Collagen Hybridizing Peptide (CHP) staining is the process used to detect the denatured or degraded collagen that occur in different tissues. It is based on a synthetic short peptide which mimic the natural collagen sequence having 6 to 10 repeating units of Gly-Xaa-Yaa (such as Gly-Pro-Hyp). This peptide is rich in Proline and Hydroxyproline which gives it a strong tendency to form a triple helical structure. It is the process in which the single-stranded CHP binds to the unfolded collagen strands and forms a hybridized triple helix. This is referred to as collagen hybridization.
The binding is made possible because the monomeric CHP recognizes the exposed Gly-Xaa-Yaa sequence of the damaged collagen. This mechanism is similar to DNA fragments annealing to their complementary strands. It is very specific because the intact collagen triple helix does not provide any free strands for binding so the affinity is negligible.
In this technique the CHP must undergo a thermal activation step. The peptide solution is heated (about 80°C for few minutes) so that the self-assembled trimers are separated into active single strands. It is then cooled quickly to prevent further damage of tissue. When the activated peptide is incubated with the tissue, usually at 4°C, it interacts strongly with the denatured collagen regions. The hybridized triple helix is stable at this lower temperature. Different reporter molecules like Fluorescein (F-CHP), Cy3 (R-CHP), or Biotin (B-CHP) are attached to the CHP so that the stained regions can be visualized using fluorescence microscopy or other detection systems.
CHP staining is important because collagen is the major structural protein of the body and it undergoes denaturation during enzymatic degradation, mechanical damage, thermal injury, or pathological remodeling. These changes occur in diseases like pulmonary fibrosis, myocardial damage, bone disorders, and skin aging.
Conventional stains like Masson’s trichrome or Picrosirius red detect only total collagen and therefore cannot distinguish intact collagen from degraded collagen. CHP staining is used to visualize only the damaged collagen which gives better information for tissue remodeling studies. It is used in histology, immunofluorescence, in-gel collagen detection, and in studying mechanical injuries of connective tissues.
Principle of Collagen Hybridizing Peptide Staining
The principle of Collagen Hybridizing Peptide (CHP) staining is based on a molecular process called collagen hybridization. It is the process in which a single–stranded synthetic peptide mimicking the Gly-Xaa-Yaa (Gly-X-Y) repeat of collagen binds to the denatured collagen strands present in damaged tissues. The CHP contains 6 to 10 repeating units of this triplet (such as Gly-Pro-Hyp) which gives it the tendency to form a triple-helical conformation. When collagen is intact it remains in a tightly packed triple helix, and no binding sites are exposed for the peptide. When collagen is degraded by enzymes (MMPs or Cathepsin K), mechanical stress, or heat, the triple helix becomes unfolded. This exposes the individual collagen chains. The monomeric CHP strand can now recognize these unfolded regions and hybridize with them forming a new stable triple helix. This mechanism is similar to DNA strands annealing during PCR.
It is the selective binding that makes the principle unique because the peptide shows negligible affinity toward intact collagen molecules. The specificity occurs due to the requirement of a free collagen strand for forming hydrogen bonding and chain assembly. The peptide is neutral and hydrophilic so it remains inert toward non-specific proteins or biomolecules in the matrix. For this hybridization to occur, the CHP must be present in the monomeric form. During storage the peptide slowly reassembles into inactive trimers which cannot bind to damaged collagen. Therefore it is heated (about 80°C for few minutes) to dissociate these trimers. Immediate cooling is required so that the active single strands do not re-form trimers. These activated strands hybridize with any unfolded collagen chains during incubation, usually at low temperature (4°C) where the triple helix formation is more stable.
The binding depends on the conserved secondary structure of Gly-X-Y repeats rather than a specific epitope. Because of this, the CHP can detect unfolded chains from almost all collagen types and across different species. This principle allows the technique to be used in detecting molecular collagen damage, in visualizing extracellular matrix remodeling, and even in SDS-PAGE gels for observing collagen bands without the need of a western blotting step.
Objective
Collagen Hybridizing Peptide Staining is used to detect the Denatured collagen ligaments.
Requirement
Preparation of Active CHP Monomers
- The lyophilized CHP powder is dissolved in pure water or in PBS to prepare a stock solution (about 100 μM).
- The stock solution is kept at 4°C.
- It is the step where trimeric CHP is dissociated because the peptide forms inactive triple helices during storage.
- The diluted working solution is heated at 80°C for around 5 minutes in sealed tubes.
- Rapid quenching is done immediately in an ice-water bath for about 15–90 seconds.
- The solution is then applied to the tissue quickly as the active monomers lose activity with longer delay.
CHP Working Solution and Concentration
- The stock solution is diluted in PBS just before staining.
- The general recommended concentration is around 20 μM.
- In most samples it can vary between 5 μM to 30 μM.
Tissue Preparation
- Frozen and paraffin-embedded sections are used for CHP staining.
- Paraffin or OCT compound is removed by standard laboratory steps.
- Antigen retrieval is not required because the binding depends on denatured collagen chains.
- Fixation is usually not important for this staining. If fixation is needed, formaldehyde is preferred for reducing auto-fluorescence.
Incubation Conditions
- The incubation is carried out at 4°C as the binding affinity is higher at lower temperature.
- Minimum incubation time is about 2 hours. Overnight incubation at 4°C is used for better staining.
Detection and Counterstaining
- CHP must be conjugated with a detectable motif (F-CHP for green fluorescence, R-CHP for red fluorescence, B-CHP for biotin labeling).
- After staining, slides are washed in PBS for 5 minutes, repeated three times.
- CHP staining is compatible with antibody staining. Primary antibodies can be added directly into the quenched CHP solution.
- Blocking may be skipped due to low non-specific binding, but it is used when antibodies are involved.
Control Measures
- A negative control is prepared without the pre-heating step.
- This control keeps CHP in its inactive trimeric form, and it helps verify that the staining is due to collagen hybridization rather than non-specific attachment.
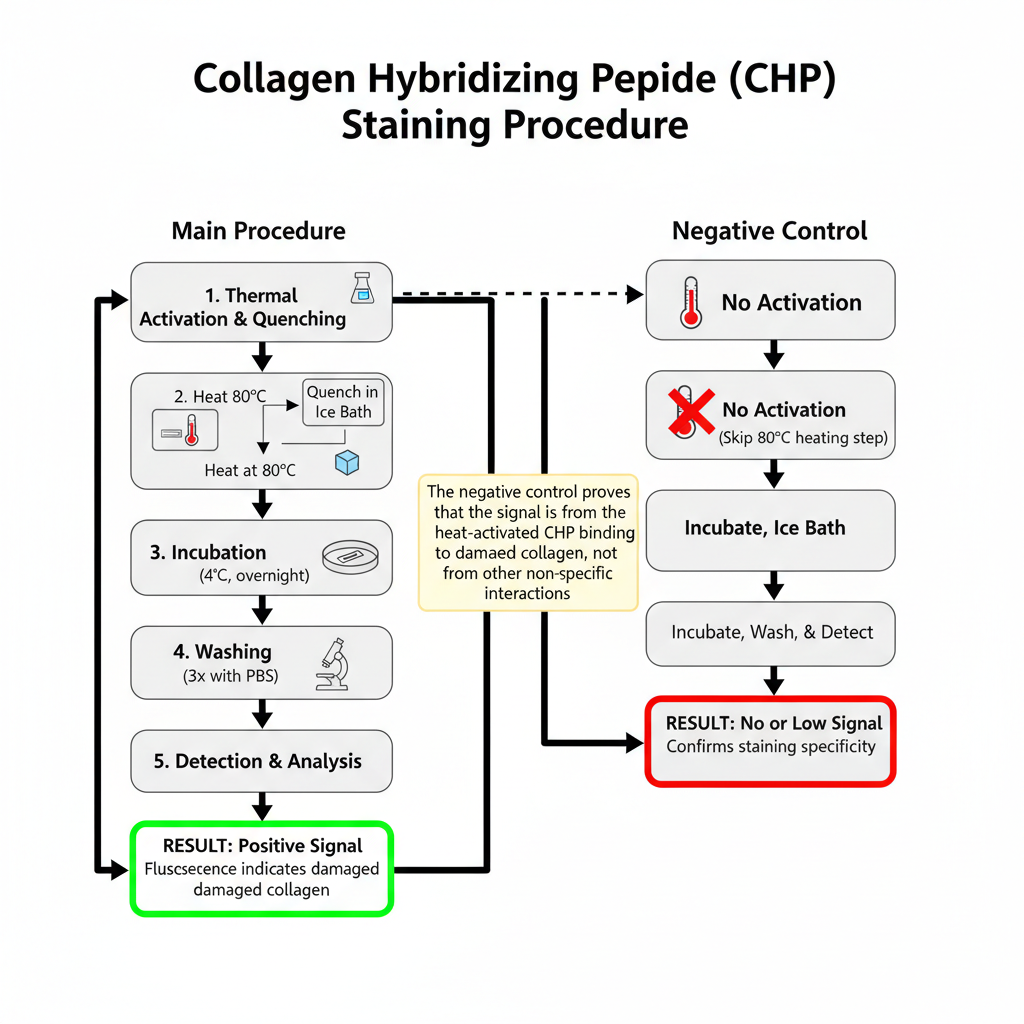
Procedure of Collagen Hybridizing Peptide Staining
I. Preparation of CHP Stock Solution
- The lyophilized CHP powder is dissolved in pure water or PBS to make a stock solution of about 100 μM.
- The prepared stock solution is stored at 4°C, and fluorescent forms are kept protected from light.
II. Sample Preparation (Tissue Sections)
- Frozen sections and paraffin-embedded sections are used for CHP staining.
- The embedding material (OCT or paraffin) is removed by standard laboratory steps.
- Antigen retrieval is not required in this method.
- Formaldehyde fixation can be used when needed because it gives less autofluorescence in fluorescent imaging.
- Blocking with serum or BSA is optional and used only when antibody co-staining is planned.
III. Thermal Activation and Quenching
- The stock solution is diluted in PBS buffer to form the working concentration (usually around 20 μM).
- The diluted solution is heated at 80°C for 5 minutes inside sealed tubes to dissociate the trimeric CHP.
- Immediate quenching is done by placing the tube in an ice-water bath for around 15–90 seconds.
- The quenched CHP is then placed on the tissue quickly, keeping the delay around 1–3 minutes.
– Primary antibodies can be mixed directly with the quenched solution when co-staining.
IV. Incubation and Washing
- The slides are incubated with the staining mixture at 4°C. A minimum of 2 hours is required, and overnight incubation is usually preferred.
- After incubation, the slides are washed in PBS for 5 minutes, repeated three times to remove unbound peptide.
V. Detection and Analysis
- F-CHP and R-CHP stained tissues are viewed directly under fluorescence microscopes using appropriate filters.
- B-CHP labeled tissues are detected by incubating with labeled streptavidin (about 0.005 mg/mL) for 1 hour at room temperature.
- Antibody co-staining is detected with secondary antibodies either mixed with streptavidin (for B-CHP) or added after CHP staining (for F-CHP or R-CHP).
VI. Negative Control
- A negative control is prepared by skipping the 80°C heating step so the CHP remains in its inactive trimeric structure.
- This control should show no or very low signal, confirming that the staining in the activated sample is due to collagen hybridization.
Result and interpretation of Collagen Hybridizing Peptide Staining
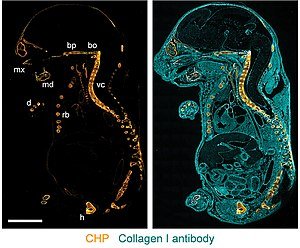
It is the process where denatured or unfolded collagen chains is specifically visualized by the binding of Collagen Hybridizing Peptide. The staining shows the distribution of damaged collagen which has lost its native triple-helical structure. The signal is usually fluorescent depending on the conjugate used, and it is taken as a direct indication of collagen degradation or mechanical injury in the tissue.
In most samples, the CHP signal appears only in regions where collagen is partially degraded, while intact collagen does not show any significant binding. This is referred to as the selective affinity of CHP towards the single-stranded collagen fragments. These are important because the common stains like Picrosirius Red or Masson’s Trichrome stain the total collagen, but CHP only detects the damaged regions.
The reaction is visualized according to the label attached to the peptide. F-CHP gives green fluorescence (Ex 494 nm/ Em 512 nm), R-CHP gives red fluorescence (Ex 548 nm/ Em 563 nm) and B-CHP is usually detected through avidin or streptavidin systems. The colourimetric detection through HRP is also used for reducing background.
Interpretation
The interpretation is based on the intensity and localization of the CHP binding. A strong signal means a high amount of collagen denaturation in that region. It is the major source of understanding tissue damage, inflammation and remodeling.
Some of the main features are–
- It indicates that the triple helix of collagen is opened and the strands become accessible to the peptide.
- It shows specific areas where disease-associated remodeling is occurring.
- It also identifies mechanical damage in load-bearing tissues like bone.
In quantitative studies, the CHP fluorescence intensity correlates strongly with the percentage of denatured collagen. In cortical bone, the value shows a very high linear relationship with the Trypsin-Hydroxyproline (T-H) assay (r² = 0.99) which confirms reliable measurement. It is the process that allows non-destructive quantification.
In mechanically damaged bone, R-CHP fluorescence is increased in the regions that undergo post-yield deformation (around 42% increase compared to pre-yield). This confirms that collagen denaturation occurs during plastic deformation. In fractured bone surfaces also, high CHP intensity is seen, which is referenced to the severely damaged collagen fibrils.
CHP reveals subtle levels of damage which the bulk stains cannot detect. This is the reason why it is considered more sensitive than Picrosirius Red.
Pattern in Physiological and Pathological Conditions
These are the important observations–
- Myocardial Infarction
CHP signal is visible at day 3, reaches maximum at day 7 and continues up to day 14. It is localized near macrophage-rich sites. This indicates active collagen degradation during inflammation and cardiac repair. - Glomerulonephritis
Strong CHP binding is detected only inside the diseased glomeruli. Normal kidney shows negligible staining. It signifies that type IV collagen in the basement membrane is unfolded or degraded in the lesion. - Pulmonary Fibrosis (Bleomycin model)
Damaged collagen shows a spotty and highly localized CHP pattern. High MMP2 areas often show reduced CHP intensity. This shows that denatured collagen is produced and then digested by collagenolytic enzymes. - Endochondral Ossification
CHP binding is seen in the hypertrophic cartilage and developing bone regions. No binding occurs in nonskeletal tissues. It confirms collagen remodeling during normal bone development. - Skin Aging
Higher CHP signals are observed in aged dermis. This is the evidence of collagen fragmentation in chronological aging. - SDS-PAGE (In-gel detection)
Fluorescent collagen bands appear only in lanes containing collagen protein. It is because CHP binds to heat-denatured single collagen strands inside the gel. Other protein lanes do not show any band.
Importance of Controls
The correct interpretation must include a negative control. The unheated trimeric form of CHP is used as the negative control because it cannot bind denatured collagen.
If the heated (monomeric) CHP shows a high signal and the unheated form shows no signal, then the staining is confirmed as specific. In heat-denatured ligament sections the signal is clear, while intact ligament shows no signal. This is taken as proof of correct hybridization.
Advantages of Collagen Hybridizing Peptide Staining
- It shows high specificity as CHPs bind only to denatured or unfolded collagen chains.
- The staining gives a clear signal with very low background because non-specific binding is negligible.
- It is superior to the common stains (Picrosirius Red and Masson’s Trichrome) which stain total collagen without distinguishing healthy and damaged parts.
- CHP acts as a functional biomarker showing the quality and remodeling status of collagen.
- It allows accurate quantification because the binding is based on a defined molecular mechanism.
- The staining is highly reproducible and removes observer-dependent variation.
- It has higher sensitivity and detects small or early collagen damage that bulk stains may miss.
- The fluorescence intensity of CHP correlates strongly with the destructive Trypsin-Hydroxyproline assay, so it is taken as a reliable non-destructive method.
- CHP binds to denatured collagen of all types because it depends on the Gly-X-Y backbone, so it works across many tissues and species.
- It is suitable for histology, biochemical assays like SDS-PAGE, live cells, whole tissue and 3D culture systems.
- The small size of the peptide helps in deep tissue penetration and whole-specimen staining.
- No antigen retrieval step is needed for section staining.
- It can be easily combined with any antibody because there is no species limitation.
- CHPs are compatible with automated staining platforms which improves workflow and consistency.
- Different detection labels like Fluorescein, Cy3 and Biotin are available for both brightfield and fluorescence imaging.
- It helps in detecting mechanical damage at molecular level in tissues like tendon, ligament and bone.
- It is useful for quality control of extracellular matrix scaffolds in tissue engineering.
- Radiolabeled CHP can be used for in vivo imaging to monitor active collagen remodeling.
- It also has potential in supporting collagen fibrillogenesis which may help in regenerative studies.
Limitations of Collagen Hybridizing Peptide Staining
- CHP does not bind to intact collagen, so it cannot show the total collagen amount unless all collagen is artificially denatured.
- It cannot distinguish between different collagen types because the binding depends on the Gly-X-Y structure only.
- Some non-collagenous proteins having short triple-helical regions may also bind when they unfold.
- The peptide must be thermally activated before use, and this makes the procedure more complex.
- The active monomer slowly converts back to the inactive trimer, so the handling time must be kept short.
- Fluorescence intensity can vary between experiments, making comparison difficult without a reference.
- Quantitative analysis needs an internal calibration which may require destroying part of the sample.
- Autofluorescence from tissues like bone can interfere with CHP fluorescence, especially with green labels.
- Penetration into dense or mineralized tissues is limited and needs higher temperature staining for improvement.
- Heat-based antigen retrieval can denature all collagen and produce staining of total collagen instead of damaged collagen.
- Slides previously treated with some immunohistochemistry methods may not show reliable CHP binding.
Uses of Collagen Hybridizing Peptide Staining
- It is used in histology to detect and localize degraded collagen in tissues.
- It helps in studying inflammation and tissue damage in different diseases.
- It is used for identifying collagen remodeling in fibrosis, myocardial infarction and glomerular injury.
- It helps in assessing collagen degeneration in bone, cartilage, tendon and ligament disorders.
- It is used for detecting denatured collagen during cancer progression and metastasis.
- It helps in studying developmental processes like endochondral ossification.
- It is used to detect collagen fragmentation in skin aging.
- It works on frozen and paraffin-embedded tissue sections.
- It is used to detect mechanical collagen damage in load-bearing tissues like bone, cartilage, tendons and arteries.
- It helps in evaluating extracellular matrix scaffolds for regenerative medicine.
- It is used in 3D cell cultures to visualize pericellular matrix turnover.
- It can detect collagen directly in SDS-PAGE gels as an in-gel Western-type staining method.
- It is used in in vivo imaging when conjugated with fluorophores or radiotracers to monitor collagen remodeling.
- It helps to study disease activity in models of pulmonary or liver fibrosis.
- It can be used to deliver conjugated therapeutic or imaging agents to sites of collagen damage.
- It may also help in modulating collagen fibril growth in vitro for tissue engineering studies.
- 3Helix Inc. (n.d.). CHPs: 3Helix’s superior collagen staining vs Picrosirius Red ….
- 3Helix Inc. (n.d.). F-CHP | Collagen Hybridizing Peptide, 5-FAM Conjugate.
- 3Helix Inc. (n.d.). Superior collagen staining – CHPs vs Masson’s Trichrome.
- Advanced BioMatrix, Inc. (2019). Directions for use collagen hybridizing peptide (CHP) (Rev 01).
- Echelon Biosciences. (n.d.). Collagen Hybridizing Peptide – Fluorescein Conjugate.
- Huang, S., Ng, N., Vaez, M., Hinz, B., Leong, I., & Bozec, L. (2025). Collagen hybridizing peptides promote collagen fibril growth in vitro. ACS Applied Bio Materials, 8(3), 2003–2014. https://doi.org/10.1021/acsabm.4c01509
- Hwang, J., Huang, Y., Burwell, T. J., Peterson, N. C., Connor, J., Weiss, S. J., Yu, S. M., & Li, Y. (2017). In situ imaging of tissue remodeling with collagen hybridizing peptides. ACS Nano, 11(10), 9825–9835. https://doi.org/10.1021/acsnano.7b03150
- IQ Products. (n.d.). Collagen hybridizing peptide.
- Li, X., Zhang, Q., Yu, S. M., & Li, Y. (2023). The chemistry and biology of collagen hybridization. J Am Chem Soc, 145(20), 10901–10916. https://doi.org/10.1021/jacs.3c00713
- Wikipedia contributors. (n.d.). Collagen hybridizing peptide.
- Woolley, W., Chin, N., Yu, S. M., & Acevedo, C. (2025). Fluorescent collagen hybridizing peptide for quantifying collagen denaturation in cortical bone. Bone Research, 26, 101855. https://doi.org/10.1016/j.bonr.2025.101855