What is Ames Test?
- The Ames test, developed by Bruce Ames and his team at the University of California, Berkeley, is a biological assay designed to evaluate the mutagenic potential of various chemicals, drugs, or implant devices. This test specifically assesses the ability of these substances to induce mutations in the DNA of the test organism, primarily using histidine auxotrophs of Salmonella typhimurium or tryptophan auxotrophs of Escherichia coli.
- In the context of the Ames test, these mutant strains of Salmonella cannot grow or form colonies on a medium that lacks histidine due to a specific mutation that inhibits histidine biosynthesis. However, when exposed to mutagenic chemicals, these substances may reverse the mutation in the bacterial cells, enabling them to grow on a histidine-deficient medium. This reversal indicates the mutagenic nature of the chemical.
- It’s crucial to understand that while not all mutagens are carcinogens, many chemicals that are mutagenic also possess carcinogenic properties, meaning they can cause cancer in humans and other animals. Therefore, identifying a compound as mutagenic through the Ames test serves as an early warning sign of its potential carcinogenic effects.
- Subsequently, chemicals that demonstrate mutagenic properties in the Ames test can undergo further testing to determine their carcinogenic effects in animals. This test offers a rapid and cost-effective alternative to traditional carcinogen assays on animals, which are both time-consuming and expensive.
- In essence, the Ames test provides a preliminary assessment of the mutagenic and potential carcinogenic properties of chemicals, acting as a vital tool in the field of toxicology and public health. The consistent third-person point of view and objective tone emphasize the test’s significance and functionality in assessing chemical safety.
Definition of Ames Test
The Ames test is a biological assay developed by Bruce Ames, used to assess the mutagenic potential of chemical compounds by observing their ability to induce mutations in specific strains of bacteria, primarily Salmonella typhimurium. A positive result indicates that the chemical may be mutagenic and potentially carcinogenic.
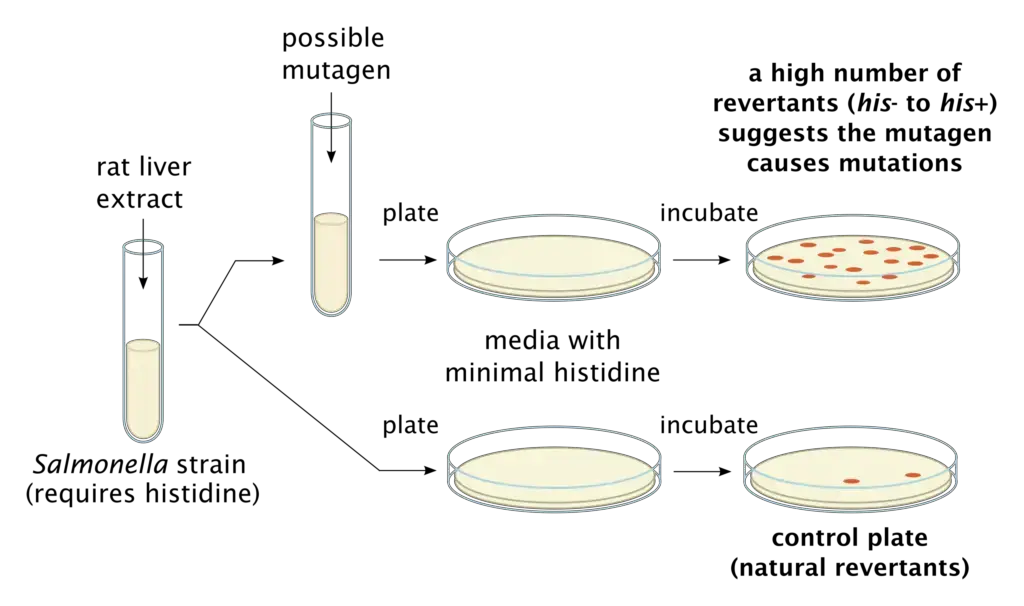
Principle of Ames Test
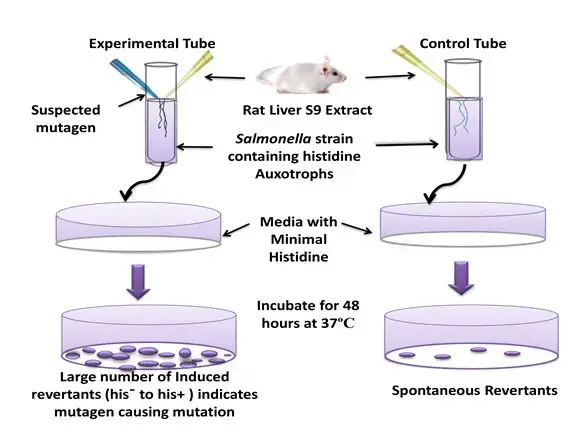
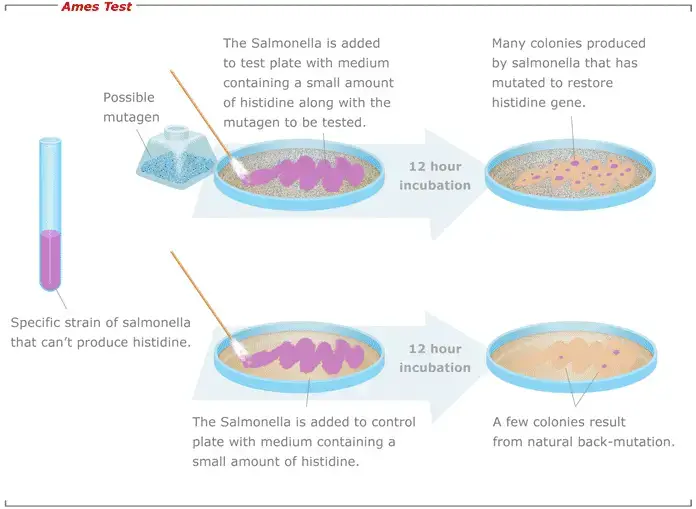
- The principle of the Ames test revolves around the use of specific bacterial strains, primarily histidine auxotrophs of Salmonella typhimurium, which are unable to synthesize the essential amino acid histidine due to a mutation. When these bacteria are exposed to a medium lacking histidine, they cannot grow. However, if a chemical being tested induces a reverse mutation (reversion) that allows the bacteria to regain their ability to produce histidine, they can grow and form colonies on the histidine-deficient medium.
- In the Ames test, the suspected mutagen is introduced to the medium. If the rate of reversion, evidenced by the appearance of more colonies, increases in the presence of the suspected mutagen, it indicates the mutagenic potential of the chemical. The greater the number of colonies formed, the higher the mutagenicity of the test substance.
- Furthermore, the Ames test can also be conducted using tryptophan auxotrophs of Escherichia coli. In this variation, the medium lacks the amino acid tryptophan. The principle remains the same: if the bacteria revert to a state where they can synthesize tryptophan (due to the action of a mutagen), they will grow on the tryptophan-deficient medium.
- It’s essential to understand that not all mutations are harmful. The Ames test specifically identifies those mutations that can revert the bacteria to their original, wild-type state, allowing them to synthesize the missing amino acid. Chemicals that induce such reverse mutations are considered mutagenic. After identification in the Ames test, these mutagenic compounds are further evaluated for their potential carcinogenic effects in animal models.
Strains recommended for use in Ames test
Materials and Reagents Required for Ames Test
Materials:
- Sterile Petri plates with a diameter of 90 mm and a depth of 15 mm.
- Tips available in various volumes: 1,000 µl, 200 µl, and 10 µl.
- Erlenmeyer flasks and beakers in sizes of 10 ml, 250 ml, and 500 ml.
- Eppendorf tubes in capacities of 1.5 ml and 2.0 ml.
- A metal loop holder, specifically the metal loop Ch-2.
- L-shaped spreader, essential for spreading bacterial cultures evenly on agar plates.
Mutagens: These are substances that induce mutations. For the Ames test, specific mutagens are used as controls to validate the test’s sensitivity. They include:
- Sodium azide
- 4-Nitroquinoline N-oxide
- 2-Aminofluorene
- Benzo(a)pyrene
- Mitomycin C
- 2,4,7-Trinitro-9-fluorenone
- 4-Nitro-o-phenylenediamine
Reagents: Reagents play a crucial role in the Ames test, ensuring the growth of bacterial strains and the activation of certain test substances. The following reagents are required:
- Oxoid nutrient broth No. 2
- 70% ethanol
- Magnesium sulphate heptahydrate
- Citric acid monohydrate
- Potassium phosphate (dibasic, anhydrous)
- Sodium ammonium phosphate tetrahydrate
- D-biotin and L-histidine, which are essential for the growth of the bacterial strains used in the Ames test.
- Hydrochloric acid, used for pH adjustments.
- Potassium chloride and magnesium chloride hexahydrate, which serve as essential salts.
- Sodium dihydrogen phosphate monohydrate and disodium hydrogen phosphate.
- NADP (sodium salt) and D-glucose-6-phosphate (monosodium salt), which are essential cofactors for certain enzymatic reactions.
- Ampicillin trihydrate, sodium hydroxide, and crystal violet.
- Agar-Agar and nutrient broth, which serve as growth media for bacteria.
- Tetracycline and dimethylsulfoxide.
Equipments
- Orbital Shaking Incubator (Remi, model: RIS-24(BL)): This equipment facilitates the growth of microbial cultures by providing a controlled environment with consistent shaking. The orbital motion ensures uniform mixing of the culture medium, promoting optimal growth conditions.
- Laminar Flow Hood (Bio safety cabinet) (Deepak Meditech Pvt Ltd., Steri clean): Essential for maintaining a sterile environment, the laminar flow hood ensures that the air flow is unidirectional and free from contaminants. This is crucial when working with sensitive biological samples to prevent contamination.
- Pipettes (Eppendorf): Precision in liquid handling is achieved using pipettes. The mentioned models, such as Research® plus with catalog numbers 3120000062 (1,000 μl), 3120000046 (200 μl), and 3120000020 (10 μl), are designed for accurate and consistent volume dispensing.
- Vortex Mixer (Labnet International, catalog number: S0100): For thorough mixing of samples, the vortex mixer is employed. It ensures that the contents of a vial or tube are homogeneously mixed by creating a vortex motion.
- Hot Water Bath (Daiki Sciences, catalog number: KBLee2001): Used for incubating samples at a specific temperature, the hot water bath provides a consistent and controlled heat source.
- Autoclave (TSC): Sterilization is a critical aspect of biological research. The autoclave uses pressurized steam to sterilize equipment and other items, ensuring they are free from microbial contamination.
- Automatic Colony Counter (Sonar): To quantify the number of microbial colonies on an agar plate, the automatic colony counter is used. It provides a rapid and accurate count, streamlining the process.
- Refrigerator Centrifuge (Thermo Fisher Scientific, Thermo ScientificTM, model: Heraeus Biofuge Primo R): This equipment is used to separate components of a sample based on their densities. The refrigeration feature ensures that the samples remain at a specific temperature during the centrifugation process.
- pH Meter (Labindia Analytical Instruments, model: PICO pH Meter, catalog number: PC13330101): Accurate measurement of pH is crucial in many biological experiments. The pH meter provides a precise reading of the acidity or alkalinity of a solution.
- Tissue Tearor (Bio Spec Products, catalog number: 985370-04): For the disruption and homogenization of tissues, the tissue tearor is employed. It ensures that tissues are broken down into their constituent parts for further analysis.
Procedure of Ames Test
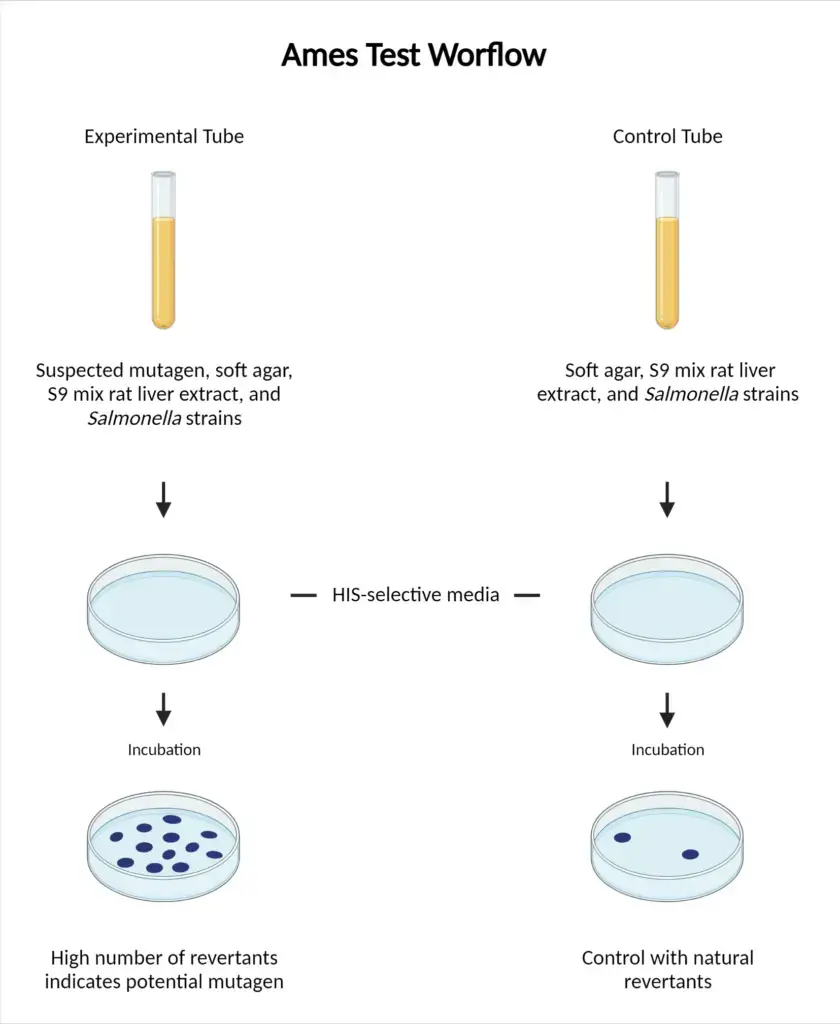
- Setting Up New Colonies: Begin by establishing new colonies for each strain of S. typhimurium. These strains should be incubated overnight in Oxoid nutrient broth #2 at a temperature of 37°C. Ensure that they are placed on a shaker set to 120 rpm for optimal aeration.
- Preparation of Stock Solutions: On the day the experiment is to be conducted, formulate stock solutions of the positive control mutagens. These solutions will differ based on whether the tubes contain S9 or not.
- Labeling and Tube Preparation: Appropriately label 10 mL Falcon tubes for each strain, referring to Table 2 for the required conditions. Concurrently, get the minimal glucose plates ready by aliquoting 20 mL into each 90mm x 15 mm Petri plate. All plates should be labeled accordingly.
- Agar Solution Preparation: Prepare a heated agar solution and maintain its temperature around 45°C using a water bath. Subsequently, aliquot 5 mL of this heated agar into each of the 10 mL Falcon tubes.
- Addition of Components: To each tube containing the heated agar, add the following components in sequence:
- 100 µL of the cultured strain from step 1.
- 200 µL of the 0.5 mM histidine/biotin solution.
- Either 500 µL of the S9 mixture or 500 µL of the sodium phosphate buffer, depending on the requirements.
- 100 µL of the sample, which could be a positive control, negative control, or the test sample.
- 100 µL of dH2O.
- Mixing and Distribution: Vortex each of the 10 mL tubes swiftly to ensure a homogenous mixture. Then, distribute this mixture evenly onto the minimal glucose plates. Allow the mixture to cool and solidify for approximately 2-3 minutes.
- Protection and Incubation: Shield the plates with aluminum foil to protect them from light exposure. Then, incubate these plates at 37°C for a duration of 48 hours.
- Observation: After the incubation period, examine the plates for colony formation. Ideally, the colonies should be evenly distributed across the plate.
Recipes
1. Vogel-Bonner medium E (50x) for Minimal agar (Recipe 9)
- Ingredients: This recipe requires warm distilled H2O, magnesium sulfate, citric acid monohydrate, potassium phosphate, and sodium ammonium phosphate.
- Procedure: Salts are sequentially added to warm water in a flask. After each salt dissolves, the solution is transferred to glass bottles and autoclaved. Once cooled, the bottles are capped and stored at 4 °C.
2. 0.5 mM histidine/biotin solution for mutagenic bioassay
- Ingredients: D-Biotin and L-Histidine·HCl are the primary components.
- Procedure: Biotin is dissolved in hot distilled water, autoclaved, and stored at 4 °C.
3. Salt solution (1.65 M KCl + 0.4 M MgCl2) for S9 hepatic fraction
- Ingredients: Potassium chloride and magnesium chloride are essential.
- Procedure: All components are dissolved in water, autoclaved, and stored at 4 °C.
4. 0.2 M sodium phosphate buffer, pH 7.4 for S9 hepatic fraction
- Ingredients: Sodium dihydrogen phosphate and disodium hydrogen phosphate are used.
- Procedure: The pH is adjusted to 7.4, and the buffer is sterilized by autoclaving.
5. 1 M nicotinamide adenine dinucleotide phosphate (NADP) solution for S9 hepatic fraction
- Ingredients: NADP is the primary component.
- Procedure: NADP is dissolved in distilled water, mixed, and stored in an ice bath for up to six months.
6. 1 M glucose-6-phosphate for S9 hepatic fraction
- Ingredients: Glucose-6-phosphate is essential.
- Procedure: It is dissolved in distilled water, mixed, and stored in an ice bath for up to six months.
7. Ampicillin solution (4 mg/ml)
- Ingredients: Ampicillin trihydrate and sodium hydroxide are used.
- Procedure: Ampicillin trihydrate is dissolved in NaOH, mixed, and stored in an ice bath.
8. Crystal violet solution (0.1%)
- Ingredients: Crystal violet is the main component.
- Procedure: It is dissolved in distilled water.
9. Minimal glucose plates for Mutagenic bioassay
- Ingredients: Agar, distilled H2O, VB salts, and glucose are required.
- Procedure: Agar is dissolved in distilled water, autoclaved, and the salts and glucose are added gently.
10. Histidine/Biotin plates
- Ingredients: Agar, distilled H2O, VB salts, glucose, histidine, and biotin are essential.
- Procedure: Each solution is autoclaved separately and combined post-cooling.
11. Ampicillin and tetracycline plates*
- Ingredients: Agar, distilled H2O, VB salts, glucose, histidine, biotin, ampicillin solution, and tetracycline solution are used.
- Procedure: Each solution is autoclaved separately and combined post-cooling.
12. Nutrient agar plates
- Ingredients: Nutrient agar and distilled H2O are required.
- Procedure: Agar is dissolved in distilled water and poured into Petri plates post-autoclaving.
13. S9 mix (Rat Liver Microsomal Enzymes + Cofactors)
- Ingredients: Mice liver, MgCl2-KCl salts, glucose-6-phosphate, NADP, phosphate buffer, and distilled H2O are essential.
- Procedure: Ingredients are added in reverse order, and the mix is stored without refreezing.
14-17. Sodium azide, 2-Nitrofluorine, Mitomycin, and 2-Anthramine for Mutagenicity assay
- Ingredients: Each reagent requires its respective compound and autoclaved distilled H2O.
- Procedure: The compound is dissolved in distilled water to achieve the desired concentration.
Uses of Ames Test
- Screening of Chemical Mutagens: The primary application of the Ames Test is to screen and identify chemical mutagens that have the potential to cause mutations. These mutations can be carcinogenic, posing a risk to both humans and animals. For instance, certain chemicals, such as the food additive AF-2 and the flavoring agent Safrole, have been identified as both mutagenic and carcinogenic.
- Identification of Drug Mutagenicity: Some drugs, like Isoniazid, which is used to treat tuberculosis, have been found to be mutagenic. Therefore, the Ames Test plays a crucial role in the pharmaceutical industry, ensuring that drugs are safe for consumption.
- Adaptation for Eukaryotic Cells: While the Ames Test primarily uses the bacterium Salmonella typhimurium, it has been adapted to test mutagens using eukaryotic cell cultures, yeast cells, and even animal models. This adaptation is essential because Salmonella may not always be the most suitable organism for testing human mutagens. Some chemicals, like sodium nitrate (NaNO3), are not inherently mutagenic but can transform into mutagens when metabolized by the body. For instance, sodium nitrate becomes a potent mutagen, nitrous oxide (HNO2), when acted upon by HCL in the stomach.
- High Sensitivity Detection: The Ames Test is highly sensitive and can detect suitable mutants even in a vast population of bacteria. This sensitivity ensures that even minute quantities of mutagens are identified, ensuring comprehensive safety evaluations.
- Emphasis on Mutagenicity Over Carcinogenicity: It’s essential to note that the Ames Test primarily tests for mutagenicity and not carcinogenicity. However, a significant correlation exists, as over 90% of the mutagens detected by the Ames Test have been found to cause cancer.
- Bacterial Reverse Mutation Assay: The Ames Test operates on the principle of bacterial reverse mutation. This means that a defective gene in the bacteria can be mutated back into a functional gene, allowing for the identification of mutagenic substances.
- Environmental Screening: The Ames Test is invaluable in detecting and screening potentially hazardous chemicals present in our environment, food, or drugs. This ensures that harmful substances are identified and controlled, protecting public health.
- Pharmaceutical Industry Application: The pharmaceutical industry heavily relies on the Ames Test. Before clinical trials are initiated, various drugs and chemicals are tested using the Ames Test to ensure their safety and non-mutagenic nature.
Limitations
- Model Organism Limitations: The primary organism used in the Ames Test is Salmonella typhimurium, a prokaryote. Therefore, it is not an ideal model for humans, who are eukaryotic organisms. This difference in biological complexity can lead to discrepancies in the test results when extrapolated to human conditions.
- Metabolic Differences: To simulate mammalian metabolic conditions, the Ames Test uses the rat liver S9 fraction. This fraction is utilized to assess the mutagenic potential of metabolites formed in the hepatic system. However, there are inherent differences between human and rat metabolism, which can influence the mutagenicity of the substances being tested. Although the use of human liver S9 fraction might improve the test’s accuracy, it was previously limited by its availability. However, with its commercial availability, its use may become more widespread.
- Eukaryotic Adaptations: Given the limitations of using a prokaryotic model, there have been adaptations of the Ames Test for eukaryotic cells, such as yeast. While this offers a more complex biological model, it still does not fully replicate human cellular conditions.
- Mutagenicity vs. Carcinogenicity: A significant limitation of the Ames Test is that it primarily identifies mutagens, not necessarily carcinogens. Just because a substance is mutagenic does not mean it will cause cancer. Therefore, any potential carcinogen identified in the Ames Test requires further testing to confirm its carcinogenic potential.
- False Positives: Certain compounds, especially those containing the nitrate moiety, might produce false-positive results in the Ames Test. For instance, nitrate compounds can generate nitric oxide, a crucial signaling molecule, leading to false positives. An example of this is Nitroglycerin, which tests positive in the Ames Test but is still used in medical treatments today.
- Nitrate Complications: While some nitrates in drugs might be safe, nitrates in food can be reduced by bacterial action to nitrites. These nitrites can generate carcinogens by reacting with amines and amides, complicating the interpretation of Ames Test results.
- Need for Further Testing: A positive result in the Ames Test does not conclusively indicate a substance’s safety or danger. Long toxicology and outcome studies are essential for compounds that produce positive Ames Test results to determine their actual impact on human health.
Safety Considerations
- Biosafety Guidelines: It is crucial to always follow standard biosafety guidelines when handling S. typhimurium. This includes the use of plugged pipettes to prevent accidental spills and ensuring proper sterilization methods, such as using 70% ethanol and autoclaving all materials that come into contact with the bacterium.
- Use of Biosafety Cabinet: All handling of chemicals and strains should be conducted within a biosafety cabinet. This enclosed workspace provides a controlled environment that minimizes the risk of contamination. Before and after each use, the cabinet must be thoroughly sterilized using 70% ethanol. Additionally, it should be exposed to UV light for at least 15 minutes to ensure the elimination of any residual pathogens.
- Personal Protective Equipment (PPE): To protect oneself from potential chemical exposure and bacterial contamination, it is essential to wear appropriate personal protective equipment. This includes wearing laboratory gowns, protective eyeglasses, and gloves. These items not only shield the individual from direct contact with hazardous materials but also prevent the spread of contaminants to other areas.
- Proper Disposal: After any experiment or procedure involving S. typhimurium, all contaminated materials, such as test tubes, pipettes, pipette tips, gowns, and gloves, must be collected for proper disposal. Before discarding, these materials should be autoclaved to ensure complete sterilization. Autoclaving is a high-pressure steam treatment that effectively kills all microorganisms, ensuring that no pathogens are released into the environment.
- Avoiding Chemical Exposure: When working with chemicals, care must be taken to prevent any form of exposure. This means ensuring that all containers are securely sealed when not in use and handling chemicals with caution to avoid spills or splashes.
References
- Föllmann, W., Degen, G., Oesch, F., & Hengstler, J. G. (2013). Ames Test. Brenner’s Encyclopedia of Genetics, 104–107. doi:10.1016/b978-0-12-374984-0.00048-6
- Hengstler, J. G., & Oesch, F. (2001). Ames Test. Encyclopedia of Genetics, 51–54. doi:10.1006/rwgn.2001.1543
- Jain, A. K., Singh, D., Dubey, K., Maurya, R., Mittal, S., & Pandey, A. K. (2018). Models and Methods for In Vitro Toxicity. In Vitro Toxicology, 45–65. doi:10.1016/b978-0-12-804667-8.00003-1
- Vijay U, Gupta S, Mathur P, Suravajhala P, Bhatnagar P. Microbial Mutagenicity Assay: Ames Test. Bio Protoc. 2018 Mar 20;8(6):e2763. doi: 10.21769/BioProtoc.2763. PMID: 34179285; PMCID: PMC8203972.
- Mortelmans K, Zeiger E. The Ames Salmonella/microsome mutagenicity assay. Mutat Res. 2000 Nov 20;455(1-2):29-60. doi: 10.1016/s0027-5107(00)00064-6. PMID: 11113466.
- Ames Test. (2021, August 10). https://bio.libretexts.org/@go/page/63340
- https://www.biotoxicity.com/index.php/ebpi-toxicity-tests/ames-tests
- http://www.sci.sdsu.edu/~smaloy/MicrobialGenetics/topics/rev-sup/ames.html
- https://www.news-medical.net/life-sciences/The-Ames-Test.aspx
- https://www.cyprotex.com/toxicology/genotoxicity/amestest
- http://legacy.genetics-gsa.org/education/pdf/GSA_DeStasio_Ames_Student_Resources.pdf
- https://www.xenometrix.ch/miniaturized-ames-test.html
- https://thebumblingbiochemist.com/365-days-of-science/the-ames-test-for-mutagenicity/
- https://www.nelsonlabs.com/testing/genotoxicity/
- https://bienta.net/ames-test/
- https://revive.gardp.org/resource/ames-test/?cf=encyclopaedia
- https://www.bionity.com/en/encyclopedia/Ames_test.html
- https://www.vivotecnia.com/ames-test/
- https://www.synbiosis.com/application-notes/ames-test/
- https://www.eionet.europa.eu/gemet/en/concept/370
- https://www.aatbio.com/resources/faq-frequently-asked-questions/What-is-the-principle-of-the-Ames-test
- https://microbeonline.com/ames-test-mutagenicity-carcinogens/
- https://www.davuniversity.org/images/files/study-material/BCH526-3.pdf
- https://www.mbbiosciences.com/ames-test
- https://www.criver.com/products-services/safety-assessment/toxicology-services/genetic-toxicology/ames-test?region=3701
- https://www.mun.ca/biology/scarr/4241_Ames_Test.html
- https://www.onlinebiologynotes.com/ames-test-a-test-for-mutagenicity-principle-procedure-and-application/
- https://www.xenometrix.ch/ames-test-scientific-background.html
- https://www.eurofins.com.au/biopharma-services/genetic-toxicology/the-ames-test/
- https://www.sciencedirect.com/science/article/pii/B0123694000000570
- https://www.sciencedirect.com/science/article/pii/B9780323983679000135
- https://microbiologyinfo.com/ames-test/
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.