The Casein Hydrolysis Test is a biochemical test that is used to determine the ability of microorganism to produce and secrete extracellular proteolytic enzyme known as caseinase. It is the process in which casein protein of milk is broken down into smaller soluble products. Casein is the main protein present in milk and it is present in the form of large colloidal particles which gives milk its white and opaque appearance. These molecules are too large to pass through the bacterial cell membrane.
In this process, the bacteria releases caseinase enzyme into the surrounding medium. This enzyme hydrolyzes the peptide bonds of casein and converts it into smaller peptides and amino acids which can be absorbed by the cell and used for energy and growth. This breakdown of casein results in loss of opacity of the medium. This process occurs only when the organism is capable of producing extracellular protease.
The test is performed using Skim Milk Agar (SMA) medium which contains skim milk as the source of casein. After inoculation and incubation, a positive result is indicated by the formation of a clear transparent zone around the bacterial growth. This clear zone is referred to as zone of proteolysis and it shows that the casein has been hydrolyzed. In case of a negative result, no clear zone is formed and the medium remains white and opaque around the colony.
The Casein Hydrolysis Test is important in the identification of proteolytic bacteria such as Bacillus species, Pseudomonas aeruginosa and Serratia marcescens. It is also useful in differentiating microorganisms based on their ability to hydrolyze proteins. This test is commonly used in microbiology laboratories for characterization of bacteria producing extracellular enzymes.
Objectives of Casein Hydrolysis Test
- To determine the ability of microorganism to produce extracellular proteolytic enzyme known as caseinase.
- To check whether the organism can hydrolyze casein protein into smaller soluble products like peptides and amino acids.
- To differentiate microorganisms based on their ability or inability to degrade casein.
- To assist in identification of proteolytic bacteria belonging to families such as Bacillaceae and Enterobacteriaceae.
- To differentiate aerobic Actinomycetes such as Nocardia, Streptomyces and Actinomadura species.
- To help in identification of organisms like Pseudomonas aeruginosa especially in milk and water related studies.
- To characterize microorganisms that are capable of growing and utilizing protein present in milk and milk products.
Principle of Casein Hydrolysis Test
The principle of Casein Hydrolysis Test is based on the ability of microorganism to hydrolyze casein protein present in milk. Casein is a large phosphoprotein which exists in the form of colloidal micelles and it is responsible for white opaque appearance of milk. Due to its large molecular size, casein cannot pass directly through the bacterial cell membrane.
In this process, the organism secretes extracellular proteolytic enzymes known as caseinase or protease into the surrounding medium. These enzymes hydrolyze the peptide bonds (CO–NH) present in the casein molecule and convert it into smaller soluble compounds such as peptones, polypeptides and amino acids. These smaller molecules can easily enter the cell and are utilized as source of carbon and nitrogen for metabolic activities.
The principle is demonstrated on Skim Milk Agar medium. When the organism produces caseinase, the casein present around the growth is digested which results in disappearance of opacity of the medium. This forms a clear transparent zone around the colony known as zone of proteolysis. If the organism does not produce the enzyme, no hydrolysis occurs and the medium remains white and opaque, indicating a negative result.
Requirements for Casein Hydrolysis Test
- Culture medium
- Skim Milk Agar (SMA) medium
- Components of Skim Milk Agar
- Skim milk powder (source of casein)
- Tryptone or pancreatic digest of casein
- Yeast extract
- Dextrose or glucose
- Agar
- Distilled water
- Bacterial cultures
- Test organism (18–24 hours old culture)
- Positive control organism (caseinase producer such as Bacillus species or Pseudomonas aeruginosa)
- Negative control organism (non caseinase producer such as Escherichia coli)
- Laboratory equipment
- Sterile inoculating loop
- Sterile Petri plates
- Incubator
- Bunsen burner or spirit lamp
- Autoclave
- Weighing balance
- Optional reagent
- 10% Trichloroacetic acid (TCA) solution (used to enhance visibility of clearing zone)
Composition of Skim Milk Agar
| Ingredients | Gram per liter |
| Skim milk powder | 28.0 |
| Trypton | 5.0 |
| Yeast extract | 2.50 |
| Dextrose | 1.0 |
| Agar | 15.0 |
Preparation of Skim Milk Agar
- Except for the agar, each part of the medium is dissolved in 500 ml of distilled water in 1000 ml flasks. The pH of the medium is set to 7.0.
- Then, the agar powder is added, and the medium is filled to 1000 ml.
- The medium is then autoclaved at 121°C for 15 min at 15 psi.
- The medium that has been heated in an autoclave is then poured into clean Petri dishes and left to harden. Alternatively, ready-made mediums can be used.
Procedure of Casein Hydrolysis Test
- A sterile Skim Milk Agar plate is taken and labeled properly.
- Using a sterile inoculating loop, a well isolated colony is picked from a fresh (18–24 hours old) culture of the test organism.
- The organism is streaked on the surface of Skim Milk Agar plate in a straight line or zig-zag pattern.
- The inoculated plates are incubated at suitable temperature (generally 35–37°C) for 24–48 hours.
- For slow growing organisms, incubation is carried out at 25–30°C for a longer duration.
- After incubation, the plates are observed for formation of a clear transparent zone around the growth.
- If the clearing is not clearly visible, the plate is flooded with 10% Trichloroacetic acid (TCA) and observed again for zone of proteolysis.
Result of Casein Hydrolysis Test
Positive result (+)
A clear transparent zone is formed around the bacterial growth.
This indicates that the organism produces extracellular proteolytic enzyme caseinase.
The casein present in the medium is hydrolyzed into smaller soluble products, resulting in loss of opacity.
Examples include Pseudomonas aeruginosa, Bacillus subtilis, Serratia marcescens and Streptomyces species.
Negative result (−)
No clear zone is observed around the growth.
The medium remains white and opaque near the colony.
This indicates that the organism does not produce caseinase enzyme.
Examples include Escherichia coli and Enterococcus faecalis.
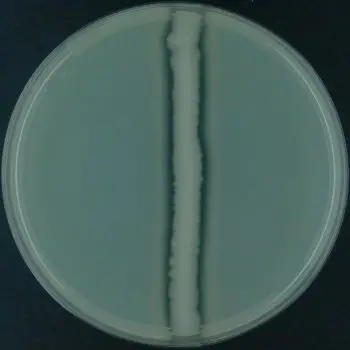
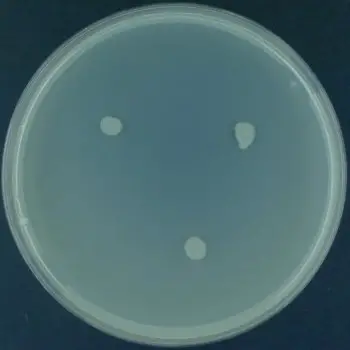
List of organisms showing Casein Hydrolysis Test result
- Casein hydrolysis positive organisms
- Bacillus subtilis
- Bacillus cereus
- Bacillus megaterium
- Bacillus altitudinis
- Pseudomonas aeruginosa
- Pseudomonas fluorescens
- Serratia marcescens
- Serratia liquefaciens
- Lactococcus lactis
- Yersinia intermedia
- Chryseobacterium oncorhynchi
- Clostridium perfringens
- Streptomyces species
- Actinomadura species
- Nocardia brasiliensis
- Nocardia pseudobrasiliensis
- Casein hydrolysis negative organisms
- Escherichia coli
- Enterococcus faecalis
- Micrococcus roseus
- Clostridium innocuum
- Nocardia asteroides
- Nocardia farcinica
- Nocardia nova
- Nocardia otitidiscaviarum
- Nocardia transvalensis
Uses of Casein Hydrolysis Test
- To determine the ability of microorganism to produce extracellular proteolytic enzyme caseinase.
- To differentiate bacteria based on their proteolytic activity on casein.
- To assist in identification and differentiation of members of families such as Bacillaceae and Enterobacteriaceae.
- To differentiate aerobic Actinomycetes such as Streptomyces, Nocardia and Actinomadura species.
- To help in identification of Nocardia brasiliensis from other Nocardia species.
- To detect and confirm the presence of Pseudomonas aeruginosa in water samples.
- To identify proteolytic microorganisms involved in spoilage of milk and milk products.
- To characterize microorganisms capable of growing and hydrolyzing protein present in milk.
Advantages of Casein Hydrolysis Test
- It helps in differentiation of aerobic Actinomycetes such as Nocardia, Streptomyces and Actinomadura species.
- It is useful in identification of pathogenic species like Nocardia brasiliensis from Nocardia asteroides group.
- It serves as a confirmatory test for identification of Pseudomonas aeruginosa.
- It aids in differentiation of bacterial families such as Bacillaceae and Enterobacteriaceae.
- It is useful in detection of proteolytic bacteria responsible for spoilage of milk and dairy products.
- It helps in screening of microorganisms producing industrially important proteolytic enzymes.
- It is a simple and easy test with clear visual interpretation based on formation of zone of proteolysis.
Limitations of Casein Hydrolysis Test
- It is not a confirmatory test and cannot identify the organism at species level.
- It must be used along with other biochemical tests for final identification.
- Fastidious organisms may not grow properly on Skim Milk Agar medium.
- A negative result does not always indicate absence of caseinase enzyme.
- Some organisms require longer incubation period to show hydrolysis.
- Weak or slow hydrolysis may produce faint clearing zone which is difficult to interpret.
- Use of additional reagents like TCA may be required to visualize the result clearly.
Precautions
- A heavy inoculum should be used while streaking the organism on Skim Milk Agar plate.
- The plate should not be reported negative at early stage of incubation.
- Proper incubation time must be given as some organisms require longer duration to show hydrolysis.
- Strict aseptic technique should be followed throughout the procedure.
- While inoculation, streaking should be done at the center of the plate without touching the edges.
- Skim Milk Agar medium should be stored at 2–8°C and protected from light and moisture.
- Media showing cracks, discoloration or dehydration should not be used.
- Positive and negative control organisms should be used along with test organism.
- This test should not be used alone for confirmation and must be supported by other tests.
- All contaminated materials and cultures should be properly sterilized before disposal.
- Acharya, S. (2022, August 23). Casein hydrolysis test: Principle, procedure, and uses. Microbe Online. https://microbeonline.com/casein-hydrolysis-test-principle-procedure-and-uses/
- American Society for Microbiology. (2013, December 1). Casein hydrolysis. https://asm.org/Image-Gallery/Casein-Hydrolysis
- Becton, Dickinson and Company. (n.d.). Skim milk • Skim milk medium [Instructions for use]. Grosseron. https://www.grosseron.com/oo/Assets/client/FTP/GROSSERON/FT/FT232100.pdf
- Becker, B., Lechevalier, M. P., Gordon, R. E., & Lechevalier, H. A. (1964). Rapid differentiation between Nocardia and Streptomyces by paper chromatography of whole-cell hydrolysates. Applied Microbiology, 12(5), 421–423. https://doi.org/10.1128/am.12.5.421-423.1964
- BenchChem. (2025). A comparative guide to bacterial identification: The litmus milk test vs. modern alternatives. https://www.benchchem.com/pdf/A_Comparative_Guide_to_Bacterial_Identification_The_Litmus_Milk_Test_vs_Modern_Alternatives.pdf
- Children’s Online Books. (n.d.). Litmus milk. http://www.childrensonlinebooks.com/bi284/differentialMedia/litmus_milk.htm
- Clinical Gate. (2015, February 8). Nocardia, Streptomyces, Rhodococcus, and similar organisms. https://clinicalgate.com/nocardia-streptomyces-rhodococcus-and-similar-organisms/
- Dahal, P. (2023, April 13). Casein hydrolysis test- Principle, procedure, results. Microbe Notes. https://microbenotes.com/casein-hydrolysis-test/
- Davie, J. R., Graur, O., & Sheaffer, J. (n.d.). Final diagnosis — Case 226. UPMC. https://path.upmc.edu/cases/case226/dx.html
- Fackrell, B. (n.d.). Litmus milk. University of Windsor. https://web2.uwindsor.ca/courses/biology/fackrell/Methods/Litmus_Milk.htm
- Hardy Diagnostics. (2020). Skim milk agar [Instructions for use]. https://hardydiagnostics.com/media/assets/product/documents/SkimMilkAgar.pdf
- Hussein, N. N. A., Ibrahim, A. I., Kamar, F. H., & Nechifor, A. C. (2020). Caseinase production and media optimization from Bacillus subtilis. Revista de Chimie, 71(11), 1–9. https://doi.org/10.37358/RC.20.11.8368
- MacKenzie, E. (2025, October 15). 31.2: Skim milk test. Biology LibreTexts. https://bio.libretexts.org/Courses/Irvine_Valley_College/IVC_Microbiology_Lab_Manual/31%3A_METABOLIC_TESTING/31.02%3A_Skim_Milk_Test
- Mukhida, S., Chavan, S., Pandya, V., & Pandya, J. (2025). Preparation and validation of skim milk-based method for preservation of bacterial strain at resource-limited setup. Journal of Family Medicine and Primary Care, 14(5), 1833–1842. https://doi.org/10.4103/jfmpc.jfmpc_1626_24
- Remel. (2013). Litmus milk medium [Instructions for use]. https://assets.fishersci.com/TFS-Assets/MBD/Instructions/IFU61274.pdf
- Reynolds, J. (2024, February 6). 30: Casein hydrolysis. Biology LibreTexts. https://bio.libretexts.org/Learning_Objects/Laboratory_Experiments/Microbiology_Labs/Microbiology_Labs_I/30%3A_Casein_Hydrolysis
- Santana, E. H. W. de, Luiz, L. L., Pasquim, P. da S., Pinto, L. de F. B., Pereira, F. de A. B., Gasparini, G. B. F. B., Lorenzetti, E., Bruzaroski, S. R., & Eleodoro, J. I. (2020). Psychrotrophic microorganisms in raw milk and the cheese quality. Research, Society and Development, 9(9), e127997217. https://doi.org/10.33448/rsd-v9i9.7217
- Saubolle, M. A., & Sussland, D. (2003). Nocardiosis: Review of clinical and laboratory experience. Journal of Clinical Microbiology, 41(10), 4497–4501. https://doi.org/10.1128/JCM.41.10.4497-4501.2003
- Tantray, J. A., Mansoor, S., Wani, R. F. C., & Nissa, N. (2023). Casein hydrolysis test. In Basic Life Science Methods (pp. 185–187). Elsevier. https://doi.org/10.1016/B978-0-443-19174-9.00047-7
- Wang, W., Liang, Q., Zhao, B., Chen, X., & Song, X. (2024). Functional peptides from yak milk casein: Biological activities and structural characteristics. International Journal of Molecular Sciences, 25(16), 9072. https://doi.org/10.3390/ijms25169072
- Yalew, K., Pang, X., Huang, S., Zhang, S., Yang, X., Xie, N., Wang, Y., Lv, J., & Li, X. (2024). Recent development in detection and control of psychrotrophic bacteria in dairy production: Ensuring milk quality. Foods, 13(18), 2908. https://doi.org/10.3390/foods13182908
- Yalew, K., Zhang, S., Gebreyowhans, S., Xie, N., Wang, Y., Lv, J., Li, X., & Pang, X. (2025). Development of multiplex qPCR method for accurate detection of enzyme-producing psychrotrophic bacteria. Foods, 14(11), 1975. https://doi.org/10.3390/foods14111975
- Yuan, L., Sadiq, F. A., Liu, T., Li, Y., Gu, J., Yang, H., & He, G. (2018). Spoilage potential of psychrotrophic bacteria isolated from raw milk and the thermo-stability of their enzymes. Journal of Zhejiang University-SCIENCE B, 19(8), 630–642. https://doi.org/10.1631/jzus.B1700352