Endospore Staining: Staining Procedure to Detect Bacterial Endospores.
- Hartline, R. (n.d.). 1.12: Endospore Stain. In Microbiology Laboratory Manual. Biology LibreTexts. Retrieved from https://bio.libretexts.org/Bookshelves/Microbiology/Microbiology_Laboratory_Manual_(Hartline)/01%3A_Labs/1.12%3A_Endospore_Stain
- Hussey, M. A., & Zayaitz, A. (2007). Endospore Stain Protocol. American Society for Microbiology.
- Matthew. (2025, November 21). Why Heat is Crucial for Effective Endospore Staining. Smart.DHgate.
- MBBS NAIJA. (n.d.). Endospore Stain (Schaeffer Fulton Method Or Dorner Method) ; Staining Technique in Microbiology [Video]. YouTube.
- Merck KGaA. (2018). 04551 Schaeffer and Fulton Spore Stain Kit (Spore Stain Kit according to Schaeffer and Fulton).
- Miller, E. (2024). Acid-Fast and Endospore Staining. In Microbiology Laboratory Manual. Open Oregon Educational Resources.
- Petersen, J., & McLaughlin, S. (n.d.). 4.2: Procedures. In Laboratory Exercises in Microbiology (McLaughlin and Petersen). Biology LibreTexts. Retrieved from https://bio.libretexts.org/Learning_Objects/Laboratory_Experiments/Microbiology_Labs/Laboratory_Exercises_in_Microbiology_(McLaughlin_and_Petersen)/04%3A_Acid_fast_and_Endospore_Staining/4.02%253A_Procedures
- Watson, R. (n.d.). Endospore Stain. The Virtual Edge, University of Wyoming.