What is Voges Proskauer (VP) Test?
- The Voges-Proskauer (VP) Test, originating from the foundational work of Voges and Proskauer in 1898, serves as a pivotal biochemical assay in microbiology, particularly in discerning the metabolic pathways utilized by bacteria during glucose fermentation.
- This test is integral to the IMViC test series, which is employed to differentiate and identify Gram-negative bacteria, with a specific emphasis on the Enterobacterales order.
- The VP Test is meticulously designed to detect the capability of bacteria to metabolize pyruvate, a product of glycolysis, into a neutral intermediate product known as acetylmethylcarbinol or acetoin.
- The metabolic divergence in bacterial species, specifically in their approach to glucose fermentation, is underscored by their choice between two primary pathways: the mixed acid fermentation pathway, which converts pyruvate into a mixture of stable organic acids, and the butylene glycol pathway, which leads to the production of acetoin and butanediol.
- In the historical context, the VP Test has undergone several modifications since its inception. The initial methodology, which involved the addition of KOH to cultures grown in glucose peptone media, was characterized by the emergence of a red fluorescent color in some organisms, indicative of acetoin production.
- However, the exact nature of this coloration and the chemical reactions leading to it were not fully understood at the time. Subsequent research by Arthur Harden and further modifications by researchers like O’Meara and Barritt have refined the test, enhancing its sensitivity and reliability.
- Barritt’s 1936 modification, which introduced the use of α-naphthol, is widely recognized as the standard procedure today, utilized to detect acetoin as a metabolic intermediate in the fermentation of glucose via the butanediol pathway.
- The VP Test employs a specific medium, commonly referred to as MRVP broth, and utilizes two reagents, KOH and α-naphthol, to facilitate the detection of acetoin. The test is executed by inoculating the organism into the MRVP broth, which is then subjected to incubation.
- Following incubation, the reagents are added, and the presence of a red or pink color indicates a positive result, signifying the presence of acetoin and, by extension, the utilization of the butanediol pathway by the tested organism.
- In the broader context of microbial classification, the VP Test, especially when used in tandem with other biochemical tests such as the Methyl Red (MR) Test, provides a nuanced understanding of the metabolic strategies employed by different bacterial species.
- It is noteworthy that some bacteria, such as Escherichia coli, are typically MR positive and VP negative, indicating a propensity for mixed acid fermentation, while others, such as members of the Enterobacter-Klebsiella group, tend to be MR negative and VP positive, signifying a preference for the butanediol pathway.
- In conclusion, the Voges-Proskauer Test stands as a testament to the evolution of microbiological methodologies, bridging the historical explorations of bacterial metabolism with contemporary practices in bacterial identification and classification. The test not only provides insights into the metabolic predilections of bacteria but also serves as a crucial tool in the taxonomic differentiation of bacterial species within the expansive realm of microbiology.
Definition of Voges Proskauer (VP) Test
The Voges-Proskauer (VP) Test is a biochemical assay used to detect the ability of certain bacteria to metabolize glucose into a neutral intermediate product called acetoin during fermentation. Positive results are indicated by the development of a red or pink color after the addition of specific reagents. This test aids in the differentiation and identification of Gram-negative bacteria, particularly within the Enterobacterales order.
Principle of Voges Proskauer (VP) Test
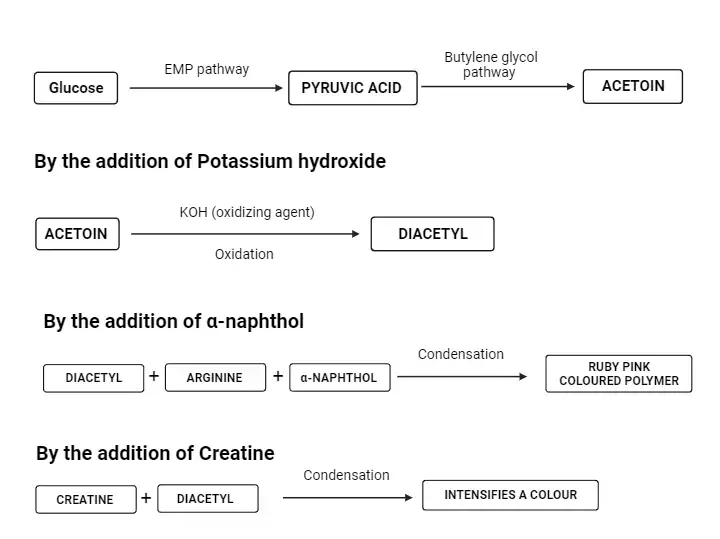
The Voges-Proskauer (VP) Test is a biochemical procedure employed to ascertain the metabolic capabilities of specific microorganisms in fermenting glucose to produce neutral end products, predominantly acetoin. This test is an integral component of the IMViC series, a suite of tests designed for the differentiation of Enterobacteriaceae based on their enzymatic reactions and biochemical properties.
At the core of the VP test is the detection of acetoin, an intermediate produced when bacteria ferment sugars via the butanediol pathway. The chemical equation representing this metabolic pathway is as follows:
2 pyruvate→acetoin+2CO2
acetoin+NADH+H+→2,3−butanediol+NAD+
In the presence of potassium hydroxide (KOH), acetoin undergoes oxidation to form diacetyl. This reaction is catalyzed by α-naphthol. Subsequently, diacetyl interacts with the guanidine group, which is derived from the peptone present in the medium, resulting in the formation of a pinkish-red compound. The α-naphthol, as utilized in Barritt’s modification of the VP test, acts as a color enhancer, intensifying the visual output of the reaction.
The VP test employs Barritt’s reagent, a concoction of alcoholic α-naphthol and a 40% solution of potassium hydroxide. The detection of acetoin mandates its oxidation to diacetyl, a process facilitated by the α-naphthol catalyst and a guanidine group intrinsic to the peptone in the MR-VP medium. The culmination of this reaction is the emergence of a pink complex, rendering a rose hue to the medium. A pronounced rose coloration within 15 minutes post the addition of Barritt’s reagent signifies a positive result, indicating the presence of acetoin. Conversely, the lack of such a coloration denotes a negative outcome.
In essence, the Voges-Proskauer test serves as a pivotal tool in clinical laboratories, offering a means to categorize strains of Enterobacteriaceae based on their acetoin production capabilities. This test, in conjunction with others in the IMViC series, provides a comprehensive understanding of the metabolic pathways and fermentation capabilities of various bacterial strains.
Requirements for VP Test
- Culture Media:
- MR-VP Broth (Glucose Phosphate Broth): This broth is specifically designed for the VP test.
- Composition (per 1000 ml):
- Buffered Peptone: 7.00 grams
- Dextrose (Glucose): 5.00 grams
- Dipotassium Phosphate: 5.00 grams
- Final pH: 6.9±0.2 at 25°C
- Preparation of MR-VP Broth:
- Take the designated quantity of MR-VP broth media powder (17.0 grams for 1000 mL) and dissolve it in the requisite volume of water within a conical flask or glass bottle.
- Ensure thorough mixing using a magnetic stirrer or manual stirring. Apply heat if necessary to facilitate complete dissolution.
- Dispense approximately 5 to 10 mL of the prepared broth into sterile test tubes, sealing them with a cap or cotton plug.
- Sterilize the test tubes using an autoclave at 121°C under 15 lbs pressure for a duration of 15 minutes. Allow the tubes to cool to a temperature range of 40 – 45°C prior to inoculation.
- Composition (per 1000 ml):
- MR-VP Broth (Glucose Phosphate Broth): This broth is specifically designed for the VP test.
- Reagents:
- 5% Alpha-naphthol Solution (Barritt’s Reagent A):
- Preparation: Dissolve 5 grams of α-naphthol in 100 mL of 95% ethanol. Store the resultant reagent in a dark environment at temperatures between 4 to 8°C for a maximum duration of 3 weeks.
- 40% KOH or NaOH Solution (Barritt’s Reagent B):
- Preparation: Dissolve 40 grams of KOH pellets in 100 mL of sterile distilled water. This reagent can be preserved for up to 3 weeks at temperatures ranging from 4 to 8°C.
- 5% Alpha-naphthol Solution (Barritt’s Reagent A):
- Equipment:
- Test tubes
- Incubator
- Dropper
- Autoclave
- Bunsen burner
- Weighing machine
- Inoculating loop
- Test Organism: The bacterial sample under investigation.
- Control Organisms:
- Escherichia coli ATCC 25922
- Klebsiella pneumoniae ATCC 13883
In summary, the Voges-Proskauer test necessitates a specific culture medium, designated reagents, essential laboratory equipment, a test organism, and control organisms to ensure accurate and reliable results. The meticulous preparation and adherence to the outlined requirements are paramount for the successful execution of the test in a scientific setting.
Quality Control of Voges Proskauer Test
Ensuring the accuracy and reliability of the Voges Proskauer (VP) Test necessitates rigorous quality control measures. These measures are paramount to validate the efficacy of the test and to ensure that the results obtained are both consistent and reproducible. Here are the essential steps and considerations for quality control in the VP test:
- Media Inspection:
- Prior to utilization, the broth must be meticulously inspected for any signs of contamination.
- It is imperative to check for any indications of dehydration or degradation. Such anomalies can compromise the integrity of the test and lead to erroneous results.
- Quality Control Testing:
- Every new batch of media or reagent should undergo quality control testing. This is to ensure that the batch meets the requisite standards and is free from any defects or inconsistencies.
- The testing should involve the use of control organisms. Specifically, one organism that is known to yield a positive VP reaction and another that is known to yield a negative VP reaction. This dual-testing approach ensures that the media or reagent can reliably differentiate between positive and negative results.
- Control Organisms:
- Klebsiella pneumoniae ATCC 13883: This organism is known to produce a positive VP reaction, resulting in a red coloration. It serves as the positive control in the quality control process.
- Escherichia coli ATCC 25922: This organism does not produce a positive VP reaction and thus shows no color change. It is used as the negative control to validate that the media or reagent can accurately identify organisms that do not produce acetoin.
In conclusion, the quality control process for the Voges Proskauer Test is a systematic and rigorous procedure designed to uphold the highest standards of scientific accuracy. By employing both positive and negative control organisms and by regularly inspecting and testing new batches of media and reagents, laboratories can ensure that the VP test remains a reliable tool for differentiating bacterial metabolic capabilities.
Procedure of of Voges Proskauer (VP) Test
The Voges Proskauer (VP) Test is a biochemical assay employed to detect the presence of acetoin, an intermediate in the butanediol fermentation pathway. The procedure is meticulous and requires adherence to specific steps to ensure accurate results. Here is a detailed breakdown of the procedure:
- Inoculation:
- Begin by sterilizing an inoculating loop. Once sterilized, use it to pick well-isolated colonies from a culture that is 18 to 24 hours old.
- Inoculate the MR-VP broth with the selected colonies.
- Incubation:
- Place the inoculated tubes in an incubator set at 35±2°C.
- Allow the tubes to incubate aerobically for a duration of 18 to 24 hours.
- Preparation for Testing:
- After the incubation period, transfer 2 mL of the broth from the incubated tube to a clean test tube. Ensure sterility during this transfer to prevent contamination.
- Reagent Addition:
- To the test tube containing the broth, add 6 drops of Reagent A, which is a 5% α-naphthol solution. Mix the contents thoroughly by shaking the tube.
- Subsequently, introduce 2 drops of Reagent B, a 40% KOH solution, to the tube. Again, ensure proper mixing by shaking the tube.
- Observation:
- Monitor the test tube for any color changes. Specifically, look for the emergence of a red-pink hue at the surface of the medium. This observation should be made within a 30-minute window.
- During this period, it’s crucial to shake the tube vigorously at regular intervals to facilitate the reaction.
- Further Incubation (if required):
- If no color change is observed, indicating a negative reaction, the broth should be re-incubated for an additional 24 hours.
- After this extended incubation, the test should be repeated to ascertain the result.
In summary, the Voges Proskauer Test is a systematic procedure that hinges on the accurate addition of reagents and careful observation of color changes. Adherence to the outlined steps ensures the reliable detection of acetoin, aiding in the differentiation of bacterial species based on their metabolic pathways.
Results and Interpretation of Voges Proskauer (VP) Test

The Voges Proskauer (VP) Test is a diagnostic tool employed to discern bacteria based on their metabolic products. The test specifically detects the presence of acetoin, an intermediate compound in certain fermentation pathways. The results of the VP Test can be interpreted as follows:
- Positive Result:
- Indication: The appearance of a ruby pink coloration at the surface of the medium signifies a positive VP test.
- Interpretation: The presence of this pink-red hue indicates that the bacterium has metabolized glucose to produce acetoin.
- Examples of Organisms: Bacteria that typically yield a positive result include Serratia marcescens, Vibrio eltor, Listeria species, and Enterobacter species.
- Negative Result:
- Indication: A negative VP test is denoted by the absence of the pink-red coloration at the medium’s surface. In some instances, a copper color may manifest, which also indicates a negative result.
- Interpretation: The lack of color change suggests that the bacterium does not produce acetoin from glucose metabolism.
- Examples of Organisms: Organisms that typically yield a negative result encompass Streptococcus mitis, Salmonella species, Citrobacter species, Yersinia species, and Shigella species.

Voges Proskauer (VP) Test Results of Some Common Bacteria
The Voges-Proskauer (VP) test is a biochemical assay employed in microbiology to differentiate bacterial species based on their ability to produce acetoin from glucose fermentation. The presence or absence of a pink-red color after the addition of specific reagents indicates a positive or negative VP reaction, respectively. This test is instrumental in distinguishing between various bacterial species, especially within the Enterobacteriaceae family.
VP Positive Bacteria:
- Klebsiella spp.: A genus of non-motile, rod-shaped bacteria commonly found in the environment and the human gut.
- Enterobacter spp.: Ubiquitous bacteria that inhabit various environments, including the human intestinal tract.
- Viridans Streptococci: A group of streptococci, with exceptions like S. mitis and S. vestibularis, which do not produce a positive VP reaction.
- Proteus mirabilis: A motile bacterium known for its swarming behavior on agar plates.
- Hafnia spp.: Less commonly known bacteria that can be isolated from various sources, including food and clinical specimens.
- Serratia spp.: Recognized for some species’ ability to produce a red pigment.
- Staphylococcus aureus: A gram-positive bacterium commonly found on the skin and nasal passages of humans.
- Listeria spp.: Rod-shaped bacteria that can cause a severe foodborne illness called listeriosis.
- V. cholerae: The causative agent of cholera, a severe diarrheal disease.
VP Negative Bacteria:
- Escherichia spp.: E. coli is the most well-known species, commonly found in the intestines of warm-blooded animals.
- Proteus vulgaris: Another species of the Proteus genus, distinct from P. mirabilis.
- Citrobacter freundii: A bacterium that can be found in the environment and the intestines of humans and animals.
- Morganella morganii: Known to cause urinary tract infections and other opportunistic infections.
- Shigella spp.: Causative agents of shigellosis, a form of dysentery.
- Yersinia spp.: Some species, like Y. pestis, are responsible for severe diseases like the plague.
- V. parahaemolyticus: A bacterium that can cause gastrointestinal illness, especially after consuming undercooked seafood.

Voges-Proskauer (VP) Positive Organisms of Enterobacteriaceae family are
The Enterobacteriaceae family comprises a diverse group of gram-negative bacteria, many of which inhabit the gastrointestinal tract of humans and animals. Among these bacteria, certain species exhibit a positive Voges-Proskauer (VP) reaction, indicating their ability to ferment sugars to produce acetoin, a specific metabolic end product. The VP test is a valuable diagnostic tool that aids in the differentiation and identification of these bacteria based on their metabolic profiles.
Within the Enterobacteriaceae family, the following organisms are known to yield a positive VP reaction:
- Klebsiella species: These are rod-shaped, non-motile bacteria that are commonly found in the environment and can also inhabit the human gut. They are known to cause various infections, including pneumonia, urinary tract infections, and bloodstream infections.
- Enterobacter species: These bacteria are commonly found in the human intestinal tract and are also present in various environmental sources. Some species, like Enterobacter aerogenes, are opportunistic pathogens and can cause infections in immunocompromised individuals.
- Hafnia species: Hafnia is a lesser-known genus within the Enterobacteriaceae family. Hafnia alvei is the primary species and has been isolated from various sources, including food, water, and clinical specimens. It can occasionally cause infections in humans.
- Serratia species: Serratia marcescens is the most well-known species within this genus. It is recognized for its ability to produce a red pigment. Serratia species can be found in various environments and are also opportunistic pathogens, causing infections like urinary tract infections, respiratory infections, and wound infections.
In summary, the Voges-Proskauer test serves as a critical tool in microbiological diagnostics, enabling the differentiation of various members of the Enterobacteriaceae family based on their metabolic characteristics. The aforementioned organisms are notable for their positive VP reaction, highlighting their unique metabolic pathways within this diverse bacterial family.
Precautions of Voges Proskauer (VP) Test
The Voges-Proskauer (VP) test is a crucial biochemical assay used in microbiology to discern bacterial species based on their metabolic capabilities. To ensure the accuracy and reliability of the VP test results, several precautions must be observed:
- Inoculum Size: It is imperative to regulate the size of the bacterial inoculum. Over-inoculation can hinder bacterial growth. Ideally, the bacterial concentration should not exceed 10^9 cells per mL of broth to ensure optimal growth and accurate test results.
- Reagent Freshness: Always employ freshly prepared reagents for the test. Stale or degraded reagents can compromise the test’s accuracy.
- Reagent Storage: If there’s a need to store reagents for future use, it’s essential to store them in a dark environment. Exposure to light can degrade certain chemicals, affecting their efficacy.
- Proper Mixing: Thorough mixing of the medium and reagents is crucial. Vigorous shaking ensures the proper dissolution of oxygen, which is vital for the test’s chemical reactions.
- Observation Time: After adding the reagents, it’s essential to wait for up to 30 minutes, shaking continuously, before declaring a negative result. Immediate observations might lead to premature and inaccurate conclusions.
- Avoid Over-Incubation: Incubating the test samples for an extended period, especially more than three days, can result in weak or false-negative reactions. It’s essential to adhere to the recommended incubation time to ensure accurate results.
- Reagent Quantity: Using an excessive amount of KOH can yield a weakly positive reaction. It’s crucial to use reagents in their recommended quantities to avoid skewed results.
In summary, meticulous adherence to these precautions ensures the reliability and accuracy of the VP test, making it a valuable tool in bacterial identification and differentiation in microbiological studies.
Applications of Voges Proskauer (VP) Test
The Voges-Proskauer (VP) Test is a biochemical assay that serves as a pivotal tool in the realm of microbiology. Its applications are rooted in its ability to detect specific metabolic reactions characteristic of certain bacterial groups. Here are the primary applications of the VP Test:
- Identification of Gram-Negative Bacteria: One of the primary applications of the VP Test is the identification of Gram-negative bacteria. This is especially pertinent for the Enterobacteriaceae family, a large and diverse group of Gram-negative bacteria that includes several pathogenic species. By determining the presence or absence of specific metabolic products, the VP Test aids in distinguishing between members of this family.
- Differentiation of Actinobacteria: Actinobacteria is a significant phylum of Gram-positive bacteria known for its diverse metabolic capabilities and its role in soil ecology. The VP Test assists in differentiating members of the Actinobacteria phylum based on their metabolic reactions.
Limitations of Voges Proskauer (VP) Test
The Voges-Proskauer (VP) Test, a biochemical assay employed for the differentiation of bacterial species based on their metabolic properties, has several inherent limitations:
- Non-Confirmatory Nature: The VP test alone is not definitive for bacterial identification. It requires the integration of results from other biochemical tests for a comprehensive and accurate identification.
- Delayed Positive Results: In some instances, a positive result may manifest between 30 minutes to 1 hour. Interpreting results after 1 hour can lead to false positive outcomes.
- Reagent Specificity: The order and quantity in which reagents are added are crucial. Deviations can result in weak-positive or false-negative reactions.
- Potential for False Positives: The reaction between KOH and α-naphthol can produce a copper-like hue, potentially leading to false positive interpretations if read after 1 hour.
- Acetoin Destruction: Some bacterial species can degrade acetoin, rendering the VP test results inconclusive.
- Necessity for Agitation: Vigorous shaking of the test tubes can enhance the VP reaction, making it a crucial step in the procedure.
- Prolonged Incubation Issues: Extended incubation beyond 3 days can alter the medium’s pH, leading to weak positive or false-negative reactions.
- Reagent Order: Reversing the order of reagent addition can compromise the test’s accuracy.
- Precipitate Formation: The potassium hydroxide reagent solution might form a precipitate, though this has not been proven to impact the reagent’s effectiveness.
- Concurrent Positive Results: Some bacteria, like Hafnia alvei and Proteus mirabilis, might yield positive results for both the MR and VP tests, complicating interpretations.
- Reading Timeframe: The VP test should be read at 48 hours. Extended incubation can affect result interpretation due to changes in the broth’s pH.
- Raw Material Interference: Certain raw materials might contain acetoin, diacetyl, or related compounds. Therefore, it’s recommended to use media with low concentrations of these substances, like MR-VP media, for the test.
Quiz
FAQ
What is the Voges-Proskauer (VP) Test?
The VP Test is a biochemical assay used to detect the presence of acetoin in bacterial cultures, aiding in the identification and differentiation of certain bacterial species.
Why is the VP Test important in microbiology?
The VP Test is crucial for distinguishing between bacteria that produce acetoin from those that do not, especially within the Enterobacteriaceae family and Actinobacteria phylum.
What indicates a positive VP Test result?
A pink-red color appearing on the surface of the medium indicates a positive VP Test result.
How is the VP Test different from the MR Test?
While both tests are used to differentiate bacteria based on fermentation reactions, the MR Test detects acid production, whereas the VP Test identifies acetoin production.
What are the main reagents used in the VP Test?
The primary reagents are 5% Alpha-naphthol Solution (Barritt’s Reagent A) and 40% KOH or NaOH solution (Barritt’s Reagent B).
Are there any limitations to the VP Test?
Yes, the VP Test is not a standalone confirmatory test. It requires the results of other biochemical tests for accurate bacterial identification. Additionally, factors like over-incubation or incorrect reagent amounts can influence the results.
Which bacteria are typically VP positive?
Bacteria such as Klebsiella spp., Enterobacter spp., and Serratia spp. are examples of VP positive organisms.
How long should the VP Test be read after adding the reagents?
The VP Test should be read within 1 hour of adding the reagents to avoid potential false-positive interpretations.
What precautions should be taken while performing the VP Test?
It’s essential to avoid over-inoculation, use freshly prepared reagents, mix the medium and reagent properly, and ensure the reagents are added in the specified order and amount.
Can the VP Test be used for Gram-positive bacteria?
While the primary application is for Gram-negative bacteria, especially within the Enterobacteriaceae family, it can also be used to differentiate certain Gram-positive bacteria like Actinobacteria.
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.