Xanthoproteic test
Proteins are polymers of amino acids. They are complex organic compounds containing nitrogen, hydrogen, carbon and oxygen. Proteins are abundant in our everyday food e.g. egg, soya bean, pulses, fish, milk etc. The presence of proteins can be confirmed qualitatively by usign several tests, Xanthoproteic test is one of them. Due to the presence of characteristic side chains in them, certain amino acids exhibit typical color reactions that form the basis for their identification.
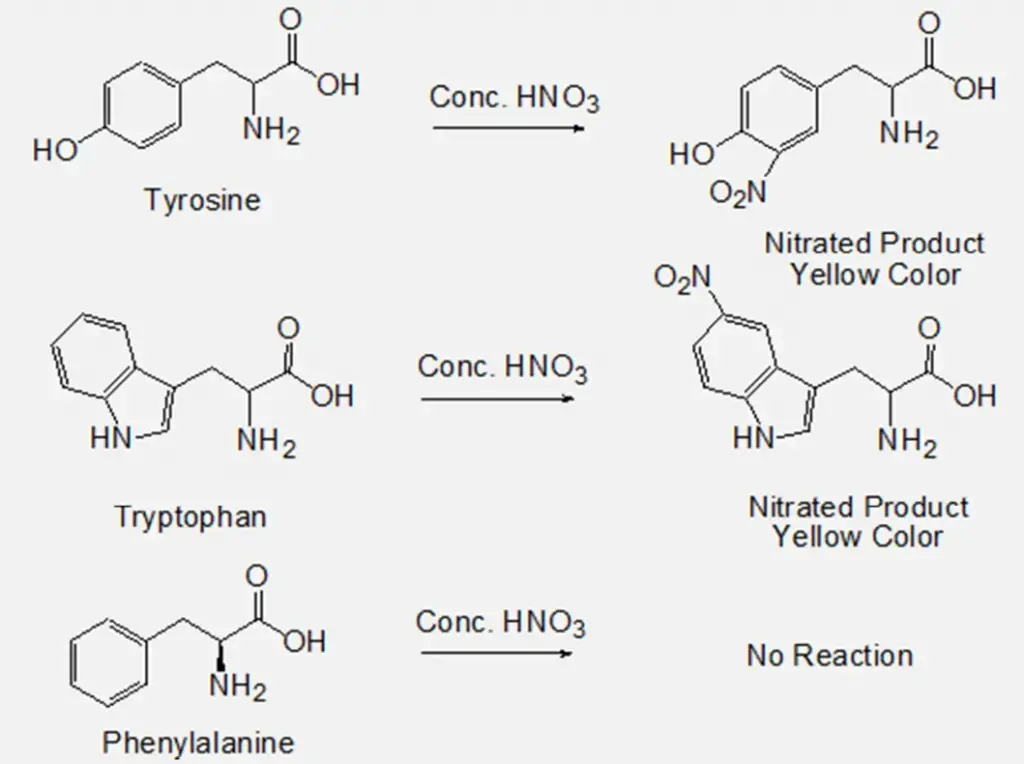
Xanthoproteic test is a biochemical method, which is used for the identification of amino acids containing phenolic or indolic groups like phenylalanine, tyrosine, and tryptophan (aromatic amino acids). The end product of this reaction is yellow in colour and the name comes from Greek word ‘Xanthos’ which means yellow.
This test is also referred to as the Yellow Protein Test. The benzene rings containing amino acids or other aromatic groups also give a positive result in Xanthoproteic test. It is a type of qualitative test which only shows the presence or absence of the amino acids.
Aim of Xanthoproteic Test
- To distinguish tyrosine and tryptophan from other amino acids.
- To detect the presence of aromatic groups-containing amino acids like tyrosine and tryptophan.
Principle of Xanthoproteic Test
The Xanthoproteic test utilizes a nitration reaction that identifies the presence of an activated benzene ring. Aromatic groups of either the free amino acid or protein undergo nitration on heating with concentrated nitric acid (HNO3) and forms yellow coloured product. The salts of these derivatives are orange in colour. On the addition of alkali, however, the residue turns orange due to the formation of a salt of the tautomeric form of the nitro compound.
Benzene ring-containing phenylalanine does not provide any positive result in this test because the phenyl group in phenylalanine is very stable, which doesn’t react with nitric acid in the conditions of this test. But, after an extended period of heating phenylalanine might give a positive result.
Reagent and Material Required
- Reagent: Concentrated Nitric acid, 40% NaOH, Test solution.
- Other Materials: Test tubes, Test tube stand, Pipettes.
Procedure of Xanthoproteic Test
- Take a clean test tube and add 1 ml of the sample solution within it.
- After that add 1 ml of concentrated nitric acid to it.
- Keep the test tube at room temperature and allow it to cool down.
- If the test sample is a protein solution then a white precipitate of the denatured proteins will be developed.
- After that add 1 ml of 40% NaOH solution to the test tube.
- Observed for color change.
Result of Xanthoproteic Test
- Positive Test: Indicated by the appearance of a dark yellow or orange-colored solution. It confirms the presence of aromatic groups in the proteins and amino acids.
- Negative result: Indicated by the appearance of a dark yellow or orange-colored solution. It confirms the absence of aromatic groups in proteins and amino acids.

Application of Xanthoproteic Test
- This test is used to detect proteins and amino acids.
- Xanthoproteic Test is used for differentiation of aromatic amino acids from non-aromatic amino acids.
Limitations
- Phenylalanine doesn’t give a positive Xanthoproteic test, though it is an aromatic group-containing amino acid. Because Phenylalanine contains a highly stable phenyl group.
Registration
- https://www.onlinebiologynotes.com/xanthoproteic-test-objective-principle-reagents-procedure-and-result/
- https://www.mlsu.ac.in/econtents/1848_Xanthoproteic-test.pdf
- https://microbenotes.com/xanthoproteic-test/
- https://en.wikipedia.org/wiki/Xanthoproteic_reaction
- http://biocheminfo.com/2020/04/04/xanthoproteic-test-principle-reaction-reagents-procedure-and-result-interpretation/
- https://himedialabs.com/td/htbc004.pdf
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.