It is a biochemical test used for identification and differentiation of bacteria based on their ability to decarboxylate certain amino acids. This test is mainly used for the differentiation of members of Enterobacteriaceae family. It is the process in which specific amino acids like lysine, ornithine, and arginine are broken down by bacterial enzymes to form alkaline amines and carbon dioxide. The enzyme involved in this test is decarboxylase or dihydrolase enzyme, which removes the carboxyl group (–COOH) from the amino acid. The test is performed using Moeller’s decarboxylase broth which contains glucose, a specific amino acid, and pH indicator.
In this test, the medium is first acidified due to fermentation of glucose by the organism, which changes the colour of the medium from purple to yellow. This acidic condition is required for induction of decarboxylase enzyme. If the organism possesses the enzyme, the amino acid is decarboxylated and alkaline amines are produced, which neutralizes the acid and the medium again turns purple. This is referred to as a positive decarboxylase test. If the organism does not produce the enzyme, the medium remains yellow indicating a negative result. A control tube without amino acid is always used to confirm glucose fermentation and viability of the organism.
Principle of Decarboxylase Test
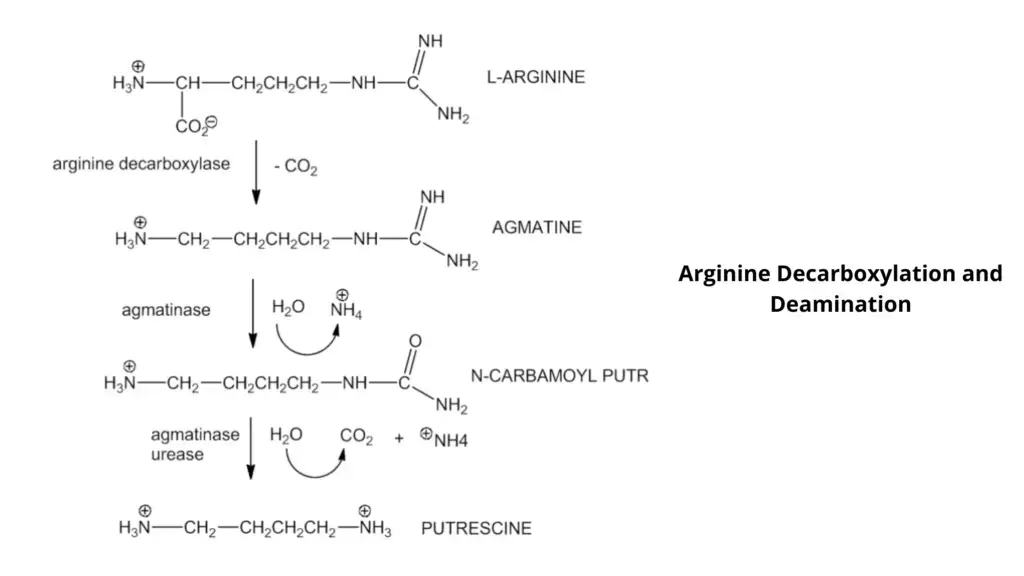
It is the process by which the decarboxylase test is used to detect the ability of an organism to produce specific decarboxylase enzymes. These enzyme is responsible for removing the carboxyl group (–COOH) from amino acids such as lysine, ornithine or arginine. During this reaction carbon dioxide is released and alkaline amines are formed, which results in increase of pH of the medium. This is referred to as decarboxylation reaction.
In this test, the medium contains glucose, a specific amino acid and a pH indicator such as bromcresol purple or cresol red. Initially the organism ferments glucose and acid is produced which lowers the pH of the medium turning it yellow. This acidic condition is essential as it helps in inducing the production of decarboxylase enzymes. If the organism possess the specific decarboxylase enzyme, the amino acid is decarboxylated and alkaline amines like cadaverine or putrescine is formed which neutralize the acid.
As a result of amine production the pH of the medium rises and the colour of the indicator changes back to purple indicating a positive result. In the absence of decarboxylase activity no amines are produced and the medium remains acidic and yellow. To maintain the acidic condition required for enzyme induction, the medium is overlaid with mineral oil which creates anaerobic condition and prevents oxidation.

Objective of Decarboxylase Test
- To determine the ability of an organism to produce specific decarboxylase enzymes (lysine, ornithine and arginine) which removes the carboxyl group (–COOH) from amino acids and form alkaline reacting amines.
- To differentiate members of Enterobacteriaceae family from other gram negative rods on the basis of decarboxylase enzyme activity.
- To distinguish closely related bacterial genera within Enterobacteriaceae group by their specific enzymatic reaction pattern.
- To assist in identification of specific pathogens by using individual decarboxylase reactions–
- Lysine decarboxylase test helps in identification of Salmonella (positive) and differentiation from Shigella (negative).
- Ornithine decarboxylase test is used to differentiate Klebsiella (ODC negative) from Enterobacter (ODC positive) and also Proteus mirabilis from Proteus vulgaris.
- Arginine decarboxylase test helps in differentiating enteric bacteria with similar physiological characters and aiding in identification of organisms like Pseudomonas aeruginosa.
Requirements
- Test organism– A fresh pure culture of the organism is required. The culture used is generally 18–24 hours old.
- Control strains– Known positive and negative control organisms are used to compare the reaction and to confirm reliability of the test.
- Decarboxylase broth base (Moeller’s medium)– It contains peptone and beef extract as nutrient source, glucose for fermentation and pyridoxal which acts as enzyme co-factor.
- Amino acid substrate– Specific amino acid such as L-lysine, L-ornithine or L-arginine is added depending on the test to be performed.
- Control broth– A basal medium without amino acid is required to check glucose fermentation and viability of organism.
- pH indicator– Bromcresol purple or cresol red is used to detect change in pH of the medium during reaction.
- Anaerobic sealant– Sterile mineral oil or liquid paraffin is used to overlay the medium to create anaerobic condition.
- Test tubes– Screw cap tubes are preferred for proper sealing during incubation.
- Inoculating tools– Sterile inoculating loop or needle is required for transfer of culture.
- Pipette– Sterile pipette is used for adding mineral oil overlay.
- Incubator– Incubator maintained at 35–37°C is required for incubation up to 3–4 days.
Procedure of Decarboxylase Test
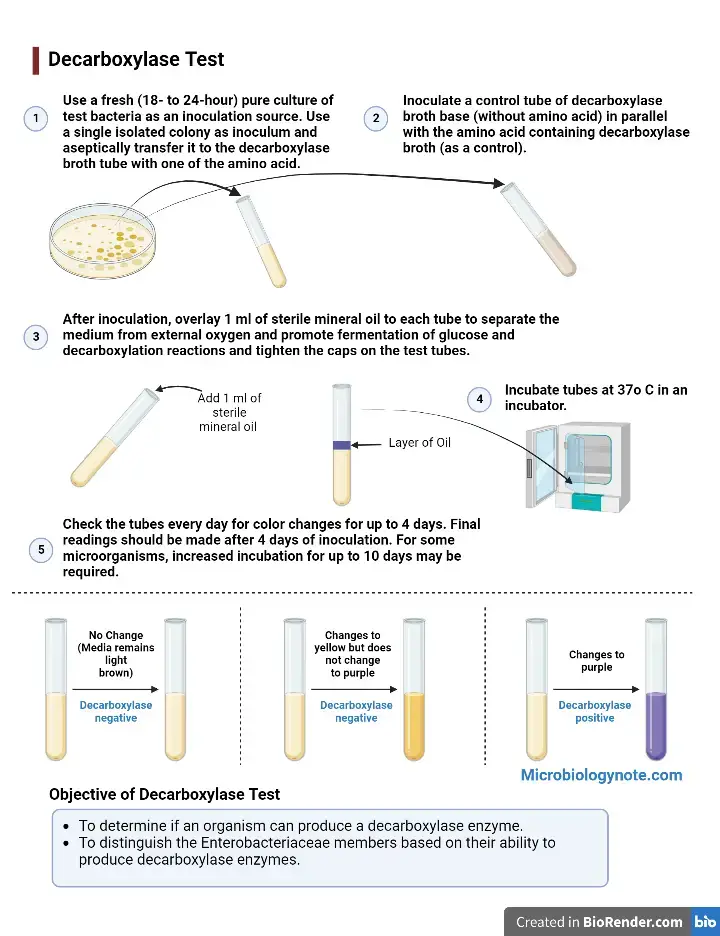
- Preparation
- Tubes of decarboxylase broth containing specific amino acids (L-lysine L-arginine and L-ornithine) is taken.
- One tube of decarboxylase base broth without amino acid is taken as control.
- All tubes is labeled properly with name of organism and amino acid used.
- Inoculation
- A fresh pure culture of 18–24 hours old is used for inoculation.
- Each tube including control is inoculated aseptically with test organism.
- For glucose fermenting organisms a light inoculum is sufficient.
- For glucose non fermenting organisms a heavy suspension (McFarland No. 5) is used.
- Anaerobic Seal
- After inoculation each tube is overlaid with sterile mineral oil (about 4–5 mm).
- This step is important to prevent entry of oxygen and to allow fermentation.
- Incubation
- Caps of tubes is tightened properly.
- Tubes is incubated at 35–37°C in ambient air.
- Observation and Timing
- Tubes is observed daily for colour change.
- Results is not read before 18–24 hours as initial glucose fermentation is required.
- Incubation is continued up to 4 days and some organisms may require longer time.
- Reading of Results
- Colour of amino acid tubes is compared with control tube.
- Purple or greyish purple colour indicates positive decarboxylase reaction.
- Yellow colour or no change indicates negative result.
- If control tube turns purple the test is considered invalid.
Result Interpretation of Decarboxylase Test
- Positive Result (+)– The medium turns purple or violet in colour. It indicates that the organism produces specific decarboxylase enzyme which acts on the amino acid present in the medium and forms alkaline amines such as cadaverine or putrescine. Initially the medium turns yellow due to glucose fermentation and acid production. Later the accumulated amines neutralize the acid and raise the pH resulting in purple colour.
- Negative Result (–)– The medium turns yellow and remains yellow after incubation. This shows that the organism ferments glucose producing acidic end products but does not produce the specific decarboxylase enzyme required to reverse the acidity of the medium.
- Negative Result (Glucose Non-Fermenter)– The medium shows no colour change and remains light brown or same as the uninoculated control. It indicates that glucose is not fermented and the acidic condition necessary for induction of decarboxylase enzyme is not produced.
- Invalid Test– If the control tube containing basal medium without amino acid turns purple or shows alkaline reaction the test is invalid. The control tube must remain yellow or unchanged to confirm that the colour change in test medium is due to amino acid decarboxylation only and not due to other reactions such as oxidative deamination.
| Media color | Bacterial reaction |
| No Change (Media remains light brown) | Decarboxylase negative (-) |
| Changes to yellow but does not change to purple | Decarboxylase negative (-) |
| Changes to purple | Decarboxylase positive (+) |




Decarboxylase Test Result of Some organisms
| Test organism | Lysine decarboxylase test | Arginine decarboxylation test | Ornithine decarboxylase test |
| Escherichia coli | Positive Result | Positive Result | Positive Result |
| Enterobacter cloacae | Negative Result | Positive Result | Positive Result |
| Klebsiella pneumoniae | Positive Result | Negative Result | Negative Result |
| Klebsiella oxytoca | Positive Result | Negative Result | Negative Result |
| Enterobacter aerogenes | Positive Result | Negative Result | Positive Result |
| Proteus vulgaris | Negative Result | Negative Result | Negative Result |
| Pseudomonas aeruginosa | Negative Result | Positive Result | Negative Result |
| S. serotype typhi | Positive Result | delayed positive reaction or negative reaction,yellow colour | Negative Result |
| Serratia marcescens | Positive Result | Negative Result | Positive Result |
| Shigella flexneri | Negative Result | delayed positive reaction or negative reaction,yellow colour | Negative Result |
| Vibrio cholerae | Negative Result | Positive Result | Positive Result |
Precautions of Decarboxylase Test
- A fresh pure culture of 18–24 hours old should be used as old cultures may give false negative result.
- After inoculation the medium must be overlaid immediately with sterile mineral oil to maintain anaerobic condition. Absence of oil layer may cause false positive reaction.
- Caps of the test tubes should be tightened properly during incubation to prevent entry of air.
- A control tube without amino acid should always be inoculated and incubated along with test tubes to check validity of result.
- Results should not be read before 18–24 hours as initial glucose fermentation is required for induction of decarboxylase enzyme.
- Incubation should be continued for sufficient time as some organisms show delayed reactions.
- Proper amount of inoculum should be used. Very light inoculum may give false negative result while heavy inoculum may interfere with colour change.
- Tubes showing two colour layers should be gently shaken before interpretation.
- Colour change should always be compared with control tube for correct interpretation.
Uses of Decarboxylase Test
- It is used for identification and differentiation of members of Enterobacteriaceae from other Gram-negative rods.
- It helps in separation of Klebsiella species and Enterobacter species by ornithine decarboxylase test.
- It is useful in distinguishing Salmonella species from Shigella species by lysine decarboxylase reaction.
- It is used for differentiation of Proteus mirabilis and Proteus vulgaris based on ornithine decarboxylase activity.
- It helps in identification of specific Salmonella serotypes such as Salmonella Paratyphi A which is lysine decarboxylase negative.
- It aids in speciation of Enterobacter species such as Enterobacter aerogenes and Enterobacter cloacae.
- It is useful in identification of non glucose fermenting Gram-negative rods such as Pseudomonas, Acinetobacter, Stenotrophomonas and Burkholderia.
- It helps in identification of other genera like Aeromonas, Plesiomonas and Vibrio species.
- It is used in differentiation of Enterococcus species by arginine dihydrolase test.
Advantages of Decarboxylase Test
- It is a simple and reliable biochemical test used for differentiation of enteric bacteria especially members of Enterobacteriaceae.
- It helps in separation of closely related genera such as Salmonella and Shigella based on lysine decarboxylation reaction.
- It is useful in differentiation of Klebsiella species and Enterobacter species by ornithine decarboxylase activity.
- It aids in speciation of Proteus species such as differentiation of Proteus mirabilis and Proteus vulgaris.
- It is helpful in identification of non fermenting Gram negative bacilli like Pseudomonas, Acinetobacter and Aeromonas.
- The standard Moeller’s method shows high sensitivity and specificity and gives good taxonomic value.
- It has importance in food microbiology as it helps in detection of bacteria producing biogenic amines responsible for food spoilage and poisoning.
- Rapid modified methods of decarboxylase test are available which can give result within few hours.
Limitations of Decarboxylase Test
- Results should not be interpreted before 18–24 hours of incubation as early reading may give false negative result due to initial glucose fermentation. Some organisms may require prolonged incubation for positive reaction.
- Proper anaerobic condition is required for the test. If mineral oil seal is not applied correctly oxygen may enter and cause false positive alkaline reaction due to oxidative deamination.
- Glucose non fermenting organisms may give weak or indefinite colour change as the acidic condition required for induction of decarboxylase enzyme is not produced.
- The control tube must remain yellow or unchanged. If the control tube turns purple the test result is considered invalid.
- Very light inoculum may not produce sufficient alkaline amines to overcome the acidity resulting in false negative reaction.
- Sometimes colour change may be unclear such as grey or faint purple colour which makes interpretation difficult and requires careful comparison with control.
- Variation in glucose concentration of the medium can affect the result. Excess glucose may produce more acid which cannot be neutralized by amines.
- The test detects only phenotypic enzyme activity and should not be used as the sole method for identification of organisms.
- Biolab Diagnostics Laboratory Inc. (2016). Culture media for amino acid decomposition studies [Technical sheet].
- Biolife Italiana S.r.l. (2025). Decarboxylase Moeller base broth [Instructions for use].
- Bonev, S. I., Zakhariev, Z., & Gentchev, P. (1974). Comparative study of media for determination of lysine decarboxylase activity. Applied Microbiology, 27(3), 464–468.
- Comparative biochemical and genetic analysis of amino acid decarboxylation: Standardizing the decarboxylase test for clinical and diagnostic microbiology. (n.d.). [Source text provided].
- Cooper, C. R., Jr. (2018). BIOL 3702 lab exercise: Decarboxylation test. Youngstown State University.
- Difco & BBL. (n.d.). Lysine decarboxylase broth. In Difco & BBL manual (2nd ed.).
- HiMedia Laboratories. (2015). Decarboxylase test medium base (Falkow) [Technical data].
- HiMedia Laboratories. (2024). Decarboxylase broth base, Moeller [Technical data].
- Kanjee, U., Gutsche, I., Alexopoulos, E., Zhao, B., El Bakkouri, M., Thibault, G., Liu, K., Ramachandran, S., Snider, J., Pai, E. F., & Houry, W. A. (2011). Linkage between the bacterial acid stress and stringent responses: The structure of the inducible lysine decarboxylase. The EMBO Journal, 30(5), 931–944.
- Key Scientific Products. (n.d.). Decarboxylase and dihydrolase tests [Package insert].
- Lal, A., & Cheeptham, N. (2015, September 2). Decarboxylase broth protocol. American Society for Microbiology.
- Liofilchem. (2023). Lysine decarboxylase test [Instructions for use].
- Rao, T. V. (n.d.). Biochemical tests in Enterobacteriaceae [PowerPoint slides]. Scribd.
- Sandoval, M., & Shah, D. D. (2025). Diversity and distribution of amino acid decarboxylase enzymes in the human gut bacteria—a bioinformatics investigation. Frontiers in Microbiology, 16, 1616635.
- Sapkota, A. (2022, January 20). Decarboxylase test: Principle, procedure, results, uses. Microbe Notes.
- Seputiene, V., Suziedelis, K., Normark, S., & Melefors, Ö. (2004). Transcriptional analysis of the acid-inducible asr gene in enterobacteria. Research in Microbiology, 155(7), 535-542.
- Shi, X., Waasdorp, B. C., & Bennett, G. N. (1993). Modulation of acid-induced amino acid decarboxylase gene expression by hns in Escherichia coli. Journal of Bacteriology, 175(4), 1182–1186.
- Sigma-Aldrich. (2018). D2935 Decarboxylase broth base, Moeller [Product information].
- Tankeshwar, A. (n.d.). Decarboxylation test: Types, principles, uses. Microbe Online.
- Uyirgene International. (n.d.). Decarboxylases test.
- Viala, J. P. M., Méresse, S., Pocachard, B., Guilhon, A.-A., Aussel, L., & Barras, F. (2011). Sensing and adaptation to low pH mediated by inducible amino acid decarboxylases in Salmonella. PLoS ONE, 6(7), e22397.
- VUMIE. (2022). Lysine decarboxylase test [Software].
- VUMIE. (2022). Ornithine decarboxylase test [Software].
- Wauters, G., Avesani, V., Charlier, J., Janssens, M., & Delmée, M. (2004). Histidine decarboxylase in Enterobacteriaceae revisited. Journal of Clinical Microbiology, 42(12), 5923–5924.