A bench top centrifuge is a laboratory instrument which is used for separation of components present in a liquid sample based on their density. It is a compact device that is designed to be placed on laboratory bench and is commonly used in routine laboratory work. It is the process in which centrifugal force is applied to separate heavier particles from lighter liquid components. This instrument is widely used in clinical, research and industrial laboratories.
The working of bench top centrifuge is based on rapid spinning of samples with the help of an electric motor. When the samples are rotated at high speed, the denser particles are forced towards the bottom of the centrifuge tube forming a pellet while the lighter liquid remains above as supernatant. The speed of rotation generally ranges from a few hundred to several thousand revolutions per minute depending on the model and application. Different types of rotors such as fixed angle and swing out rotors can be used which makes the centrifuge suitable for different sample volumes.
Bench top centrifuges are commonly used for separation of blood components, collection of cells, precipitation of proteins and nucleic acids, and clarification of solutions. Some models are also provided with temperature control to protect heat sensitive samples like enzymes and proteins. Due to its small size, ease of operation and cost effectiveness, bench top centrifuge is considered as an essential equipment in most biological and medical laboratories.
Principle of Benchtop Centrifuges
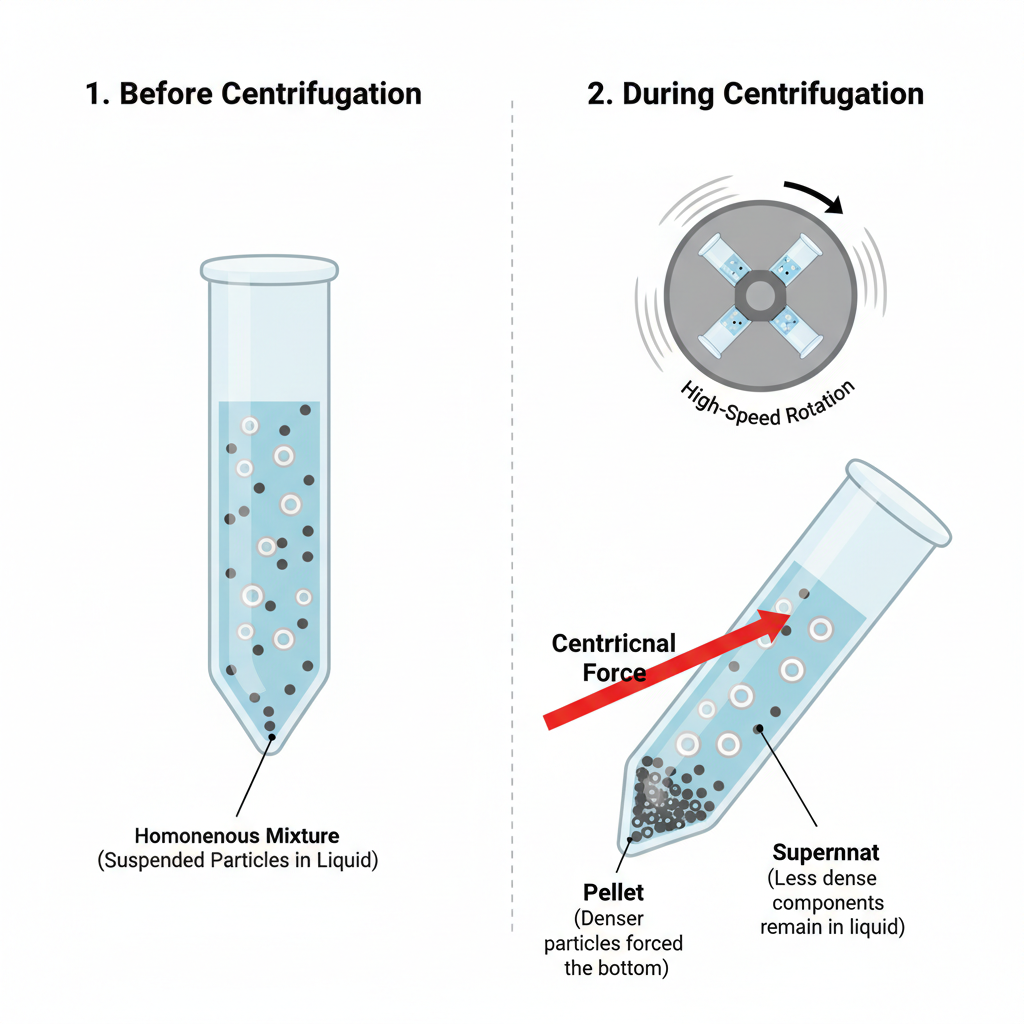
Benchtop centrifuges works on the principle of sedimentation under the influence of centrifugal force. It is the process in which suspended particles present in a liquid medium are separated on the basis of their density size and shape. When the centrifuge is operated, the samples are subjected to high speed rotation which generates a force many times greater than gravity. This force accelerates the natural settling of particles which otherwise would take a long time under normal gravitational conditions.
During centrifugation, the denser and heavier particles move away from the axis of rotation and are forced towards the bottom or outer side of the centrifuge tube. These particles gradually accumulate and form a compact mass which is referred to as pellet. At the same time, the lighter and less dense components remain in the liquid portion which stays above the pellet and is known as supernatant. The separation takes place efficiently due to continuous application of centrifugal force for a fixed period of time.
This principle is commonly applied in laboratories for separation of cellular components blood constituents precipitated proteins and nucleic acids. The efficiency of separation depends upon factors such as speed of rotation radius of the rotor time of centrifugation and nature of the sample. Thus, benchtop centrifuge is based on simple physical principle of sedimentation but provides rapid and effective separation in routine laboratory applications.
Types of Benchtop Centrifuges
The different types of benchtop centrifuges are classified on the basis of their design speed capacity and application. These are commonly used in routine laboratory work as well as specialized procedures.
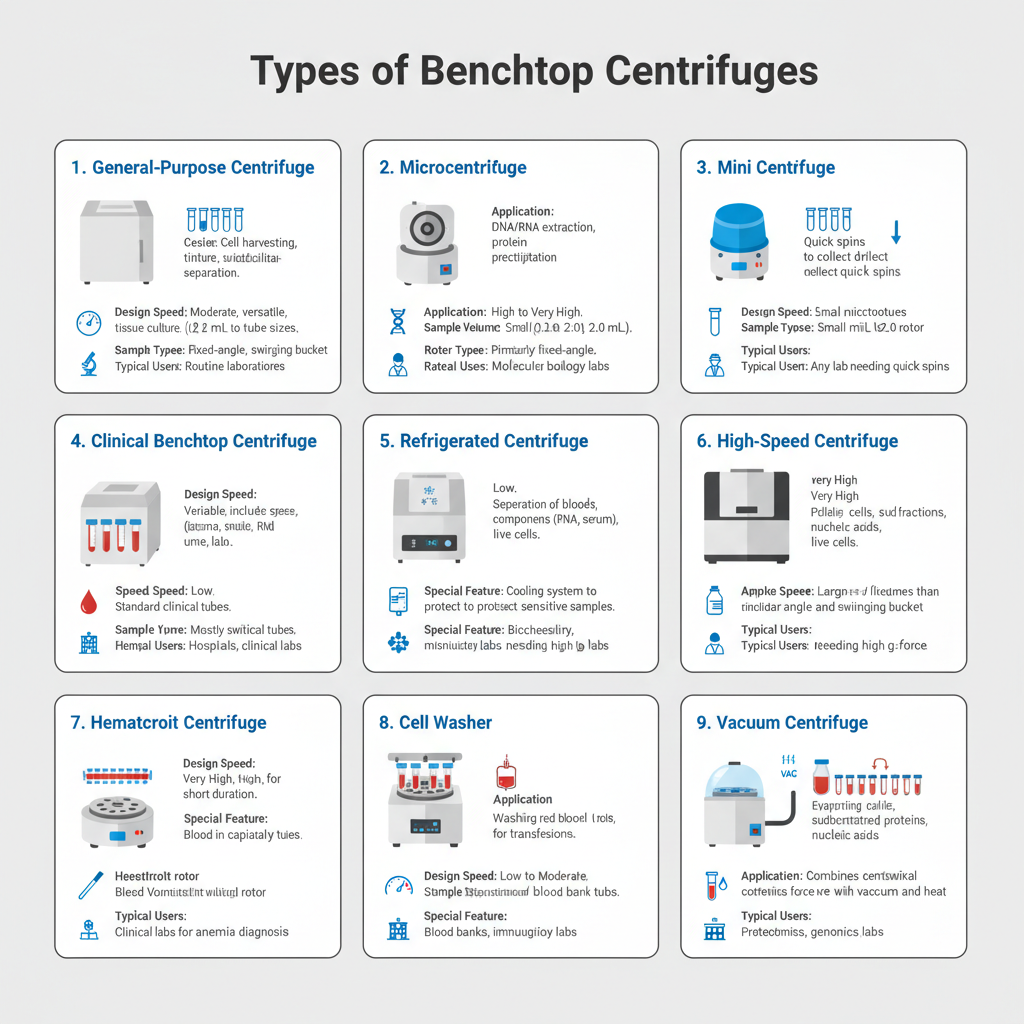
- General-Purpose (Multipurpose) Benchtop Centrifuges– These are the commonly used centrifuges in laboratory and are considered as workhorse units. It is designed for routine laboratory applications and can accommodate different types of rotors such as fixed-angle and swinging bucket rotors. These centrifuges handle wide range of tube sizes and are used for cell harvesting tissue culture and subcellular separation.
- Microcentrifuges– These are small sized benchtop centrifuges designed for handling small volume samples usually ranging from 0.2 mL to 2.0 mL microtubes. It can operate at very high speeds and is widely used in molecular biology laboratories. These centrifuges are used for DNA RNA extraction protein precipitation and plasmid isolation.
- Mini centrifuges – These are simple and compact type of microcentrifuges. It is mainly used for quick spin applications to collect liquid at bottom of tube and for basic separation steps.
- Clinical Benchtop Centrifuges– These centrifuges are used mainly in hospitals and clinical laboratories. It generally operates at low speed and are designed for diagnostic purpose. Swing-out rotors are mostly used to obtain proper separation layers. These are used for separation of blood components plasma serum and urine samples.
- Refrigerated Benchtop Centrifuges– These centrifuges are provided with cooling system to maintain low temperature during centrifugation. It is used when samples are temperature sensitive. These centrifuges are important for processing enzymes proteins RNA and live cells which may degrade due to heat produced during high speed spinning.
- High-Speed Benchtop Centrifuges– These centrifuges are capable of operating at very high rotational speed as compared to regular benchtop centrifuges. It is used for pelleting cells subcellular fractions and nucleic acid purification in larger volumes. These centrifuges act as intermediate between standard benchtop and floor standing centrifuges.
- Hematocrit Centrifuges– These are special type of benchtop centrifuges used for determination of hematocrit value. It spins capillary tubes containing whole blood at high speed for short duration. This helps in separation of red blood cells from plasma and is used for diagnosis of anemia and dehydration.
- Cell Washers– These are low speed centrifuges mainly used in blood banks and clinical laboratories. It is designed to wash red blood cells to remove unwanted proteins and cellular debris. This process is necessary during cross-matching and blood transfusion tests.
- Vacuum Centrifuges (Centrifugal Concentrators)– These centrifuges are different from conventional centrifuges. It uses centrifugal force along with vacuum and sometimes heat. It is used to evaporate solvents and concentrate samples like proteins and nucleic acids for further analysis.
Parts of Benchtop Centrifuges
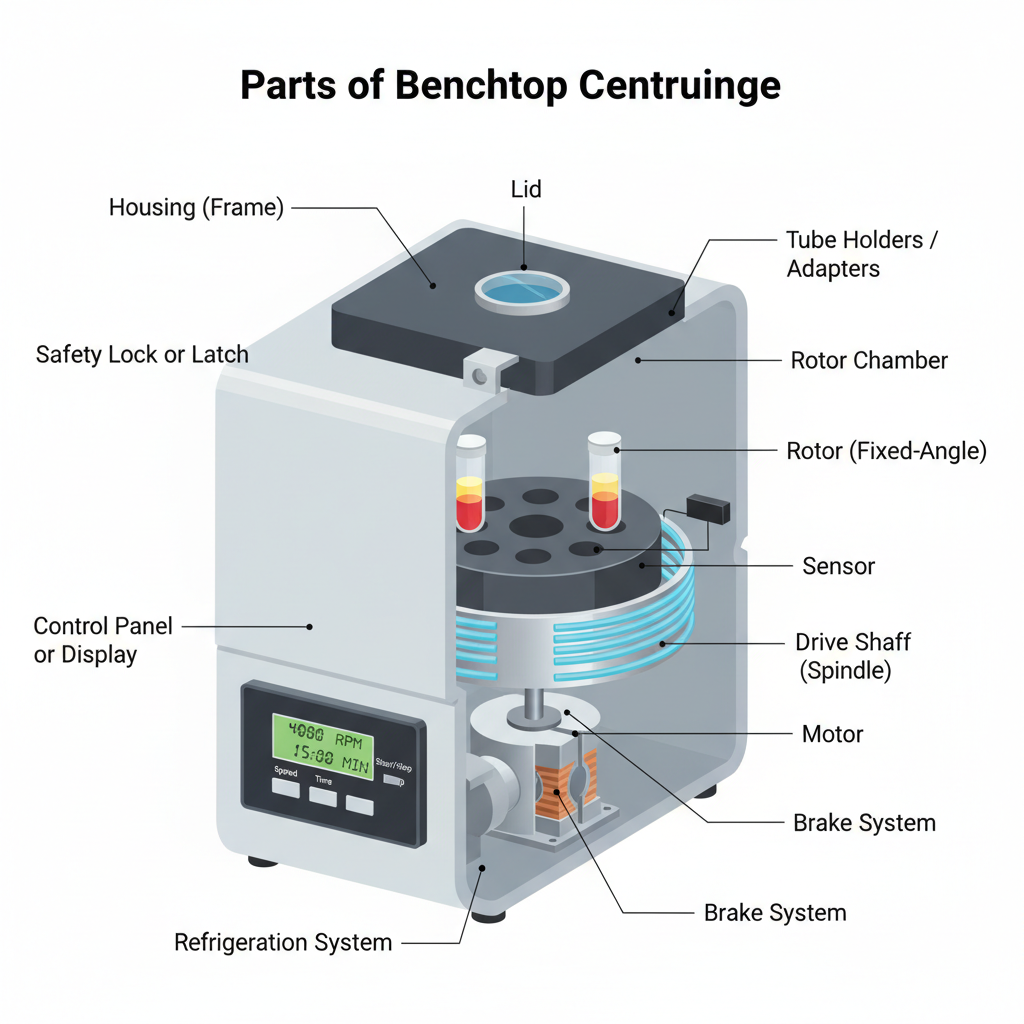
- Motor– The motor is the main component of the centrifuge. It converts electrical energy into mechanical energy and helps in rotating the rotor at required speed. The speed of rotation is controlled by this unit.
- Rotor– The rotor is the rotating part where sample tubes are placed. It generates centrifugal force during spinning which causes separation of sample components based on density. Rotors are of different types such as fixed-angle rotor and swinging bucket rotor.
- Rotor Chamber– This is the enclosed compartment in which the rotor spins. It provides protection to the user and helps in controlling airflow during centrifugation. In refrigerated centrifuges it also helps in maintaining required temperature.
- Lid– The lid is the upper cover of the centrifuge which seals the rotor chamber. It ensures safe operation during high speed rotation. Some lids are provided with viewing window for observation.
- Safety Lock or Latch – This part prevents the centrifuge from operating when the lid is open. It also keeps the lid locked during operation to avoid accidents due to sudden opening at high speed.
- Control Panel or Display– The control panel is used to set operating parameters such as speed time and temperature. It allows monitoring of centrifugation conditions during the run.
- Brake System – The brake system helps in slowing down and stopping the rotor after completion of centrifugation. Controlled braking is important to prevent remixing of separated layers.
- Tube Holders / Swing Buckets / Adapters– These components are attached to the rotor to hold tubes and bottles securely. Different adapters are used for accommodating tubes of various sizes.
- Sensors– Sensors are internal monitoring units that detect speed temperature and imbalance. If the samples are not balanced properly the centrifuge is automatically stopped to avoid damage.
- Drive Shaft (Spindle)– The drive shaft connects the motor to the rotor. It transfers rotational motion from the motor to the rotor assembly.
- Housing or Frame– This is the outer body of the centrifuge. It supports all internal components and provides mechanical protection to the instrument.
- Refrigeration System (in refrigerated centrifuges)– The refrigeration system helps in maintaining low temperature during centrifugation. It is used for protecting heat sensitive samples such as enzymes proteins and nucleic acids.
Operating Procedure of Benchtop Centrifuges
1. Pre-run inspection and preparation
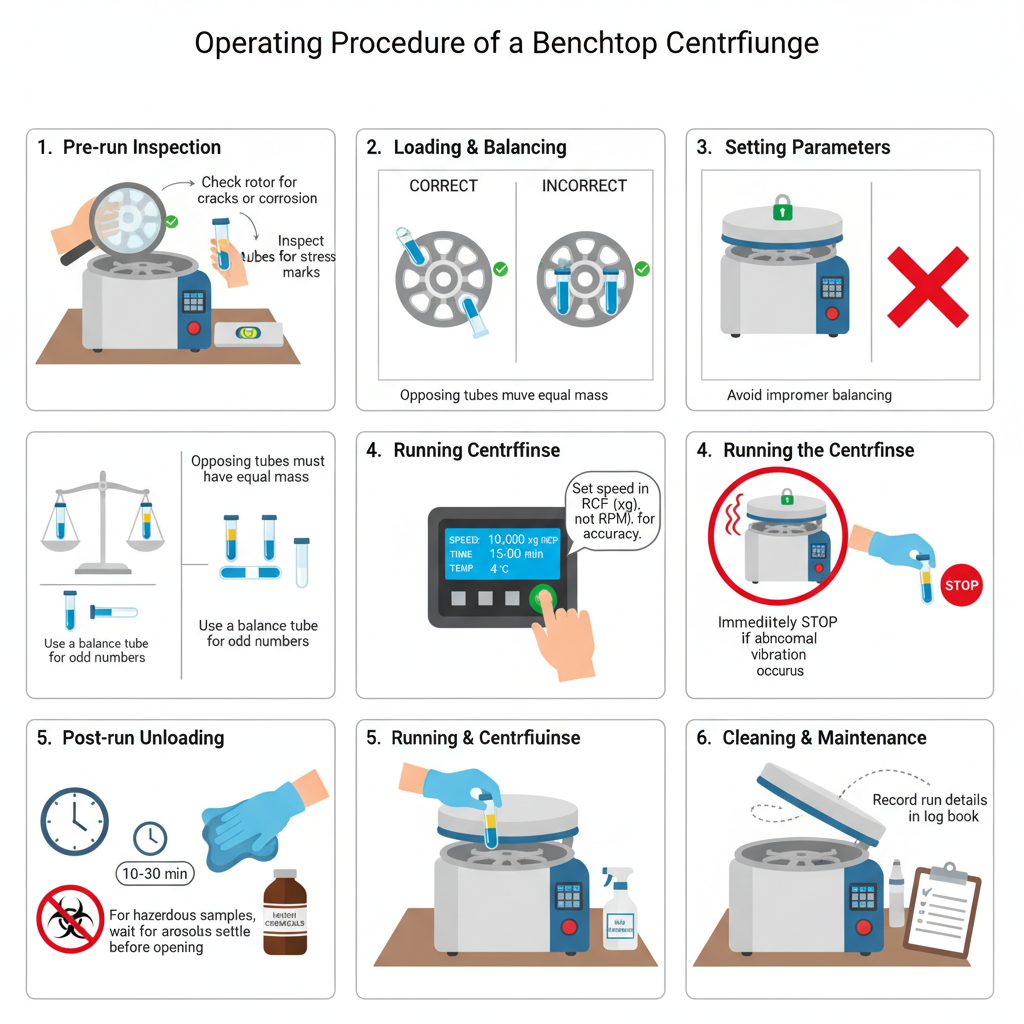
- It is ensured that centrifuge is placed on a firm and level surface before switching on.
- The rotor is inspected for cracks corrosion or any visible defect. Tubes are also checked for stress marks or breakage.
- It is confirmed that rotor used is compatible with the centrifuge and is properly seated on drive shaft.
- The tubes used is ensured to be suitable for required speed (g-force) and temperature of the experiment.
- In refrigerated centrifuge the rotor is pre-cooled to required temperature (usually 4°C) before loading the samples.
2. Loading and balancing of samples
- This is the most critical step and improper balancing is avoided.
- The tubes lids and adapters are weighed using balance so that opposing tubes have equal mass.
- The tubes are placed symmetrically around the center of rotation.
- In fixed-angle rotor tubes are placed directly opposite to each other.
- In swinging-bucket rotor all buckets are loaded even if some are empty to avoid damage.
- If odd number of samples is present a balance tube filled with water or similar liquid is used.
- The rotor lid is tightened properly to prevent leakage or aerosol formation.
3. Setting of operational parameters
- Speed is set preferably in terms of RCF (× g) rather than RPM to maintain accuracy.
- If only RPM option is present conversion is done based on rotor radius.
- The run time and temperature is entered through control panel.
- Acceleration and deceleration settings are selected as required. Slow braking is selected for gradient samples.
4. Running the centrifuge
- The centrifuge lid is closed properly until safety lock engages.
- The start button is pressed and centrifuge is observed till it reaches full speed.
- Any abnormal noise vibration or wobbling is immediately addressed by pressing stop button.
5. Post-run handling and unloading
- The lid is opened only after rotor comes to complete stop.
- For hazardous or infectious samples centrifuge is allowed to stand for 10–30 minutes before opening to allow aerosols to settle.
- The chamber buckets and tubes are checked for any leakage or spill.
- Sealed safety cups are opened only inside biological safety cabinet if required.
6. Cleaning and maintenance
- Spills are cleaned immediately using mild detergent and approved disinfectant.
- Harsh or caustic chemicals are avoided especially on aluminum rotors.
- The centrifuge lid is kept open after use to allow drying and prevent corrosion.
- Details such as speed time and rotor type are recorded in log book for routine maintenance and rotor life monitoring.
Uses of Benchtop Centrifuges
- It is used for separation of blood components such as red blood cells white blood cells and plasma or serum for clinical diagnosis.
- It is used in urinalysis to sediment cells crystals and other solid particles present in urine samples.
- It is used for hematocrit determination where packed cell volume of red blood cells is measured.
- It is used for washing of red blood cells in blood bank laboratories to remove plasma proteins and debris.
- It is used for isolation of DNA and RNA during nucleic acid extraction procedures in molecular biology laboratories.
- It is used for quick spin during PCR preparation to collect reagents at bottom of microcentrifuge tubes.
- It is used for protein precipitation concentration and purification from biological samples.
- It is used for processing of spin columns during plasmid miniprep and other purification kits.
- It is used for pelleting of bacterial yeast and mammalian cells from culture media.
- It is used for subcellular fractionation to separate nucleus mitochondria and other organelles by differential centrifugation.
- It is used for phase separation during phenol–chloroform extraction of nucleic acids.
- It is used for density gradient centrifugation to separate viruses organelles and macromolecules based on density.
- It is used for clarification of samples by removing suspended particles from liquid supernatant.
- It is used in environmental laboratories for separation of contaminants from water and soil samples.
- It is used in food and dairy laboratories for quality control such as cream separation and juice clarification.
- It is used in industrial laboratories for oil chemical and suspension analysis.
Advantages of Benchtop Centrifuges
- It has a compact size and occupies less space which allows it to be placed easily on laboratory workbench.
- It does not require floor space and hence suitable for laboratories with limited working area.
- It is economical as compared to floor standing centrifuges and requires lower initial investment.
- It has low maintenance requirement and long operational life which reduces overall running cost.
- It does not need special infrastructure such as reinforced flooring or dedicated power supply.
- It is versatile in nature and can be used for different laboratory applications.
- It can accommodate different types of rotors such as fixed-angle and swing-out rotors.
- It supports wide range of tube sizes from microcentrifuge tubes to larger volume tubes.
- It provides rapid acceleration and deceleration which saves processing time.
- It is easy to operate and convenient for routine laboratory work.
- It is lightweight and portable which allows easy relocation within laboratory.
- High-speed models are capable of generating high centrifugal force for cell and molecular work.
- Refrigerated models allow temperature control which helps in protecting heat sensitive samples.
- It provides reliable and reproducible results for routine as well as advanced laboratory procedures.
Disadvantages of Benchtop Centrifuges
- It has limited sample capacity and cannot handle large volume samples in a single run.
- It is not suitable for high throughput work where large number of samples are required to be processed at one time.
- It cannot achieve ultra-high speed and very high g-force as compared to ultracentrifuge.
- It is not suitable for separation of very small particles such as viruses ribosomes and some subcellular components.
- It occupies laboratory bench space which may limit working area for other experiments.
- It may produce noise and vibration which can disturb nearby sensitive instruments.
- Non-refrigerated models may generate heat during operation affecting heat sensitive samples.
- Refrigerated benchtop centrifuges are bulky and more expensive as compared to normal models.
- Fixed-angle rotors form pellet on side of tube which makes pellet recovery difficult.
- Swing-out rotors have limitation in maximum speed and g-force which increases centrifugation time.
- It has limited rotor options as compared to large floor standing centrifuges.
- It is less efficient for industrial and large scale processing applications.
Precautions for operating Benchtop Centrifuges
- Proper balancing of tubes is ensured before every run as imbalance can cause damage to centrifuge.
- Tubes caps and adapters are balanced by weight and not by visual estimation.
- Tubes are placed symmetrically around the rotor and balance tubes are used if required.
- All buckets are loaded in swinging-bucket rotors even if some positions are empty.
- Tubes are checked for cracks deformation or stress marks before use.
- Damaged or weak tubes are not used as they may break during centrifugation.
- The rotor is inspected regularly for corrosion cracks or any visible defect.
- The rotor is seated properly on drive shaft and lid is tightened securely.
- O-rings and seals are checked for proper condition when aerosol-tight lids are used.
- Compatible tube material is selected according to the chemical nature of sample.
- The centrifuge is placed on a stable and level surface before operation.
- Maximum speed and load limits of rotor are not exceeded.
- The centrifuge is observed until it reaches full speed after pressing start button.
- Any unusual noise vibration or wobbling is addressed by stopping the centrifuge immediately.
- The lid is not opened until rotor comes to complete stop.
- In case of suspected spill or tube breakage lid is kept closed for some time to allow aerosols to settle.
- Safety cups or sealed rotors are used for hazardous and infectious samples.
- Spills are cleaned immediately to prevent corrosion and contamination.
- Mild detergents are used for cleaning and harsh chemicals are avoided.
- Rotors are dried properly and stored in dry condition after use.
- For high-speed use run details are recorded to monitor rotor life.
FAQ
Q1. What is a benchtop centrifuge?
A. A benchtop centrifuge is a laboratory instrument which is placed on a working bench and is used to separate components of a mixture based on their density by applying centrifugal force. It is commonly used for routine laboratory work involving small to medium volume samples.
Q2. How do I choose the right benchtop centrifuge for my laboratory?
A. The selection of a benchtop centrifuge depends on type of samples speed requirement temperature control and tube size. It is important to consider whether routine or advanced applications are to be performed before choosing the instrument.
Q3. What factors should be considered when choosing a laboratory centrifuge?
A. The major factors include sample volume maximum speed g-force rotor type temperature requirement safety features and available laboratory space. Cost and maintenance requirement are also considered.
Q4. What are the types of bench top centrifuges?
A. The types of bench top centrifuges are
• Low-speed benchtop centrifuge
• High-speed benchtop centrifuge
• Refrigerated benchtop centrifuge
• Microcentrifuge
• Hematocrit centrifuge
Q5. What are the main types of laboratory centrifuges?
A. The main types of laboratory centrifuges are
• Benchtop centrifuge
• Floor-standing centrifuge
• High-speed centrifuge
• Ultracentrifuge
• Refrigerated centrifuge
Q6. What is the principle of bench top centrifuges?
A. The principle of bench top centrifuges is sedimentation. When samples are rotated at high speed heavier particles move outward and settle at bottom of the tube while lighter particles remain in supernatant due to centrifugal force.
Q7. How does a laboratory centrifuge work?
A. A laboratory centrifuge works by spinning samples at high speed around a central axis. The centrifugal force generated causes separation of particles according to their size shape and density.
Q8. What are the parts of bench top centrifuges?
A. The main parts of bench top centrifuges are
• Motor
• Rotor
• Centrifuge tubes
• Control panel
• Lid with safety lock
• Drive shaft
Q9. What are the applications of bench top centrifuges?
A. Bench top centrifuges are used for separation of blood components nucleic acid extraction protein purification cell pelleting and sample clarification in laboratories.
Q10. Where are benchtop centrifuges used?
A. Benchtop centrifuges are used in clinical laboratories research laboratories biotechnology laboratories pharmaceutical industries food testing laboratories and educational institutions.
Q11. Do your samples need to be refrigerated for centrifugation?
A. Samples which are heat sensitive such as proteins enzymes and live cells require refrigerated centrifugation. Non-sensitive samples can be centrifuged at room temperature.
Q12. What are your speed requirements for a centrifuge?
A. Speed requirement depends on type of sample and application. Low-speed centrifuges are used for blood and urine samples while high-speed centrifuges are required for molecular biology and cell fractionation work.
Q13. What size tubes are you spinning in a centrifuge?
A. The tube size depends on sample volume and rotor type. Commonly used tubes range from microcentrifuge tubes (0.2–2 mL) to larger tubes of 15 mL 50 mL or more.
Q14. What is the maximum g-force a centrifuge can generate?
A. The maximum g-force generated by a centrifuge depends on its speed and rotor radius. Benchtop centrifuges generally generate up to 60,000 × g while ultracentrifuges generate much higher force.
Q15. What safety precautions should be taken when using a laboratory centrifuge?
A. Proper balancing of tubes is essential before operation. The rotor and tubes should be checked for damage. Maximum speed limits should not be exceeded and lid should not be opened until rotor stops completely. Hazardous samples should be handled using sealed rotors or safety cups.
- Amuza Inc. (2021). Benchtop vs. floor centrifuges: How to choose the right format for your lab.
- Beckman Coulter. (n.d.). Balancing your rotor.
- Beckman Coulter. (n.d.). Benchtop centrifuges.
- Beckman Coulter. (n.d.). Centrifuge maintenance and care.
- Beckman Coulter. (n.d.). Relative centrifugal field (RCF).
- Beckman Coulter. (n.d.). Using k-factor to compare rotor efficiency.
- Benchtop centrifuge systems: A comprehensive operational and technical guide. (n.d.).
Biocompare. (n.d.). Laboratory centrifuges. - Danaher Life Sciences. (n.d.). Function of centrifuge in DNA & RNA extraction explained.
- Eppendorf. (2019, July 22). Basics in centrifugation – Calculating relative centrifugal force and selecting a centrifuge and rotors. Lab Academy.
- Excedr. (2024, January 10). How much does a centrifuge cost in 2024?
- Fisher Scientific. (n.d.). Microcentrifuges.
- Galusha, H. (n.d.). The basics of centrifuge operation and maintenance: A laboratory guide. Lab Manager.
- GMP Plastic. (2025, March 10). How to properly balance a centrifuge.
- Goodman, T. (2008, January 1). Choosing the right centrifuge for your application. American Laboratory.
- Henderson Biomedical. (2024, April 29). What is the difference between a swing-out and fixed angle rotor?
- Hunan Xiang Yi Laboratory Instrument Development Co., Ltd. (n.d.). The economic advantages of benchtop centrifuges.
- Karki, P. (2024, November 5). Centrifuge: Principle, parts, types, and applications. Microbe Notes.
McDonald, K. (2023, June 27). What is the difference between a fixed-angle and a swing-out rotor? Laboratory Supply Network. - MRC Lab. (n.d.). How do you choose a microcentrifuge?
- New Life Scientific. (2023, July 3). Centrifuge care and maintenance.
- Pipette.com Team. (2025, August 15). 8 types of laboratory centrifuges & the purposes they serve. Pipette.com.
- Richmond Scientific. (2024, January 8). Centrifuge top tips part 1 – Types of centrifuges and rotors.
- Sanulli, S., & Narlikar, G. (2021, May). Generation and biochemical characterization of phase-separated droplets formed by nucleic acid binding proteins: using HP1 as a model system. Current Protocols, 1(5), e109.
- Stanford Environmental Health & Safety. (n.d.). Centrifuge safety. Stanford University.
- University of Georgia College of Agricultural & Environmental Sciences. (n.d.). Centrifuge and rotor training and safety.
- University of Kentucky Environmental Health and Safety. (n.d.). Centrifuges.
- Werner, A. (2022, February 14). How to balance a centrifuge: A comprehensive guide. INTEGRA Biosciences.
- Wikipedia contributors. (n.d.). Centrifugation. In Wikipedia, The Free Encyclopedia.
- Wikipedia contributors. (n.d.). Clearing factor. In Wikipedia, The Free Encyclopedia.