The X and V factor test is a laboratory diagnostic test which is used for the identification and differentiation of bacteria belonging to the genus Haemophilus. It is based on the nutritional requirement of these organisms for certain growth factors which they are unable to synthesize on their own. It is the process by which the requirement of Factor X and Factor V by the organism is determined, and this test is mainly applied in routine bacteriological diagnosis.
Factor X is hemin, which is a heat stable substance, and it is required for the synthesis of cytochromes and other heme containing enzymes. Factor V is nicotinamide adenine dinucleotide (NAD), which is a heat labile coenzyme and is essential for various oxidation reduction reactions. These fastidious organisms cannot grow on ordinary media unless these growth factors is supplied externally. This nutritional dependence forms the principle of the X and V factor test.
In this test, a nutrient deficient agar medium such as trypticase soy agar is used, which does not contain X and V factors. The test organism is inoculated on the surface of the agar, and paper discs impregnated with X factor, V factor, and both X and V factors (XV) are placed on the inoculated plate. After incubation, growth occurs only around those discs which provide the required growth factors. This is referred to as satellitism or factor dependency.
The interpretation of the test is done by observing the pattern of growth around the discs. Haemophilus influenzae requires both X and V factors, hence growth is seen only around the XV disc. Haemophilus parainfluenzae requires only V factor and shows growth around V and XV discs, while species requiring only X factor will grow around the X and XV discs. Thus, the X and V factor test is a simple and reliable method for differentiation of Haemophilus species in the laboratory.
Objectives
- To determine the requirement of X factor (hemin) and V factor (nicotinamide adenine dinucleotide) for the growth of organism.
- To identify Haemophilus species on the basis of their dependency on X factor, V factor or both.
- To differentiate various species of Haemophilus such as H. influenzae, H. parainfluenzae and H. ducreyi.
- To differentiate Haemophilus species from Aggregatibacter aphrophilus.
- To assist in identification of Bordetella species based on their growth factor requirement on basal medium.
Principle of X and V factor Test
The principle of X and V factor test is based on the inability of Haemophilus species to synthesize certain essential growth factors required for their normal metabolism. It is the process by which the requirement of Factor X (hemin) and Factor V (nicotinamide adenine dinucleotide) by the organism is determined. These organisms lack the specific enzymes for synthesis of these factors, therefore they must obtain them from the external environment for respiration and redox reactions to occur.
In this test, the organism is inoculated on a basal medium which is deficient of X and V factors, such as trypticase soy agar. Discs containing Factor X, Factor V and a combination of both (XV) are placed on the surface of the medium. As these factors diffuse into the agar, growth occurs only in the area where the required factor is supplied. This differential growth pattern is used for identification of species, for example Haemophilus influenzae grows only around the XV disc as it requires both factors, whereas Haemophilus parainfluenzae grows around V and XV discs as it requires only Factor V.
Requirements
Culture media
- Factor deficient agar medium such as trypticase soy agar, Mueller Hinton agar, brain heart infusion agar or nutrient agar.
- Blood agar or chocolate agar is not used as these media already contains X and V factors.
Reagents (discs)
- X factor disc containing hemin.
- V factor disc containing nicotinamide adenine dinucleotide (NAD).
- XV factor disc containing both hemin and NAD.
Inoculum preparation materials
- Sterile physiological saline or distilled water or suitable broth for preparing bacterial suspension.
- 0.5 McFarland turbidity standard for adjusting inoculum density.
- Sterile cotton or polyester swabs for lawn culture on agar surface.
Laboratory equipment
- Sterile forceps or tweezers for placing factor discs on the medium.
- Inoculating loop for picking and mixing colonies.
Incubation conditions
- Incubator maintained at 35–37°C.
- CO₂ enriched atmosphere (5–10%) using CO₂ incubator or candle jar.
Control organisms
- Haemophilus influenzae (requires both X and V factors).
- Haemophilus parainfluenzae (requires V factor only).
- Haemophilus ducreyi or Aggregatibacter aphrophilus as control for X factor or negative growth.
Procedure of X and V factor Test

- Select well isolated colonies from a fresh pure culture (18–24 hours old) and prepare a suspension in sterile saline or suitable broth. The turbidity is adjusted to 0.5 McFarland standard and care is taken to avoid transfer of enriched media.
- A sterile swab is dipped into the suspension and excess fluid is removed. The suspension is streaked evenly on a factor deficient agar plate to obtain a confluent lawn culture.
- The inoculated plate is allowed to dry at room temperature for about 3–5 minutes.
- Using sterile forceps, X factor disc, V factor disc and XV factor disc are placed on the agar surface at proper distance from each other to avoid overlapping diffusion.
- The plates are incubated in inverted position at 35–37°C for 18–48 hours in presence of 5–10% CO₂.
- After incubation, the plate is observed for growth around the discs.
- Growth only around XV disc indicates requirement of both factors as seen in Haemophilus influenzae.
- Growth around V and XV disc indicates requirement of V factor as in Haemophilus parainfluenzae.
- Growth around X and XV disc indicates requirement of X factor as in Haemophilus ducreyi.
- Growth over entire plate indicates no requirement of X or V factor as seen in Aggregatibacter aphrophilus.
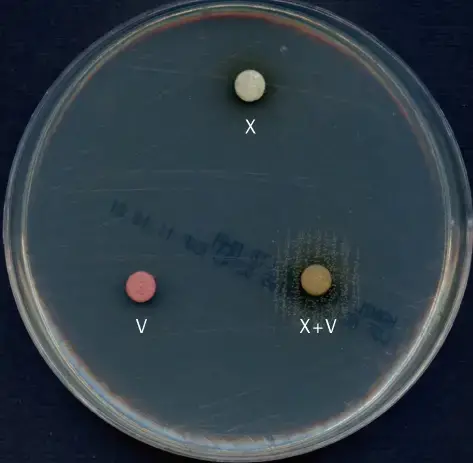
Expected Results of X and V factor Test
- Requirement of both X and V factors
- Growth is seen only around the XV disc.
- No growth is observed around X or V disc alone.
- This result indicates organisms such as Haemophilus influenzae, Haemophilus haemolyticus and Haemophilus aegyptius.
- Requirement of V factor only
- Growth is observed around V disc and XV disc.
- No growth occurs around X disc.
- This pattern is shown by Haemophilus parainfluenzae, Haemophilus parahaemolyticus and Haemophilus paraphrohaemolyticus.
- Requirement of X factor only
- Growth is observed around X disc and XV disc.
- No growth is seen around V disc.
- This result is characteristic of Haemophilus ducreyi and Haemophilus haemoglobinophilus.
- No requirement of X and V factors
- Growth is observed around all discs or over entire surface of agar plate.
- This indicates organisms independent of X and V factors such as Aggregatibacter aphrophilus.
![X and V factor Test - Principle, Purpose, Procedure, Result 4 X (hemin) and V (nicotinamide adenine dinucleotide [NAD]) factor test. A, Positive: growth
around XV disk only. B, Positive: growth around V disk.](https://biologynotesonline.com/wp-content/uploads/2024/05/image-78.png)
around XV disk only. B, Positive: growth around V disk.

| Organism | X Factor | V Factor |
| Haemophilus influenzae | + | + |
| H. influenzae biotype aegyptius | + | + |
| H. haemolyticus | + | + |
| H. parahaemolyticus | – | + |
| H. parainfluenzae | – | + |
| H. paraphrophilus | – | + |
| H. segnis | – | + |
| H. ducreyi | + | – |
| H. aphrophilus | – | – |
H. influenzae will grow only around the XV disk (i.e., the disk containing both X and V factors).
| Growth Around | Inference | |||
| X disk | V disk | XV disk | Entire Agar surface | |
| No | No | Yes | No | Requirement for both X and V factor |
| No | Yes | Yes | No | Requirement for V factor |
| Yes | Yes | Yes | Yes | No requirement for either X or V factor |
Quality Control
Organisms requiring both X and V factors (growth around XV disc only)
- Haemophilus influenzae ATCC 10211
- Haemophilus influenzae ATCC 35056
- Haemophilus influenzae NCTC 11931 or NCTC 12975
- Haemophilus influenzae ATCC 19418, 49247 or 49766
Organisms requiring V factor only (growth around V and XV discs)
- Haemophilus parainfluenzae ATCC 7901
- Haemophilus parainfluenzae NCTC 10665
- Haemophilus parahaemolyticus ATCC 10014
Organisms requiring X factor only (growth around X and XV discs)
- Haemophilus haemoglobinophilus NCTC 8540
- Haemophilus ducreyi ATCC 33940
Organisms requiring neither X nor V factors (growth around all discs)
- Aggregatibacter aphrophilus ATCC 33389

Uses
- To differentiate Haemophilus species based on their requirement of X factor, V factor or both.
- To identify Haemophilus influenzae which requires both X and V factors for growth.
- To identify Haemophilus parainfluenzae which requires only V factor.
- To identify Haemophilus ducreyi which requires only X factor.
- To differentiate Haemophilus species from Aggregatibacter aphrophilus which does not require X and V factors.
- To aid in identification of Bordetella species based on growth factor requirement.
- To differentiate Haemophilus species from other fastidious organisms such as Abiotrophia and Granulicatella.
- To assist in diagnosis of Eikenella corrodens showing X factor dependency.
Advantages
- It helps in differentiation of Haemophilus species on the basis of requirement of X factor, V factor or both.
- It is useful for presumptive identification of Haemophilus species isolated from clinical specimens.
- It helps to differentiate Haemophilus influenzae from Haemophilus parainfluenzae and Haemophilus ducreyi.
- It differentiates Haemophilus species from Aggregatibacter aphrophilus and Bordetella species.
- It is a simple, economical and easily performed laboratory test.
- It provides useful phenotypic information which helps in determining clinical significance of the isolate.
Limitations
- Transfer of inoculum from enriched media such as chocolate agar may carry X factor and give false result regarding factor requirement.
- Some basal media may contain trace amount of X factor which can support growth of X dependent strains and cause misidentification.
- V factor diffuses rapidly in agar and if discs are placed close, false growth may be seen around X disc.
- Incubation in CO₂ enriched atmosphere may sometimes produce false V dependent reaction.
- This test cannot differentiate Haemophilus influenzae from Haemophilus aegyptius as both shows similar factor requirement.
- Haemophilus haemolyticus also requires both X and V factors and may be mistaken for H. influenzae if hemolysis is not checked.
- Presence of contaminating organism may act as feeder and supply V factor leading to satellite growth.
- Other organisms such as Eikenella corrodens or Pasteurella species may show similar growth pattern and the test alone is not confirmatory.
- [Acinetobacter: HAEMOPHILUS]. (n.d.). [Source excerpt].
- Alfa, M. (2005). The laboratory diagnosis of Haemophilus ducreyi. Canadian Journal of Infectious Diseases and Medical Microbiology, 16(1), 31–34. https://doi.org/10.1155/2005/851610
- Association of Public Health Laboratories. (2020). Standard operating procedures for identification and characterization of Neisseria meningitidis, Streptococcus pneumoniae and Haemophilus influenzae.
- Carroll, K. C., Hobden, J. A., Miller, S., Morse, S. A., Mietzner, T. A., Detrick, B., Mitchell, T. G., McKerrow, J. H., & Sakanari, J. A. (2019). Haemophilus, Bordetella, Brucella, and Francisella. In Jawetz, Melnick, & Adelberg’s Medical Microbiology (27th ed.). McGraw-Hill Education.
- Centers for Disease Control and Prevention & World Health Organization. (2003). Manual for the laboratory identification and antimicrobial susceptibility testing of bacterial pathogens of public health importance in the developing world (WHO/CDS/CSR/EPH/2002.15). World Health Organization.
- Choby, J. E., & Skaar, E. P. (2016). Heme synthesis and acquisition in bacterial pathogens. Journal of Molecular Biology, 428(17), 3408–3428. https://doi.org/10.1016/j.jmb.2016.03.018
- Dahal, P. (2023, October 3). Satellitism test: Principle, media, procedure, results, uses. Microbe Notes. https://microbenotes.com/satellitism-test/
- Dalynn Biologicals. (2014). Porphyrin (ALA substrate) [Catalogue No. TP83].
- DrChika. (2023, January 2). Satellitism test. Microbiology Class. https://microbiologyclass.net/satellitism-test/
- Hardy Diagnostics. (2020). X- and V-factor disks [Instructions for use].
- HiMedia Laboratories. (2025). X+V factor [Technical data].
- Microbe Canvas. (n.d.). Satellitism test_Haemophilus influenzae. https://microbe-canvas.com/tests/tests/satelliet-groei.html
- Microbiology in pictures. (2015). Haemophilus influenzae growth on blood agar. Satelliting of H. influenzae on blood agar around Staphylococcus aureus colonies. https://www.microbiologyinpictures.com/bacteria-photos/haemophilus-influenzae-images/haemophilus-influenzae-on-blood-agar.html
- Micromaster Laboratories. (n.d.). Differentiation discs: X factor / V factor / X + V factor discs [Product specification sheet].
- Musher, D. M. (1996). Haemophilus species. In S. Baron (Ed.), Medical Microbiology (4th ed.). University of Texas Medical Branch at Galveston. https://www.ncbi.nlm.nih.gov/books/NBK8458/
- Nørskov-Lauritsen, N. (2014). Classification, identification, and clinical significance of Haemophilus and Aggregatibacter species with host specificity for humans. Clinical Microbiology Reviews, 27(2), 214–240. https://doi.org/10.1128/CMR.00103-13
- [Pathophysiological basis and methodological standards for the identification of Haemophilus species via X and V growth factor requirements]. (n.d.). [Source excerpt].
- Saeed, E. A. (n.d.). Genus: Haemophillus Morphology [Lecture notes]. Department of Clinical Laboratory Sciences.
- Schlör, S., Herbert, M., Rodenburg, M., Blass, J., & Reidl, J. (2000). Characterization of ferrochelatase (hemH) mutations in Haemophilus influenzae. Infection and Immunity, 68(5), 3007–3009.
- Smith, K. (2019, February 11). Who are the HACEK organisms? American Society for Microbiology. https://asm.org/articles/2019/february/who-are-the-hacek-organisms
- Tankeshwar, A. (n.d.). X and V factor test for Haemophilus. Microbe Online. https://microbeonline.com/x-v-factor-test-haemophilus-principle-procedure-results/
- Thermo Fisher Scientific. (2009). Haemophilus identification test kit [Instructions for use].
- Thermo Fisher Scientific. (2011). Haemophilus test medium (HTM) [Instructions for use].
- Thermo Fisher Scientific. (2011). Porphyrin test agar [Instructions for use].
- UK Health Security Agency. (2025). UK standards for microbiology investigations: Porphyrin synthesis (ALA) test (TP 29, Issue 4.1). Royal College of Pathologists.
- UK Health Security Agency. (2025). UK standards for microbiology investigations: X and V factor test (TP 38, Issue 4.2). Royal College of Pathologists.
- VetBact. (2024). X and V factor test. https://www.vetbact.org/?displayextinfo=119
- White, D. C., & Granick, S. (1963). Hemin biosynthesis in Haemophilus. Journal of Bacteriology, 85(4), 842–850. https://doi.org/10.1128/jb.85.4.842-850.1963