Capillary Electrophoresis (CE) is a type of electrophoresis technique that uses a narrow capillary to separate charged molecules (ions) based on their migration towards electrodes under the influence of an electric field. It is a high-resolution and fast method for the analysis of small molecules, DNA, and proteins.
The importance of CE lies in its ability to provide highly accurate and reproducible separations, even of complex mixtures, making it a valuable tool in various fields such as biochemistry, pharmaceuticals, and forensics.
The history of CE dates back to the late 1970s and early 1980s, when the development of microfabrication technologies paved the way for the creation of capillaries with small internal diameters. Since then, CE has evolved into a well-established analytical technique and has been increasingly used for various applications.
Principle of Capillary Electrophoresis
The velocity of an analyte migrating within a capillary under the influence of an electric field of intensity “E” is determined by the analyte’s electrophoretic mobility and the buffer’s electroosmotic mobility.
The electrophoretic mobility of a solute is dependent on the solute’s properties, such as its electric charge, molecule size, and shape, and the buffer’s properties, such as the electrolyte’s ionic strength, pH, viscosity, and additives. The solute’s electrophoretic velocity (Vep) is given by the following equation:
Vep= μepE = (q/6πηr) (V/L) where η is the viscosity of the electrolyte solution, V is the applied voltage, L= the length of the capillary, r is the Stoke’s radius of the solute, μep is its electrophoretic mobility, and q is its effective charge.
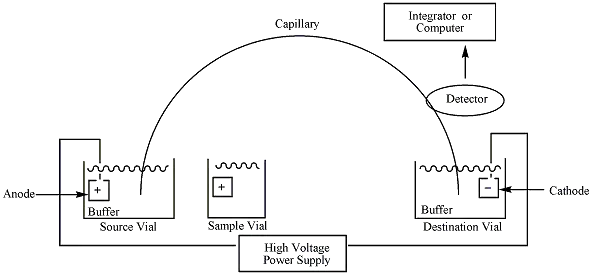
When applied through a capillary containing a buffer, the electric field induces a solvent flow within the capillary known as the electroosmotic flow. Which velocity is determined by electrophoretic mobility. Mobility is determined by the charge density of the capillary’s inner wall and the buffer’s characteristics. Electroosmotic velocity (Veo) is given by the following equation:
Veo = μeo
E = (ε𝜁/η) (V/L) where ε= dielectric constant of the buffer, 𝜁 = zeta potential of the capillary surface, η = viscosity of the electrolyte solution, V = applied voltage, μeo = electrophoretic mobility, and L = capillary length.
The velocity of the solute (V) is provided by: V = Vep + Veo.
Depending on the solute’s charge, the electroosmotic and electrophoretic mobility of the analyte can act in the same or opposite direction. In typical capillary electrophoresis, anions migrate in the opposite direction of electroosmotic flow at velocities slower than electroosmotic velocity. In contrast, the cations migrate in the same direction as the electroosmotic flow at speeds greater than the electroosmotic velocity. In settings with a fast electroosmotic rate relative to the solutes’ electrophoretic pace, cations and anions are separated in the same run. The time required (t) for the solute to migrate from the injection end of the capillary to the detection point (effective capillary length) is given by: t = l/ Vep+Veo = l(L)/ V(Vep+Veo).
Where the electroosmotic flow occurs from the anode to the cathode, uncoated fused silica capillaries with a negative charge are typically utilised. For the migration velocity of solutes to be reproducible, the electroosmotic flow must remain constant during each experiment. For certain investigations, it may be required to reduce or eliminate electroosmotic light by altering the inner wall of the capillary, the concentration, composition, or pH of the buffer solution.
After adding the sample, a zone of each analyte ion of the sample forms independently. The zone creation is a result of electrolyte migration in the backdrop. The propagation of each solute band is caused by distinct events. Under ideal conditions, molecular diffusion of the solute within the capillary is the only factor in the expansion of the zone. Here, the zone’s effectiveness is expressed as the number of theoretical plates (N), which is calculated as follows:
N= (μep+μeo) (Vl)/ 2DL, where D is the solute buffer’s molecular diffusion coefficient.
Other phenomena, such as the length of the injection plug, detector cell size, unleveled buffer reservoirs, mismatched conductivity between sample and buffer, sample adsorption onto the capillary wall, and heat dispersion, play an important part in band dispersion in general practise. The separation between two bands is achieved by adjusting the electrophoretic mobility of the analytes, the electroosmotic mobility induced in the capillary, and improving the efficiency of each analyte for the respective band.
Rs= N(μepb-μepa)/ 4(μaep+μeo), where epa and epb are the electrophoretic mobilities of the two analytes, and μaep is the average electrophoretic mobilities of the two analytes, which is calculated as: μaep= ½ ( μepb+μepa).
Instruments Required for Capillary Electrophoresis
Capillary Electrophoresis (CE) is a powerful analytical technique that enables the separation and analysis of various samples in the lab. To perform CE effectively, it is crucial to have the right instruments and set-up. In this article, we will outline the essential instruments used in Capillary Electrophoresis.
- High Voltage Power Supply: The first essential instrument in CE is a high voltage controllable direct current power supply. The power supply is responsible for generating the electric field required for the separation of samples.
- Buffer Reservoirs: Two buffer reservoirs at the same level that contain specified anodic and cathodic solutions are necessary for CE. These buffer reservoirs play a crucial role in maintaining the stability of the electric field.
- Electrodes: CE requires two electrodes, a cathode and anode, which are immersed in the buffer reservoirs. The electrodes are connected to the power supply and play a crucial role in generating the electric field.
- Capillary: The capillary used in CE is made up of fused silica, usually with a diameter of fewer than 100 microns. The capillary acts as a separation channel for the samples.
- Optical Viewing Window: CE requires an optical viewing window that is aligned to the detector. The viewing window enables the visualization of the separation process.
- Injection System: A suitable injection system for adding sample and buffer into the capillary is crucial for CE. The method of injection can be automated for precision and can be gravity, pressure or vacuum, or electrokinetic.
- Detector: CE requires a detector for monitoring the amount of substance that passes through the capillary at a given time. The detector can be based on conductimetric, fluorimetry, absorption spectrophotometry (UV and visible), amperometric, or mass spectrometric detection.
- Thermostatic System: CE requires a thermostatic system for maintaining a constant temperature inside the capillary. The constant temperature ensures the stability and accuracy of the results.
- Recorder: CE requires a recorder that records the data after the completion of electrophoresis. The recorder is responsible for storing the data for further analysis.
- Suitable Integrator or Computer: CE also needs the right integrator or computer to convert data digitally. The digital data is more convenient for analysis and interpretation.
In conclusion, the proper selection and use of instruments in Capillary Electrophoresis are crucial for obtaining accurate and reliable results. By ensuring that all the essential instruments are in place and functioning optimally, lab technicians can maximize the performance of CE and achieve their desired outcomes.
Types of Capillary Electrophoresis Method
Capillary electrophoresis is a powerful tool used in various fields such as pharmaceuticals, biochemistry, and analytical chemistry. It involves the separation of analytes in a capillary filled with a buffer or gel, allowing for efficient and precise analysis of various substances. This article will dive into the different types of capillary electrophoresis methods commonly used today.
- Capillary Zone Electrophoresis (CZE) :CZE is a method of electrophoresis that requires a capillary filled only with a buffer. The analytes are separated into bands whose velocity depends on their electrophoretic mobility and electroosmotic flow. This type of electrophoresis is efficient for analyzing substances with molecular weight ranging from small to large (less than 2000 to 100,000 molecular weight).
- Capillary Gel Electrophoresis (CGE): In capillary gel electrophoresis, a gel acts as a molecular sieve, separating molecules according to their size. Smaller molecules move through the gel network faster than larger molecules, allowing for the separation of proteins, DNA fragments, and other biological macromolecules.
- Micellar Electrokinetic Capillary Chromatography (MEKC): MEKC is a hybrid of electrophoresis and chromatography, with separation occurring in an electrolytic solution consisting of a surfactant above its critical micellar concentration. This technique can be applied to neutral and charged solutes, and the solutes are distributed based on their partition coefficient between the pseudo-stationary phase made up of micelles and the aqueous buffer.
- Capillary Electrochromatography (CEC): CEC combines the principles of capillary electrophoresis and high-performance liquid chromatography (HPLC) to achieve efficient separations. Analytes are separated based on their differences in the partition ratio between the mobile and stationary phase or their electrophoretic mobility. It is mainly used in the pharmaceutical industry to identify acidic and basic drugs and in the industrial sector for analyzing polymers.
- Capillary Isoelectric Focusing (CIEF): CIEF is a method in which charged molecules migrate due to an electric field in a pH gradient created by ampholytes dissolved in the separation buffer. This technique involves three steps: loading, focusing, and mobilization, and is mainly used for enantiomeric separations and for separating amino acids, proteins, peptides, and carbohydrates.
- Capillary Isotachophoresis (CITP): CITP involves the separation of ions based on their electrophoretic mobility in a running buffer with a continuous and rapidly decreasing conductivity gradient. It is mainly used for the separation of small ions, including DNA fragments and other biological macromolecules.
In conclusion, capillary electrophoresis is a versatile and powerful tool for analyzing various substances. Each type of capillary electrophoresis method has its strengths and weaknesses, and the method used depends on the analytes being studied and the goals of the analysis. Whether it be CZE, CGE, MEKC, CEC, CIEF, or CITP, capillary electrophoresis remains an essential tool in many fields of research and industry.
Application of Capillary Electrophoresis
Capillary electrophoresis (CE) is a separation technique used in analytical chemistry that can separate ions and molecules based on their size, charge, and hydrophobicity. CE has a wide range of applications in fields such as biochemistry, pharmaceuticals, environmental analysis, and forensics. Some common applications include:
- DNA and RNA analysis: CE can be used to separate and analyze DNA and RNA fragments, and to identify mutations and genetic variations.
- Protein analysis: CE is used to analyze proteins, including determining their size and charge.
- Pharmaceutical analysis: CE can be used to analyze drugs, and to monitor their purity and quality.
- Environmental analysis: CE can be used to analyze environmental contaminants, including toxic heavy metals and organic pollutants.
- Forensics: CE is used in forensic science to analyze and identify various substances, such as illegal drugs and poisons.
- Food technology: In food technology, capillary electrophoresis is used to identify flavonoids, vitamins, carbohydrates, proteins, pigments, and colour in a variety of foods, including beverages and fermented foods. CE may also detect DNA and RNA from bacteria and viruses, which is indicative of contamination.
- Disease diagnosis: Lipid profile analysis utilising CITP is essential for detecting cholesterol levels in clinical laboratories during disease diagnosis. In a similar fashion, capillary electrophoresis is used to analyse the vitamins and minerals in human serum.
Overall, CE is a highly sensitive and efficient separation technique that has many applications in various fields due to its ability to separate ions and molecules with high precision.
Advantages of Capillary Electrophoresis
Capillary Electrophoresis (CE) has several advantages over other separation techniques, which make it a popular choice for many applications:
- High resolution: CE can separate ions and molecules with high precision, based on their size, charge, and hydrophobicity. This makes it possible to obtain very detailed and accurate results.
- High sensitivity: CE has a high detection sensitivity, allowing trace amounts of substances to be analyzed and detected.
- High throughput: CE can be automated and performed in parallel, making it possible to analyze many samples simultaneously. This increases the throughput and efficiency of the analysis process.
- Cost-effective: CE requires relatively small sample sizes and low amounts of reagents, making it more cost-effective than other separation techniques.
- Versatility: CE can be used to analyze a wide range of substances, including ions, small molecules, proteins, and DNA/RNA.
- Non-destructive: CE is a non-destructive method, meaning that the sample can be used for additional analysis after separation.
- Minimal sample preparation: CE requires minimal sample preparation, making it a fast and convenient method for analyzing complex mixtures.
- Less time-consuming: It takes no time to read the outcome of separation if the user is conversant with computer software. Likewise, the complete procedure, from setup to separation, takes about one hour. Therefore, capillary electrophoresis has more advantages than other separation methods.
- Has the possibility of automation: Possesses the potential for automation: In capillary electrophoresis, the injection method, data analysis, buffer, and sample uploading procedures can be automated.
Overall, CE offers a combination of high resolution, sensitivity, efficiency, and versatility, making it a valuable tool for various applications in analytical chemistry.
Disadvantages of Capillary Electrophoresis
Although capillary electrophoresis is extremely useful, it has the following disadvantages:
- Changing capillaries is challenging: Due to the size of the capillaries, capillary removal and replacement can be difficult at times. Changing capillaries for an experiment therefore requires knowledge.
- Less robust: In capillary electrophoresis, minor variations such as capillary size and analyte selection can have a significant impact. Therefore, CE is less reliable than other approaches.
- Complex instrumentation: CE requires specialized instrumentation and expertise to operate, making it less accessible to some users.
- Limited separation range: CE is most effective for separating ions and molecules of similar size and charge, and may not be suitable for larger or more complex mixtures.
- Low sample capacity: CE requires small sample sizes, which may limit its ability to analyze large or complex samples.
- Interferences: CE is sensitive to contaminants in the sample and buffer, which can interfere with the separation and reduce the accuracy of the results.
- Time-consuming: CE can be time-consuming, especially for complex mixtures, and may require additional time for sample preparation and analysis.
- Cost: CE requires specialized equipment, reagents, and personnel, making it more expensive than some other separation techniques.
FAQ
What is Capillary Electrophoresis?
Capillary Electrophoresis (CE) is a laboratory technique used for separating and analyzing small molecules, such as amino acids, nucleotides, and sugars, based on their electric charge and size. In CE, samples are loaded into a narrow capillary, which serves as the separation column, and an electric field is applied to drive the migration of the analytes through the capillary. The separated components are then detected by UV absorption or fluorescence.
What are the advantages of Capillary Electrophoresis?
CE offers several advantages over other separation methods, such as gel electrophoresis or high-performance liquid chromatography (HPLC). Some of the benefits of CE include higher resolution, faster analysis times, improved sensitivity, and the ability to handle small sample volumes. CE is also a highly automated technique, which allows for the analysis of large numbers of samples in a short period of time.
What types of samples can be analyzed using Capillary Electrophoresis?
CE can be used to analyze a wide range of small molecules, including amino acids, nucleotides, sugars, peptides, and small proteins. CE is particularly well-suited for the analysis of biomolecules, such as DNA and RNA, and is commonly used in genetic analysis and sequencing applications.
What are the common detection methods used in Capillary Electrophoresis?
The most common detection methods used in CE are UV absorption and fluorescence. UV absorption is used to detect analytes that have a UV-absorbent chromophore, while fluorescence detection is used to detect analytes that have been labeled with a fluorescent probe. Other detection methods, such as mass spectrometry, can also be used in conjunction with CE for more complex analyses.
What are the potential limitations of Capillary Electrophoresis?
CE is a highly sensitive technique, but it has some limitations that should be considered. For example, CE requires the use of specialized equipment and reagents, and the separation of large or highly charged molecules can be challenging. CE is also limited by the resolution of the separation column, which can affect the accuracy of the results. Finally, CE may not be the best choice for the analysis of complex samples, such as those containing multiple analytes with similar electric charges.
References
- Robert, F., Bouilloux, J. P., & Denoroy, L. (1991). L’électrophorèse capillaire: principe et applications [Capillary electrophoresis: principle and applications]. Annales de biologie clinique, 49(3), 137–148.
- Capillary electrophoresis. (n.d.). Retrieved December 16, 2022, from https://www.usp.org/sites/default/files/usp/document/harmonization/biotechnology/harmonization-september-2019-m859.pdf
- Borst, C., Belal, F., & Holzgrabe, U. (2013). Possibilities and limitations of capillary electropherosis in pharmaceutical analysis. Die Pharmazie, 68(7), 526–530.
- Sirén, H., & Väntsi, S. (2002). Environmental water monitoring by capillary electrophoresis and result comparison with solvent chemistry techniques. Journal of chromatography. A, 957(1), 17–26. https://doi.org/10.1016/s0021-9673(02)00218-2
- Ali, I., Alharbi, O. M. L., & Marsin Sanagi, M. (2016). Nano-capillary electrophoresis for environmental analysis. Environmental chemistry letters, 14(1), 79–98. https://doi.org/10.1007/s10311-015-0547-x
- Masár, M., Hradski, J., Schmid, M. G., & Szucs, R. (2020). Advantages and Pitfalls of Capillary Electrophoresis of Pharmaceutical Compounds and Their Enantiomers in Complex Samples: Comparison of Hydrodynamically Opened and Closed Systems. International journal of molecular sciences, 21(18), 6852. https://doi.org/10.3390/ijms21186852
- Ali, I., Alharbi, O. M. L., & Marsin Sanagi, M. (2016). Nano-capillary electrophoresis for environmental analysis. Environmental chemistry letters, 14(1), 79–98. https://doi.org/10.1007/s10311-015-0547-x