- Cellulose acetate electrophoresis refer as a laboratory technique used for separation of charged molecules / like proteins and enzymes etc.
- It based on movement of molecules in electric field through a medium of cellulose acetate membrane (CAM).
- In this method the cellulose acetate act as a support medium where migration of molecules occurs by electric potential difference.
- Molecules are separated mainly according to their charge-to-mass ratio and mobility in buffer solution.
- Usually, buffer of proper pH is used to maintain ionic strength and proper migration of charged particles.
- The membrane of cellulose acetate is inert, thin and porous which allow movement of molecules easily but not interact chemically with them.
- The sample (like serum or plasma) is applied on the membrane and current is passed for some fixed time (normally 20–40 min depend on voltage).
- During electrophoresis, negatively charged molecules (like most proteins at alkaline pH) move toward the anode (+).
- The separated bands after electrophoresis are stained by dyes (like Ponceau S, Amido black, etc.) for visualization.
- Quantitative analysis sometimes done by densitometry scanning which measure intensity of each band on the membrane.
- It’s widely used for detection of abnormal protein pattern, example – Hemoglobin electrophoresis, Serum protein electrophoresis, etc.
- Advantages include simple, quick and economical method requiring small amount of sample and apparatus.
- However, resolution is moderate compared to gel electrophoresis but still satisfactory for diagnostic uses.
- The result interpretation depend upon migration distance, pattern and band intensity which represent concentration of proteins.
- Temperature and ionic strength of buffer must be controlled properly otherwise band diffusion may occur giving unclear pattern.
- Therefore, cellulose acetate electrophoresis has been utilized in clinical biochemistry for many years due to its reliability and convenience.
- The method though old, they still applied in many labs because of its sturdy and hardy performance and easy maintenance of equipment.
- Such as, it’s used in separation of lipoproteins, enzymes, hemoglobins, etc. for diagnostic and research purpose.
- Interpretation of pattern sometimes require experience person as slight variation in pH or voltage can affect results.
- Overall, this electrophoresis technique represent a basic but important analytical tool in biomedical and biochemical laboratories.
Principle of Cellulose Acetate Electrophoresis
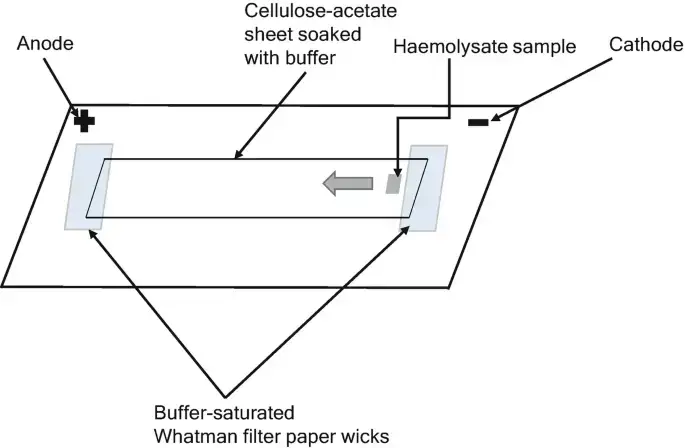
- A cellulose acetate membrane is used as support medium and saturated with running buffer before electrophoresis is started.
- In presence of electric field, charged molecules in sample are caused to migrate on that membrane toward oppositely charged electrode.
- The migration velocity is determined by net charge, size, shape, and frictional resistance in the medium.
- Because pores of cellulose acetate are relatively large, size sieving effect is minimal, so charge difference mostly govern separation.
- The buffer pH is selected so that molecules carry net charge (positive or negative) thus they will move.
- Negative molecules move toward anode, positive ones toward cathode (depending on pH) under applied voltage.
- The membrane is held by filter‐paper wicks which maintain electric contact with buffer reservoirs.
- When the migration is progressed for fixed time, bands are formed according to mobility differences.
- After run, bands are fixed and stained (by dye) to visualize separated components.
- Quantitation is done by densitometry, comparing intensity / band area with standards.
- Migration distances (relative mobility, Rf or RM) are used to identify component by comparing with known.
- Electroosmosis (flow of buffer) may interfere, diffusion broadening may occur if run too long.
- Control of voltage, time, buffer strength, temperature is necessary to avoid overheating or band distortion.
- In many cases an alkaline buffer is used (pH around 8 to 9) for proteins/hemoglobin separation.
- This principle is same in essence as zone electrophoresis with support medium, but here support is cellulose acetate.
Materials Required for Cellulose Acetate Electrophoresis
- Cellulose Acetate Membrane / Strip (support medium) is needed, often pre-cut plates or sheets.
- Electrophoresis buffer (for hemoglobin usually Tris-EDTA-Boric buffer, pH ~8.4) is required for running current.
- Electrophoresis tank / chamber (horizontal type) with adjustable bridges and reservoirs.
- Power supply capable of constant voltage/current up to required value (may reach few hundreds volts).
- Filter paper wicks (Whatman or similar) to connect membrane ends to buffer reservoir.
- Sample applicator / applicator kit (for loading sample onto membrane)
- Staining reagents / dyes (e.g. Ponceau S, other protein/hemoglobin stains)
- Clearing / fixing reagents (e.g. Clear Aid, methanol / acetic acid etc) for removing background / making bands clear.
- Control samples / standards (e.g. hemoglobin controls, protein standards) to compare band positions & intensities.
- Densitometer / scanner (or spectrophotometer) for quantitation of band intensity after staining / clearing.
- Pipettes, microtubes, gloves, lab consumables etc for handling sample, reagent mixing etc.
Steps / Procedure of Cellulose Acetate Electrophoresis
- Membrane strip (cellulose acetate) is cut to proper size and soaked / equilibrated in running buffer (for 5-15 min) to remove air bubbles.
- The electrophoresis tank is filled with buffer in both compartments and filter paper wicks are placed so ends of membrane contact buffer via the wicks.
- The membrane is placed on bridging support so that each end lies over a filter wick dipping into buffer reservoirs.
- Sample is applied (small volume, e.g. 2-5 µL) onto membrane near cathode side, leaving space between spots and from edge.
- The current / voltage is switched on (set to appropriate voltage, e.g. 100–200 V or appropriate constant current) and allowed to run for fixed time (often 20-60 min).
- Migration proceeds: molecules move according to charge toward anode or cathode.
- After completion, the current is turned off and the membrane is removed carefully from tank.
- Membrane is fixed using fixative solution (e.g. trichloroacetic acid / acetic acid mixture) to immobilize proteins / analytes.
- After fixation, membrane is rinsed / washed to remove excess fixative or salts.
- Staining is done using suitable dye (e.g. Ponceau S, Amido Black etc) to visualize the separated bands.
- Excess stain is removed by destaining / clearing (e.g. acetic acid, methanol solution) until background is light.
- Membrane is dried or kept moist and scanned / read by densitometer or scanner to get band intensities.
- Relative mobility (Rf or RM) is calculated by dividing distance moved by component by distance moved by reference (or total) to help identify bands.
- Comparison with standards / controls is done to interpret which bands correspond to known molecules.
- Clean up: buffer is drained, membrane disposed or stored, apparatus cleaned.
Cellulose Acetate as Electrophoretic Support Matrix
- Cellulose acetate membrane is used as support medium where sample molecules are carried / held in pores and migrate under electric field.
- The support is formed by interlocking fibers of cellulose acetate, making a spongy-like matrix where about 80% volume is pore/air space when dry.
- In use, those pores are filled with buffer solution so molecules move through aqueous-phase channels.
- Because the matrix is less hydrophilic than paper, less buffer is held and migration is faster, giving shorter separation time and better resolution.
- The fibers do not strongly interact chemically with proteins or analytes, so adsorption is minimized (non-specific binding is low).
- Uniform pore structure gives reproducible paths, so band pattern is more consistent / reproducible.
- Compared to paper support, cellulose acetate offers a more stable and uniform matrix for molecular separation, giving better resolution and reproducibility.
- Electroosmosis (flow of buffer within matrix) may occur because residual ionic groups in matrix push buffer, which may distort migration.
- Because support is thin, heat dissipation is efficient so overheating is less severe.
- The support is relatively rigid yet flexible enough to handle, and low background (after staining) is allowed.
- Separation is governed by charge differences rather than size sieving, because pore sizes are large compared to analytes.
- The membrane is wetted with buffer before use, so uniform ionic continuity is maintained across support.
- For hemoglobin electrophoresis the cellulose acetate support is ideal since variants with small charge differences are separated cleanly.
- Some limitations: small molecules or very similar charge species may overlap because size discrimination is weak.
- In many labs the matrix is preferred for quick / routine diagnostics because of its balance of ease, resolution, stability.
Application of Cellulose Acetate Electrophoresis
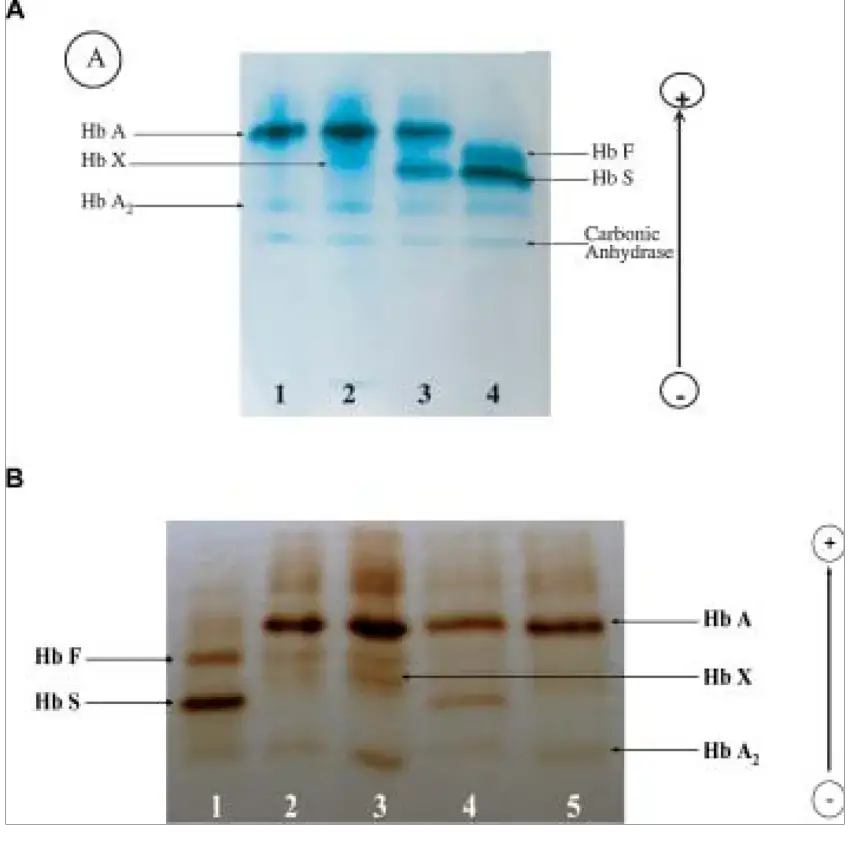
- In clinical diagnostics, hemoglobin abnormalities (like sickle cell, thalassemia) are detected by cellulose acetate electrophoresis.
- Serum protein profiling (albumin, globulins, α, β, γ fractions) is done using this method to detect abnormal protein patterns.
- In urinary protein analysis: damage in kidney is studied by isolating urinary protein bands via cellulose acetate membrane electrophoresis.
- Isoenzymes / enzyme variants are separated in research / clinical labs using this technique.
- Lipoprotein separation is another use: different classes (LDL, HDL, etc) are separated for study.
- Analysis of polypeptide dyes / mixtures (in biochemical research) is made by this method.
- Nucleic acid / RNA / DNA fragments sometimes are analysed (charged molecules) though resolution is lower for that.
- Qualitative / semi-quantitative identification of monoclonal antibodies or immunoglobulins in serum is done.
- In screening and monitoring: repeated tests of patients for hemoglobinopathy / protein disorders are done via this method.
- Because kits are made, ready diagnostic systems using cellulose acetate are commercially used in labs for routine testing.
Advantages of Cellulose Acetate Electrophoresis
- Setup is simpler and easier than gel systems so less time is wasted in preparation.
- Electrophoretic separation is faster, often completed in < 1–2 hours in many cases.
- Sharp and clear bands are produced, with less tailing and low background interference.
- Protein adsorption on support is minimal (non-specific binding is low) so loss of sample is reduced.
- The membrane can be cleared / made transparent after staining which allows densitometric quantification.
- A ready-made support matrix is available; no special casting or polymerization is required.
- Good reproducibility is allowed because uniform pore structure and stable support reduce variations.
- Heat dissipation is better because support is thin, so overheating / band diffusion is less.
- Small sample volume is sufficient (high sensitivity) so precious samples can be used.
- The method is economical (low cost relative to some advanced methods) and used routinely in many labs.
Limitation of Cellulose Acetate Electrophoresis
- Only usable under alkaline buffer conditions; other pH ranges are problematic for stability of membrane.
- Low resolving power for molecules with very small charge differences; close variants may overlap.
- Quantification is difficult; accurate measurement of low concentration components is error prone.
- Electroosmosis may occur because residual ionic groups in membrane cause buffer flow which distorts bands.
- Sample capacity is low; only small volume can be applied else bands smear or broaden.
- Thickness of support is small, mechanical handling is delicate, tearing or damage may occur.
- Very fast migration sometimes results in diffusion / band blurring if control of voltage/time is not exact.
- Certain hemoglobin variants co-migrate (e.g. HbC, E, O or Hb D, G) are not distinguished well.
- It is labor intensive and time consuming for many samples; manual steps prone to variability.
- It is less sensitive for minor / trace components; low abundance species may not visible.
Comparisons with Gel or Capillary Electrophoresis
- Gel electrophoresis (agarose / polyacrylamide) offers higher resolution especially for small molecules / peptides, whereas cellulose acetate has moderate resolution.
- Capillary electrophoresis (CE) gives much better sensitivity and separation speed, while cellulose acetate is simpler and cheaper.
- With CE, detection of minor / free light chain / cryoglobulins is improved vs cellulose acetate.
- Gel media provide sieving effect (size discrimination) whereas cellulose acetate is mostly charge-based support (pore sizes are large) so size effects are weak.
- In cellulose acetate the apparatus and setup are simpler, less expensive, low maintenance; gels (especially polyacrylamide) need polymerization, careful casting, more handling.
- Capillary systems are automated, high throughput, low sample volume, and high reproducibility; cellulose acetate is manual, more labor, more prone to human error.
- In gel or CE, heat dissipation and convection control are challenges; cellulose acetate being thin support dissipates heat better.
- For quantitation, CE is more accurate, linear dynamic range is superior; cellulose acetate often limited by dye binding, background, operator variability.
- Gel methods allow more flexibility in customizing gel % (for separating wide MW ranges), but cellulose acetate is fixed support, less adaptable.
- In clinical labs many still use cellulose acetate because cost and ease matter; but for advanced research CE / gel methods are preferred for fine separations.
FAQ on Cellulose Acetate Electrophoresis
What is cellulose acetate electrophoresis?
Cellulose acetate electrophoresis is a laboratory technique used to separate and analyze different charged species, such as proteins or nucleic acids, based on their charge and size.
How does cellulose acetate electrophoresis work?
Cellulose acetate electrophoresis works by applying an electrical field to a sample of charged species suspended in a gel. The gel matrix, made of cellulose acetate, provides a porous environment in which the charged species can move in response to the electrical field. Smaller species move faster than larger species, leading to separation of the sample based on size and charge.
What are the advantages of cellulose acetate electrophoresis?
Cellulose acetate electrophoresis has several advantages, including simplicity, low cost, ease of use, and the ability to separate a wide range of species. It is also highly versatile, allowing for the separation of a variety of charged species, including proteins and nucleic acids.
What are the limitations of cellulose acetate electrophoresis?
The limitations of cellulose acetate electrophoresis include low resolution compared to other electrophoresis techniques, such as polyacrylamide gel electrophoresis, and the tendency of some species to aggregate, making accurate separation difficult.
What is the procedure for cellulose acetate electrophoresis?
The procedure for cellulose acetate electrophoresis typically involves preparing the sample, casting the gel, loading the sample into the gel, and running the electrophoresis. The separated species are then visualized using staining or other detection methods.
What are the applications of cellulose acetate electrophoresis?
Cellulose acetate electrophoresis is widely used in molecular biology and biochemistry for a variety of applications, including protein analysis, nucleic acid analysis, and the detection of genetic disorders.
Can cellulose acetate electrophoresis be used to separate DNA?
Yes, cellulose acetate electrophoresis can be used to separate DNA based on size, with smaller fragments moving faster through the gel matrix than larger fragments.
How does the pH of the running buffer affect cellulose acetate electrophoresis?
The pH of the running buffer can affect cellulose acetate electrophoresis because it influences the charge on the species being separated. In general, a neutral pH is best for optimal separation, as this provides a neutral environment that does not interfere with the migration of the species through the gel.
What is the role of the voltage in cellulose acetate electrophoresis?
The voltage applied during cellulose acetate electrophoresis plays a critical role in the separation of the species. The voltage creates an electrical field that drives the movement of the charged species through the gel matrix, leading to separation based on size and charge.
How is the resolution of cellulose acetate electrophoresis determined?
The resolution of cellulose acetate electrophoresis is determined by the separation of closely related species. A higher resolution gel will lead to a clearer separation of species that are similar in size and charge, while a lower resolution gel will result in less distinct separation of these species.
- Forget, B. G., & Bunn, H. F. (2013). Classification of the Disorders of Hemoglobin. Cold Spring Harbor Perspectives in Medicine, 3(2), a011684–a011684. https://doi.org/10.1101/cshperspect.a011684
- Jorgenson, J. W. (1986). Electrophoresis. Analytical Chemistry, 58(7), 743A-760A. https://doi.org/10.1021/ac00298a001
- Kohn, J. (1969). Separation of haemoglobins on cellulose acetate. Journal of Clinical Pathology, 22(1), 109–111. https://doi.org/10.1136/jcp.22.1.109
- Kohn, J. (1962). Cellulose Acetate Electrophoresis. Proceedings of the Association of Clinical Biochemists, 2(1), 19–20. https://doi.org/10.1177/036985646200200114
- Kohn, J. (1957). An Immuno-electrophoretic Technique. Nature, 180(4593), 986–987. https://doi.org/10.1038/180986a0
- Marengo-Rowe, A. J. (1965). Rapid electrophoresis and quantitation of haemoglobins on cellulose acetate. Journal of Clinical Pathology, 18(6), 790–792. https://doi.org/10.1136/jcp.18.6.790
- Mashkour, M., Afra, E., Resalati, H., & Mashkour, M. (2015). Moderate surface acetylation of nanofibrillated cellulose for the improvement of paper strength and barrier properties. RSC Advances, 5(74), 60179–60187. https://doi.org/10.1039/C5RA08161K
- Nowotny, A. (1979). Immunoelectrophoresis. In Basic Exercises in Immunochemistry (pp. 235–237). https://doi.org/10.1007/978-3-642-67356-6_72
- Righetti, P. G., & Gelfi, C. (2001). 14. Electrophoresis. In Helmut Guenzler & A. Williams (Eds.), Handbook of Analytical Techniques (pp. 346–347). WILEY-VCH Verlag GmbH.
- Rocco, R. M. (2005). Joachim Kohn (1912–1987) and the Origin of Cellulose Acetate Electrophoresis. Clinical Chemistry, 51(10), 1896–1901. https://doi.org/10.1373/clinchem.2005.056572
- Walker, J. M. (2010). 10 Electrophoretic techniques. In K. Wilson & J. M. Walker (Eds.), Principles and Techniques of Biochemistry and Molecular Biology (7th ed.). Cambridge: Cambridge University Press.
- Westermeier, R., Gronau, S., Becket, P., Buelles, J., Schickle, H., & Theßeling, G. (2005). Electrophoresis in Practice: A Guide to Methods and Applications of DNA and Protein Separations (4th, revised ed.). Wiley-VCH Verlag.
- Wild, B. J., & Bain, B. J. (2004). Detection and quantitation of normal and variant hemoglobins: an analytical review. Annals of Clinical Biochemistry, 41(5), 355–369. https://doi.org/10.1258/0004563041731600