What is Vaccine?
- A vaccine is a medical agent that is created using antigens or pathogens, which are then modified to deactivate their protein synthesis mechanisms or rendered harmless through denaturation or killing. The purpose of a vaccine is to induce an immune response in the host and provide protection against specific diseases.
- The history of vaccines dates back to 1877 when Louis Pasteur developed the first vaccine using a weakened strain of the anthrax bacillus. He achieved this by attenuating the culture of the anthrax bacillus through incubation at high temperatures. Pasteur demonstrated the efficacy of the vaccine by inoculating animals with the attenuated strain, resulting in specific protection against anthrax. This breakthrough was further confirmed in 1881 when sheep, goats, and cows vaccinated with the attenuated strain survived an anthrax attack, while non-vaccinated animals succumbed to the disease.
- Louis Pasteur also played a crucial role in the development of the first vaccine against rabies in humans in 1885, which saved countless lives around the world. In honor of Edward Jenner, who pioneered the use of similar preparations for protection against smallpox, Pasteur coined the term “vaccine.” This marked the establishment of various institutions worldwide that focused on vaccine production and the study of infectious diseases, such as the Pasteur Institute in Paris.
- Vaccines are designed to provide immunity from specific diseases by utilizing different substances. These substances can range from dead microorganisms to genetically engineered antigens. The goal is to stimulate the immune system to develop antibodies that can effectively combat disease-causing microorganisms when they enter the body, thereby preventing the development of the disease.
- A vaccine may consist of live-attenuated or killed microorganisms, or their components or products, capable of eliciting a specific immune response. This response includes the production of protective antibodies and T cell immunity. To be effective, a vaccine must stimulate a sufficient number of memory T and B lymphocytes, which can differentiate into effector T cells and antibody-producing B cells when needed. In the case of viral vaccines, they should also induce the production of high titers of neutralizing antibodies.
- When a vaccine is injected into a nonimmune individual, it triggers an active immune response against the modified pathogens. This response leads to the development of active (protective) immunity, which helps the body fight off the actual disease if it is encountered in the future.
- Vaccination is the process of immunization against infectious diseases through the administration of vaccines. It aims to induce active immunity and provide protection against specific diseases in humans and animals.
- Overall, vaccines have played a crucial role in preventing the spread of diseases and saving lives. They are a cornerstone of public health efforts worldwide, and ongoing research and development continue to expand our understanding and effectiveness in combating infectious diseases.
Definition of Vaccines
Vaccines are medical preparations that stimulate the immune system to provide protection against specific diseases by promoting the production of antibodies and immune memory.
How do vaccines work in Immune System?
- Vaccines work by stimulating the immune system to recognize and respond to specific pathogens. They are typically composed of killed or weakened pathogens or parts of their surface antigens. When a vaccine is administered, the immune system identifies these antigens as foreign and initiates an immune response.
- The primary immune response is the body’s initial encounter with a pathogen, which can be slow and may result in the development of disease symptoms. However, vaccines bypass this initial response and instead trigger a secondary immune response. This response is faster, more robust, and creates a long-lasting memory of the pathogen.
- The vaccine essentially tricks the body into believing that it has been infected by the actual pathogen. As a result, the immune system produces antibodies to neutralize the antigens present in the vaccine. These immune effector antibodies are then stored as memory antibodies, ready to mount a rapid and effective response if the individual is exposed to the live pathogen in the future.
- Vaccination is a safe and common method to acquire immunity against diseases that the body has not yet encountered. By administering a vaccine containing specific antigens, the immune system can recognize and respond to those antigens, providing protection against the corresponding disease.
- Vaccination not only benefits the vaccinated individual but also plays a crucial role in achieving herd immunity. When a significant portion of the population is vaccinated and immune to a disease, it creates a barrier that prevents the spread of the disease. This protects both the vaccinated individuals and those who are unable to receive the vaccine, such as infants or individuals with compromised immune systems.
- Overall, vaccines stimulate the immune system to develop immunity against specific diseases, prevent the occurrence of those diseases, and contribute to the eradication of infectious diseases through herd immunity.
Types of Vaccines
Vaccines can be classified into three main groups based on their composition and mode of action.
- Whole-organism Vaccines:
- Inactivated (Killed) Vaccines: These vaccines contain pathogens that have been inactivated or killed using heat, chemicals, or radiation. Examples include the inactivated polio vaccine and the hepatitis A vaccine.
- Live-attenuated Vaccines: These vaccines contain weakened forms of the live pathogens. They can replicate in the body but do not cause disease. Examples include the measles, mumps, and rubella (MMR) vaccine and the yellow fever vaccine.
- Chimeric Vaccines: These vaccines are created by combining genetic material from different pathogens. They use the harmless parts of one pathogen to stimulate an immune response against another. An example is the RV1 and RV5 vaccines for rotavirus.
- Subunit Vaccines:
- Polysaccharide Vaccines: These vaccines contain purified polysaccharides from the pathogen’s surface. They are effective against diseases like pneumonia and meningitis caused by bacteria with polysaccharide capsules.
- Conjugated Vaccines: These vaccines combine polysaccharides with proteins. This linking enhances the immune response, especially in infants. The Haemophilus influenzae type B (Hib) vaccine and the pneumococcal conjugate vaccine are examples.
- Toxoid Vaccines: These vaccines use inactivated toxins produced by bacteria. They stimulate the immune system to produce antibodies against the toxins. The diphtheria and tetanus vaccines are toxoid vaccines.
- Recombinant Protein Vaccines: These vaccines use harmless proteins produced by genetic engineering techniques. The hepatitis B vaccine is a recombinant protein vaccine.
- Nanoparticle Vaccines: These vaccines use nanoparticles as carriers to deliver antigenic material to the immune system. They can enhance the immune response and target specific cells. Some COVID-19 vaccines, such as those based on mRNA technology, fall under this category.
- Nucleic Acid Vaccines:
- DNA Plasmid Vaccines: These vaccines use circular DNA molecules (plasmids) that contain genes encoding pathogen antigens. The DNA is taken up by cells, which produce the antigenic proteins, triggering an immune response. Currently, DNA vaccines are mostly under development.
- mRNA Vaccines: These vaccines use messenger RNA molecules that encode viral antigens. They instruct cells to produce the antigenic proteins, stimulating an immune response. The COVID-19 vaccines developed by Pfizer-BioNTech and Moderna are mRNA vaccines.
- Recombinant Vector Vaccines: These vaccines use harmless viruses or bacteria as vectors to deliver genes encoding pathogen antigens. The vectors infect cells and produce the antigenic proteins, inducing an immune response. The Ebola vaccine and the COVID-19 vaccine developed by Johnson & Johnson are examples of recombinant vector vaccines.
These different vaccine types are designed to mimic the pathogens and elicit an immune response without causing disease. Each type has its advantages and considerations, and the selection depends on factors such as the nature of the pathogen, target population, and desired immune response. Vaccines continue to be a critical tool in preventing infectious diseases and saving lives.
Whole-organism Vaccines
Numerous early-developed vaccines contain a pathogen that has been destroyed (inactivated) or weakened (attenuated) so that it cannot cause disease. They are referred to as whole-cell vaccines. Not all disease-causing microorganisms can be effectively targeted with a whole-organism vaccine, despite the fact that these vaccines elicit robust protective immune responses and are widely used today.
1. Inactivated (Killed) Vaccine
- Inactivated (killed) vaccines are a type of vaccine produced by rendering the pathogen (such as bacteria or virus) non-infectious through the use of chemicals, heat, or radiation. These methods kill the pathogen, making it unable to cause disease in the host. Unlike live-attenuated vaccines, inactivated vaccines do not replicate in the body.
- One advantage of inactivated vaccines is their stability. They can be stored and transported more easily than live vaccines, which require specific conditions to maintain their viability. Additionally, inactivated vaccines are generally considered safer because there is no risk of the pathogen regaining virulence or causing disease in individuals with weakened immune systems.
- However, one drawback of inactivated vaccines is that they typically elicit a weaker immune response compared to live-attenuated vaccines. As a result, multiple doses of the vaccine and sometimes booster doses are required to achieve and maintain protective immunity. This is because the immune response generated by inactivated vaccines may not be as robust or long-lasting as that generated by live vaccines.
- Examples of inactivated vaccines include the polio (salk vaccine), rabies, typhoid, cholera, pertussis, pneumococcal, hepatitis B, and influenza vaccines. These vaccines have been developed using inactivated forms of the respective pathogens, allowing the immune system to recognize and mount a response against them. Although inactivated vaccines may require more doses, they have played a crucial role in preventing the spread of various diseases and protecting individuals from harmful infections.
2. Live-attenuated vaccines
- Live-attenuated vaccines are a type of vaccine that is created by weakening the pathogenicity of a whole organism, such as a virus or bacterium. These vaccines were developed in the 1950s with the advancements in tissue culture techniques. The process of attenuation involves reducing the ability of the organism to cause disease while still allowing it to stimulate an immune response.
- One of the key advantages of live-attenuated vaccines is that they elicit strong immune responses. They closely resemble the actual disease-causing pathogen, which allows the immune system to mount a robust response. As a result, these vaccines can provide lifelong immunity with just one or two doses, making them highly effective.
- Live-attenuated vaccines are relatively easier to create for certain viruses, but more complex pathogens like bacteria and parasites pose greater challenges. It is important to note that there is a remote chance that the weakened organism in these vaccines could mutate or revert back to its full strength, potentially causing disease. Therefore, individuals with weakened or damaged immune systems should not receive live-attenuated vaccines.
- These vaccines require refrigeration and protection from light to maintain their potency. They must be stored and transported under specific conditions to ensure their effectiveness.
- Examples of live-attenuated vaccines include the Measles/Mumps/Rubella (MMR) vaccine, Influenza Vaccine Live (FluMist®), Polio (Sabin vaccine), Rotavirus, Tuberculosis, Varicella (chickenpox), and Yellow fever vaccines. The Bacillus Calmette-Guérin (BCG) vaccine, derived from the attenuated strain of Mycobacterium bovis, was developed as a vaccine for tuberculosis. It was created through a process of adaptation and attenuation in the presence of increasing concentrations of bile over 13 years.
- Live-attenuated vaccines have played a significant role in preventing diseases and providing long-lasting immunity. While they have certain limitations and precautions, their effectiveness and ability to confer robust immunity have made them important tools in vaccination programs worldwide.
3. Chimeric vaccine
- A chimeric vaccine is a type of vaccine that is created by combining genetic material from different viruses or pathogens. This is made possible through the advancements in genetic engineering techniques. Chimeric vaccines utilize a viral backbone from one virus and incorporate surface proteins or genetic components from another virus, resulting in a vaccine that displays the biological properties of both parent viruses.
- One example of a chimeric vaccine is the NIAID-developed live-attenuated chimeric vaccine that combines a dengue virus backbone with Zika virus surface proteins. This vaccine is designed to provide protection against both dengue and Zika viruses.
- Chimeric vaccines offer certain advantages over whole-organism vaccines. They can be tailored to target specific components or antigens of the pathogens, focusing the immune response on the most relevant parts for generating immunity. By incorporating specific genetic material, chimeric vaccines can induce a targeted immune response against particular pathogens or antigens.
- Moreover, chimeric vaccines address a drawback of whole-organism vaccines. Since whole-organism vaccines consist of complete pathogens, they may contain molecules that are not involved in evoking immunity. This includes contaminants or byproducts of the manufacturing process that can potentially trigger allergic or immune-disruptive reactions. Chimeric vaccines allow for a more precise selection and manipulation of components, reducing the presence of non-immunogenic or potentially harmful molecules.
- Chimeric vaccines hold promise for providing effective and targeted protection against multiple pathogens. Ongoing research and early-stage testing are aimed at further exploring and developing the potential of chimeric vaccines in preventing various infectious diseases.
Subunit Vaccines
- Subunit vaccines are a type of vaccine that is created using specific components or antigens of the pathogen. Unlike whole-organism vaccines, subunit vaccines do not contain the entire cell or organism but focus on selected parts that can stimulate the immune system to generate an appropriate immune response.
- Subunit vaccines are sometimes referred to as acellular vaccines because they lack a whole cell structure. Instead, they utilize specific antigens or components of the bacteria or virus that are essential for eliciting an immune response.
- These vaccines were developed to address the limitations and concerns associated with live attenuated and killed whole-organism vaccines. Whole-organism vaccines can sometimes cause adverse reactions in individuals, and there is a remote possibility of mutations leading to the reversion of the pathogen to its virulent form. Subunit vaccines offer a safer alternative as they contain only selected components, reducing the risk of adverse reactions and the potential for reversion.
- Subunit vaccines are considered safe and relatively easier to produce compared to whole-organism vaccines. However, they often require the use of adjuvants to enhance the immune response. Adjuvants are substances that are added to the vaccine formulation to stimulate a stronger and more durable immune response. This is because the antigen alone may not be sufficient to induce long-term immunity.
- A notable example of subunit vaccines is the pertussis vaccine. In the 1940s, an inactivated Bordetella pertussis bacteria preparation was used as a vaccine. However, this vaccine caused minor adverse reactions, such as fever and swelling at the injection site, leading to reduced vaccination rates and increased pertussis infections. Subsequently, acellular pertussis vaccines were developed, which were based on purified components of B. pertussis. These new vaccines had no associated adverse reactions and proved to be effective in preventing pertussis infections.
- Subunit vaccines represent an important advancement in vaccine development, providing a safer and targeted approach to immunization. Ongoing research continues to explore the potential of subunit vaccines in combating various infectious diseases.
Some of the subunit vaccines developed to prevent bacterial infections are based on the polysaccharides or carbohydrates that compose the outer coating of the majority of bacteria. Consequently, the following subtypes of subunit vaccines exist:
1. Polysaccharide Vaccine
- Polysaccharide vaccines are a type of vaccine that is specifically designed to target microbes that possess a polysaccharide capsule. This capsule serves as a protective barrier and allows the microbes to evade the immune system, particularly in infants and young children.
- These vaccines are developed using the sugar molecules, or polysaccharides, extracted from the outer layer of bacteria or viruses. The objective is to elicit an immune response against the molecules present in the pathogen’s capsule. However, since these molecules are typically small, they are not inherently immunogenic, meaning they cannot independently induce a robust immune response. As a result, polysaccharide vaccines are generally less effective in infants and young children, particularly those between 18-24 months of age.
- One of the limitations of polysaccharide vaccines is that they often lead to short-term immunity due to the slow immune responses and activation they induce. Additionally, these vaccines do not significantly increase antibody levels or create long-lasting immune memory.
- To enhance the effectiveness of polysaccharide vaccines, the sugar molecules are chemically linked to carrier proteins, which act as conjugate vaccines. This linkage between the polysaccharide and carrier protein helps to improve the immune response and generate a more robust and long-lasting immunity.
- Examples of polysaccharide vaccines include those targeting Meningococcal disease caused by Neisseria meningitidis groups A, C, W135, and Y, as well as Pneumococcal disease. These vaccines play a crucial role in protecting individuals against these specific pathogens and reducing the incidence of related infections.
- In summary, polysaccharide vaccines utilize the sugar molecules found in the outer layer of bacteria or viruses to trigger an immune response. While they may have limitations, such as reduced effectiveness in young children, they remain an important tool in preventing certain diseases caused by microbes with polysaccharide capsules. Ongoing research and advancements in vaccine technology continue to improve the efficacy and safety of polysaccharide vaccines.
2. Conjugated Vaccines
- Conjugated vaccines are a specific type of vaccine that addresses the challenge of immune recognition in infants and young children by linking the polysaccharides or sugar molecules found on the outer layer of bacteria to a carrier protein antigen or toxoid derived from the same microbe.
- The polysaccharide coating on bacteria disguises their antigens, making it difficult for the immature immune systems of infants and young children to recognize and mount an effective immune response against them. However, conjugate vaccines overcome this obstacle by conjugating or linking the polysaccharides with a protein component.
- By attaching the polysaccharides to a carrier protein, conjugate vaccines significantly enhance the immune response in young children. This formulation allows the immune systems of infants to recognize and respond effectively to the polysaccharides, leading to the development of protective immunity.
- An example of a conjugate vaccine is the vaccine used to protect against Haemophilus influenzae type B (Hib). By linking the polysaccharides from Hib to a carrier protein, the conjugate vaccine improves the recognition and response to the polysaccharide antigens, providing immunity against Hib infections.
- In addition to Hib, conjugate vaccines have been developed to protect against other infections caused by pathogens such as pneumococcus (Streptococcus pneumoniae) and meningococcus (Neisseria meningitidis). These vaccines have proven to be effective in preventing pneumococcal and meningococcal infections, reducing the associated morbidity and mortality.
- Conjugate vaccines represent a significant advancement in vaccine technology, particularly for protecting vulnerable populations, such as infants and young children, who may have difficulty mounting a sufficient immune response to polysaccharide antigens alone. Ongoing research and development continue to expand the application of conjugate vaccines, leading to improved protection against a range of infectious diseases.
3. Toxoid Vaccines
- Toxoid vaccines are a type of vaccine that is prepared from inactivated toxins. These toxins are treated with formalin, a solution of formaldehyde, and sterilized water to render them inactive while maintaining their antigenic properties. This process is known as detoxification, and the resulting inactivated toxin is referred to as a toxoid.
- Detoxification of the toxins is crucial in the development of toxoid vaccines as it ensures that the vaccines are safe for use. The toxins used in toxoid vaccines are obtained from bacteria that naturally secrete these toxins and are responsible for causing illness in individuals.
- When a person receives a toxoid vaccine, the harmless toxoid is introduced into their body. The immune system recognizes the presence of the toxoid as a foreign substance and mounts an immune response. The immune system produces specific antibodies that bind to and neutralize the toxin. This immune response effectively trains the immune system to recognize and combat the natural bacterial toxin if the person is later exposed to it, providing protection against the associated illness.
- Two prominent examples of toxoid vaccines are the diphtheria toxoid vaccine and the tetanus toxoid vaccine. The diphtheria toxoid vaccine provides immunity against Corynebacterium diphtheriae, the bacterium that causes diphtheria, while the tetanus toxoid vaccine protects against the toxin produced by Clostridium tetani, the bacterium responsible for tetanus.
- Toxoid vaccines have played a crucial role in preventing diseases caused by bacterial toxins. By stimulating the production of specific antibodies that neutralize the toxins, toxoid vaccines effectively confer immunity and protect individuals from the associated illnesses. These vaccines have been instrumental in reducing the incidence and severity of diseases such as diphtheria and tetanus.
4. Recombinant Protein Vaccines
- Recombinant protein vaccines are a type of vaccine that harnesses the advancements in recombinant DNA technology. This technology involves combining DNA from multiple sources to create vaccines that induce immunity against specific pathogens.
- In order for recombinant protein vaccines to stimulate an immune response, they are typically administered along with an adjuvant or expressed by a plasmid or harmless bacterial/viral vectors. The production process of these vaccines involves inserting DNA that encodes an antigen, such as a bacterial surface protein, into bacterial or mammalian cells. The cells then express the antigen, which is subsequently purified for use in the vaccine.
- One of the major advantages of recombinant protein vaccines is the ability to avoid potential concerns associated with vaccines based on purified macromolecules. By producing vaccines using recombinant technology, it is possible to eliminate contaminants that may be present in purified vaccines, thus enhancing safety. Additionally, this approach allows for the production of sufficient quantities of purified antigenic components, ensuring an adequate vaccine supply.
- The hepatitis B vaccine is a well-known example of a recombinant protein vaccine currently used in humans. The vaccine utilizes a hepatitis B virus protein produced by yeast cells into which the genetic code for the viral protein has been inserted. This recombinant protein antigen induces an immune response against the hepatitis B virus.
- Recombinant protein vaccines are also employed to prevent human papillomavirus (HPV) infections. These vaccines are prepared using recombinant protein antigens derived from the outer shell of HPV. The proteins form virus-like particles (VLPs) that closely resemble the virus. These VLPs elicit an immune response similar to that triggered by the natural virus but are non-infectious as they lack the genetic material required for viral replication.
- In addition to hepatitis B and HPV vaccines, experimental recombinant protein vaccines have been developed for other diseases. For instance, the National Institute of Allergy and Infectious Diseases (NIAID) has designed an experimental recombinant protein vaccine for chikungunya fever, a viral disease transmitted by mosquitoes.
- Overall, recombinant protein vaccines have revolutionized vaccine development by allowing for the production of specific antigens in large quantities. These vaccines have demonstrated efficacy in preventing various infectious diseases and continue to be an important tool in public health efforts.
5. Nanoparticle vaccines
- Nanoparticle vaccines are a novel approach to vaccine development that involves presenting protein subunit antigens to the immune system. These vaccines utilize microscopic particles known as nanoparticles to display protein antigens, enhancing their effectiveness in inducing an immune response.
- The National Institute of Allergy and Infectious Diseases (NIAID) has been at the forefront of nanoparticle vaccine research. They have designed an experimental universal flu vaccine using protein ferritin, which has the ability to self-assemble into nanoparticles that present a protein antigen. This vaccine has shown promising results in early-stage human trials.
- In addition to influenza, nanoparticle-based vaccines are being evaluated for their potential in combating other viral infections. The NIAID is assessing their use in developing vaccines against the Middle East Respiratory Syndrome (MERS) coronavirus, respiratory syncytial virus (RSV), and Epstein-Barr virus.
- One of the key advancements in nanoparticle vaccine development is the ability to solve the atomic structures of proteins. For instance, the NIAID has successfully determined the 3D structure of a protein from the Respiratory Syncytial Virus (RSV) bound to an antibody. This breakthrough allowed them to identify a crucial part of the protein that is highly sensitive to neutralizing antibodies. By modifying the RSV protein to stabilize this neutralization-sensitive site, they can enhance the effectiveness of the subunit vaccine.
- Nanoparticle vaccines are also being explored for their potential to provide broad protection against various infections. Researchers are developing subunit vaccines that offer immunity against diseases like malaria, Zika virus, chikungunya, and dengue fever. These vaccines often contain recombinant proteins derived from mosquito salivary glands, aiming to trigger an immune response to mosquito saliva itself rather than targeting a specific virus or parasite.
- Overall, nanoparticle vaccines represent an innovative approach to vaccine development by leveraging the unique properties of nanoparticles to enhance the presentation of protein antigens. Ongoing research and clinical trials will provide further insights into their efficacy and potential for widespread use in preventing infectious diseases.
Nucleic Acid Vaccines
- Nucleic acid vaccines are a unique approach to vaccination that involves introducing genetic material encoding the antigen of interest into host cells. By doing so, the host cells can utilize this genetic material to produce the specific antigen, which then triggers an immune response.
- One of the key advantages of nucleic acid vaccines is their ability to stimulate a broad and long-term immune response. This means that the immune system can recognize and respond to various strains or variants of the antigen, providing a more comprehensive and durable immune defense.
- Additionally, nucleic acid vaccines offer excellent stability. Compared to traditional vaccine approaches, such as live attenuated or inactivated vaccines, nucleic acid vaccines can withstand various storage conditions without losing their efficacy. This stability is particularly advantageous for vaccine distribution and stockpiling.
- Another significant advantage is the ease of large-scale vaccine manufacturing. Nucleic acid vaccines can be rapidly produced using standardized laboratory techniques, allowing for efficient and cost-effective production on a large scale. This advantage is particularly crucial in addressing epidemic or emerging diseases where a timely response is crucial.
- Nucleic acid vaccines also reduce the potential risks associated with working with live pathogens. Traditional vaccine development often involves handling the live or inactivated form of the pathogen, which carries inherent risks. In contrast, nucleic acid vaccines only encode the key antigen, eliminating the need to handle the whole pathogen, thereby enhancing safety during the manufacturing process.
- Furthermore, nucleic acid vaccines offer the advantage of encoding only the essential antigen without including other proteins. This precise targeting of the antigen of interest helps ensure that the immune response is focused solely on the desired target, potentially enhancing vaccine efficacy.
- Overall, nucleic acid vaccines hold great promise in the field of vaccination. Their ability to stimulate broad and durable immune responses, combined with excellent stability and ease of large-scale manufacturing, makes them a potential game-changer in addressing epidemic outbreaks or emerging infectious diseases. Ongoing research and development in this area will continue to advance the field of nucleic acid vaccines and their potential applications.
1. DNA plasmid vaccines
- DNA plasmid vaccines are a type of vaccine that utilizes a small circular piece of DNA called a plasmid. These plasmids contain genes that encode proteins from the specific pathogen targeted by the vaccine.
- The National Institute of Allergy and Infectious Disease (NIAID) has been at the forefront of designing experimental DNA plasmid vaccines to address viral disease threats. In 2003, during the outbreak of Severe Acute Respiratory Syndrome (SARS), DNA plasmid vaccines targeting the SARS coronavirus (SARS-CoV) were developed. Similarly, in 2005, DNA plasmid vaccines were designed to combat the H5N1 avian influenza, followed by the development of vaccines for the H1N1 pandemic influenza in 2009 and the Zika virus in 2016.
- DNA plasmid vaccines work by introducing the plasmid containing the target pathogen’s genes into host cells. Once inside the cells, the genes are expressed, leading to the production of the corresponding proteins. These proteins then trigger an immune response, stimulating the production of antibodies and activation of immune cells to recognize and combat the pathogen.
- One of the advantages of DNA plasmid vaccines is their versatility and potential for rapid development. The genetic material in the plasmid can be easily modified to encode different antigens, allowing for the adaptation of the vaccine to various viral strains or emerging pathogens. This flexibility makes DNA plasmid vaccines valuable in addressing new and evolving viral threats.
- Furthermore, DNA plasmid vaccines are considered safe because they do not contain live pathogens. They only carry specific genetic instructions for protein production, reducing the risk of causing the disease in vaccinated individuals. This safety profile makes DNA plasmid vaccines suitable for use in diverse populations, including those with weakened immune systems.
- However, it is important to note that DNA plasmid vaccines are still primarily in the experimental stage and may require additional research and development before widespread use. Despite this, they hold promise as a potential tool in combating viral diseases and responding to emerging infectious threats. Ongoing scientific advancements and clinical trials will continue to refine and evaluate the efficacy and safety of DNA plasmid vaccines in the future.
2. mRNA vaccines
- mRNA vaccines are a type of vaccine that utilize messenger RNA (mRNA), which serves as an intermediary between DNA and protein, to trigger an immune response. Recent technological advancements have addressed the stability issues of mRNA and improved its delivery into cells, leading to the development of effective mRNA vaccines.
- In the context of mRNA vaccines, synthetic mRNA molecules are designed to encode specific viral antigens. These mRNA molecules are formulated in a way that allows them to be taken up by cells in the body after vaccination. Once inside the cells, the mRNA is utilized by the cellular machinery to produce the viral antigen encoded by the mRNA.
- One of the advantages of mRNA vaccines is their ability to induce a robust immune response. By delivering the mRNA encoding the viral antigen directly into cells, mRNA vaccines stimulate the production of the antigen within the body. This prompts the immune system to recognize the viral antigen as foreign and mount an immune response, including the production of antibodies and activation of immune cells.
- Furthermore, mRNA vaccines offer flexibility and rapid development potential. Unlike traditional vaccines that may require the cultivation of viral particles or the use of inactivated or attenuated viruses, mRNA vaccines can be designed and produced synthetically in a laboratory setting. This allows for a quicker response to emerging infectious diseases or new viral strains. Additionally, mRNA vaccines can be easily modified to target different viral antigens, making them adaptable to various pathogens.
- In experimental studies, mRNA vaccines have shown promise. For instance, some mRNA vaccines have been developed and tested in mice and monkeys to protect against Zika virus infection. These vaccines have demonstrated efficacy in inducing immune responses and protecting against viral challenges, even when administered in a single dose.
- It’s important to note that mRNA vaccines have gained significant attention and success with the development of COVID-19 vaccines. The Pfizer-BioNTech and Moderna COVID-19 vaccines, both mRNA-based, have received emergency use authorization and demonstrated high efficacy in preventing COVID-19 disease.
- The use of mRNA vaccines represents an exciting and innovative approach in the field of vaccination. Ongoing research and development are focused on expanding the applications of mRNA vaccines to address other infectious diseases and potentially provide new avenues for disease prevention and control.
3. Recombinant vector vaccine
- Recombinant vector vaccines are a type of vaccine that utilizes harmless viruses or bacteria as carriers, or vectors, to introduce genetic material into cells. These vaccines are designed to elicit an immune response by delivering specific genes or antigens into the cells of the vaccinated individual.
- The concept behind recombinant vector vaccines is to use the genetic material of a harmless virus or bacterium to carry and express genes or antigens from a pathogenic virus or bacterium. By doing so, the vaccine stimulates the immune system to recognize and respond to these specific antigens, providing protection against the targeted pathogen.
- Recombinant vector vaccines have primarily been developed and approved for use in animals to protect them from various infectious diseases, such as rabies and distemper. However, they have also been developed for human use, targeting viruses like HIV, Zika virus, and Ebola virus.
- To create a recombinant vector vaccine, scientists modify the genetic material of the harmless virus or bacterium by inserting genes or antigens from the target pathogen. The modified genetic material is then incorporated into the vector, which acts as a delivery system. When the vaccine is administered, the vector carries the genetic material into cells, where it is expressed and triggers an immune response.
- One advantage of recombinant vector vaccines is their ability to induce strong and targeted immune responses. The use of viral or bacterial vectors allows for efficient delivery and expression of the genetic material, enhancing the immune system’s recognition of the antigens. This can lead to the production of specific antibodies and the activation of immune cells to combat the targeted pathogen.
- It’s important to note that the safety of recombinant vector vaccines is a critical consideration. Extensive research and testing are conducted to ensure that the vectors used in these vaccines are harmless and do not cause disease in vaccinated individuals. The genetic modifications are carefully designed to maintain the safety and efficacy of the vaccine.
- Recombinant vector vaccines have shown promise in both animal and human studies. They offer a versatile platform for vaccine development, allowing for the targeting of a wide range of pathogens. Ongoing research and advancements in this field continue to expand the applications of recombinant vector vaccines, with the aim of providing effective protection against infectious diseases in both animals and humans.
Experimental vaccines
Experimental vaccines are being developed and tested to explore new approaches in immunization. These innovative vaccines utilize various techniques and strategies to stimulate immune responses and provide protection against diseases.
- Dendritic cell vaccines combine dendritic cells with antigens to stimulate an immune reaction.
- Recombinant vector vaccines utilize the combination of one micro-organism’s physiology and another’s DNA to create immunity against diseases with complex infection processes.
- T-cell receptor peptide vaccines are being developed for diseases such as Valley Fever, stomatitis, and atopic dermatitis to modulate cytokine production and improve cell-mediated immunity.
- Targeting bacterial proteins involved in complement inhibition is explored as a strategy to neutralize key bacterial virulence mechanisms.
- Plasmid-based vaccines have shown promise in preclinical studies for cancer and infectious diseases, but their clinical efficacy needs further improvement.
- Bacterial vector vaccines employ bacteria as vectors to deliver antigens and stimulate immune responses.
- Antigen-presenting cell vaccines aim to optimize the presentation of antigens by specialized immune cells.
- Synthetic vaccines mainly consist of synthetic peptides, carbohydrates, or antigens.
- Experimental vaccines offer new approaches for immunization and hold potential for preventing a wider range of diseases.
- Continued research and clinical trials are necessary to assess the safety, efficacy, and suitability of these experimental vaccines.
Valence of Vaccines
- Vaccines can be classified as monovalent (univalent) or multivalent (polyvalent).
- A monovalent vaccine targets a single antigen or microorganism.
- A multivalent vaccine immunizes against two or more strains of the same microorganism or against multiple microorganisms.
- The valency of a multivalent vaccine is indicated by Greek or Latin prefixes such as bivalent, trivalent, or tetravalent/quadrivalent.
- Monovalent vaccines may be preferred in certain cases to rapidly develop a strong immune response.
- Interactions can occur when multiple vaccines are combined in the same formulation.
- Live attenuated vaccines are more prone to interference among their components.
- In the Sabin polio vaccine, the amount of serotype 2 virus had to be reduced to prevent interference with the response to serotype 1 and 3 viruses.
- Dengue vaccines have also demonstrated interference, with the DEN-3 serotype predominating and suppressing the response to other serotypes (DEN-1, -2, and -4).
What are the ingredients in a vaccine?
Vaccines consist of a combination of ingredients carefully selected to ensure their safety and efficacy. These ingredients can be categorized into several components.
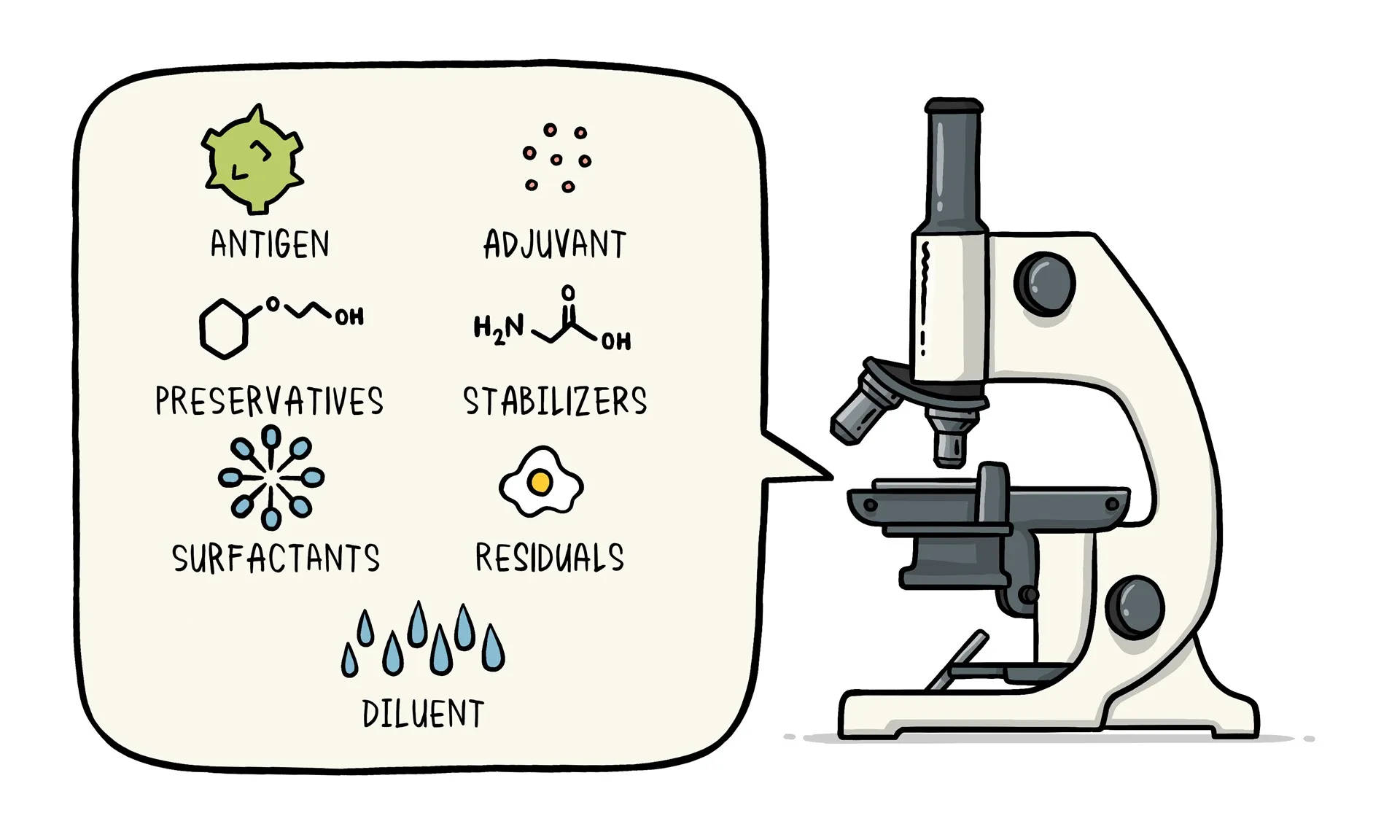
- Antigen: Every vaccine contains an active component known as the antigen. It can be a small part of the disease-causing organism (such as a protein or sugar) or the entire weakened or inactivated organism itself. The antigen stimulates the immune system to produce a response and develop immunity.
- Preservatives: Preservatives are included in vaccines to prevent contamination when multiple doses are drawn from a single vial. The most commonly used preservative is 2-phenoxyethanol, which has been extensively used in vaccines and other baby care products. It is considered safe and has low toxicity in humans.
- Stabilizers: Stabilizers are added to vaccines to prevent chemical reactions and maintain the integrity of the vaccine components. They help prevent clumping and settling of ingredients within the vaccine vial. Examples of stabilizers include sugars like lactose and sucrose, amino acids like glycine, gelatin, and recombinant human albumin derived from yeast.
- Surfactants: Surfactants are substances that ensure proper blending and dispersion of all vaccine ingredients. They prevent settling and clumping of components in the liquid form of the vaccine. Surfactants are commonly used in various food products like ice cream.
- Residuals: Residuals are trace amounts of substances used during the manufacturing or production process that are not active ingredients in the final vaccine. These substances can include egg proteins, yeast, or antibiotics, depending on the manufacturing process. However, the quantities of these residuals in the vaccine are so small that they are measured in parts per million or billion.
- Diluent: A diluent is a liquid used to dilute a vaccine to the correct concentration immediately before administration. Sterile water is the most commonly used diluent.
- Adjuvant: Some vaccines include adjuvants, which enhance the immune response to the vaccine. Adjuvants can help prolong the presence of the vaccine at the injection site or stimulate local immune cells. Examples of adjuvants used in vaccines are tiny amounts of aluminum salts like aluminum phosphate, aluminum hydroxide, or potassium aluminum sulfate. It is important to note that aluminum adjuvants have been extensively studied and have not been found to cause any long-term health problems. Humans regularly consume aluminum through food and beverages.

All vaccine ingredients undergo rigorous testing for safety during the manufacturing process. The selection and inclusion of each ingredient are carefully considered to ensure the vaccine’s effectiveness and minimize any potential risks.
How are vaccines developed?
The development of vaccines involves a rigorous process that ensures their safety and effectiveness. Here is an overview of how vaccines are developed:
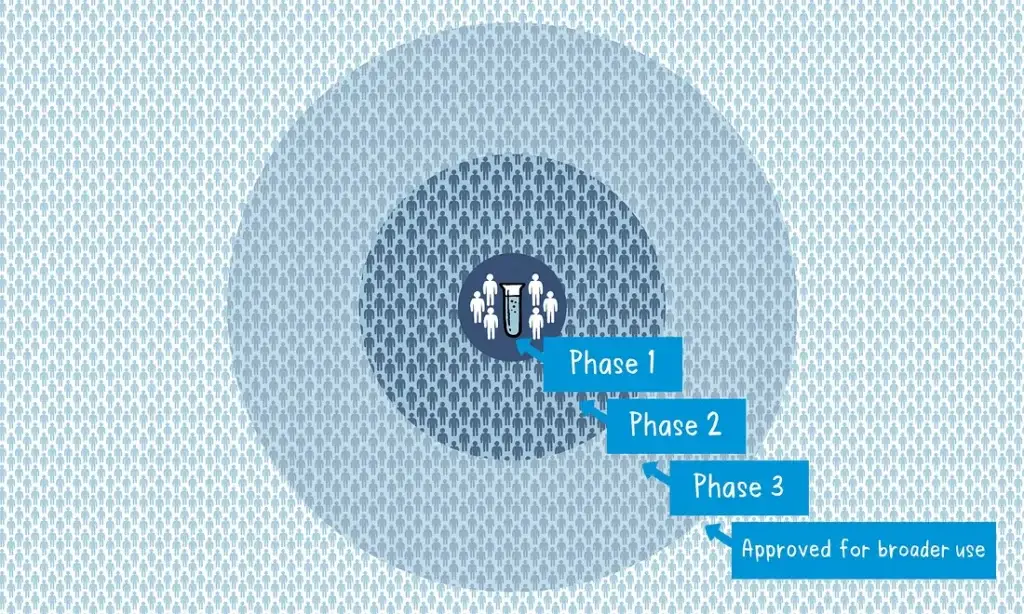
- Preclinical Phase: Scientists screen and evaluate different antigens to determine which one will be used in the vaccine. This phase is conducted using laboratory studies and animal testing to assess safety and efficacy.
- Phase 1 Clinical Trial: A small group of volunteers, usually healthy adults, receive the experimental vaccine. The primary focus is on evaluating the vaccine’s safety, dosage, and its ability to generate an immune response.
- Phase 2 Clinical Trial: The vaccine is administered to several hundred volunteers who closely resemble the target population. This phase further assesses safety, immune response, and may involve multiple trials to evaluate different age groups and formulations. A comparator group is included to determine the vaccine’s effects compared to those who didn’t receive it.
- Phase 3 Clinical Trial: This phase involves thousands of volunteers across multiple countries or sites. The vaccine is given to a large group, while another group receives a comparator product or placebo. The aim is to determine the vaccine’s effectiveness against the targeted disease and assess its safety in a broader population.
During phases 2 and 3, blinding is employed, where volunteers and scientists conducting the study are unaware of who received the vaccine or the comparator. This ensures unbiased assessment of safety and effectiveness.
- Regulatory Review and Approval: After all the clinical trial data is collected and analyzed, regulatory authorities review the efficacy and safety results. Each country’s officials carefully evaluate the study data before authorizing the vaccine for use. The vaccine must meet stringent criteria for safety and efficacy before it can be introduced into a national immunization program.
- Ongoing Monitoring: Once the vaccine is introduced, extensive monitoring systems are in place to continuously assess its safety and effectiveness. Scientists track the impact of the vaccine over time and make adjustments to optimize its usage. These monitoring systems also ensure the ongoing safety of the vaccine throughout its use.
The development of vaccines follows a thorough and meticulous process that prioritizes safety and efficacy. The data collected from clinical trials and post-approval monitoring enable scientists and regulatory authorities to make informed decisions regarding the use and impact of vaccines.
Side Effects of Vaccines
- Vaccines are generally safe and well-tolerated, with most side effects being mild and temporary. Common side effects can include soreness, swelling, or redness at the injection site, as well as mild fever, fatigue, headaches, muscle and joint aches, and fainting. These effects usually resolve within a few days and are signs that the immune system is responding to the vaccine.
- Serious side effects from vaccines are rare but can occur. Adverse reactions such as seizures or life-threatening allergic reactions are uncommon. Monitoring and analyzing the adverse effects of vaccines is an important part of vaccine safety measures, conducted by regulatory agencies such as the Food and Drug Administration (FDA) and the Centers for Disease Control and Prevention (CDC).
- Some vaccines have specific side effects associated with them. For example, the Haemophilus influenza type B (Hib) vaccine, which protects against serious infections caused by the bacteria, can cause redness, warmth, or swelling at the injection site, as well as fever in some children. These effects are generally mild and temporary.
- The smallpox vaccine, which is primarily administered to military personnel and first responders, can have more significant side effects. These can include rashes, redness, and tenderness at the injection site, fever, headaches, and in rare cases, serious effects such as loss of vision, brain damage (encephalitis), and even death. However, it’s important to note that smallpox vaccination is now uncommon and is mainly reserved for specific high-risk groups due to the eradication of natural smallpox.
- Overall, the benefits of vaccination in preventing potentially severe diseases far outweigh the risks of side effects. Vaccines undergo rigorous testing and monitoring for safety and efficacy before being approved for use. Health authorities closely monitor vaccine safety and encourage reporting of any adverse events to ensure ongoing safety evaluation.
- It’s important to consult with healthcare professionals and follow their guidance regarding vaccines. They can provide specific information about potential side effects associated with each vaccine and address any concerns or questions you may have.
FAQ
What is a vaccine?
A vaccine is a biological preparation that stimulates the immune system to recognize and fight against specific diseases or infections.
How do vaccines work?
Vaccines work by introducing a harmless part of the disease-causing organism or the blueprint for making that part into the body. This triggers an immune response, training the immune system to recognize and respond effectively if the person is later exposed to the actual disease.
Are vaccines safe?
Vaccines undergo rigorous testing and monitoring to ensure their safety. The benefits of vaccination generally outweigh the risks of adverse reactions, which are typically mild and temporary.
Do vaccines cause autism?
Extensive scientific research has found no credible evidence linking vaccines to autism. Vaccines are safe and do not cause autism.
Do vaccines contain harmful ingredients?
Vaccines may contain trace amounts of certain ingredients like preservatives, stabilizers, and adjuvants. These ingredients are carefully evaluated for safety and have been used in vaccines for many years.
Can vaccines give you the disease they are meant to prevent?
Vaccines cannot give you the disease they are designed to protect against. They may cause mild symptoms or temporary side effects as the immune system responds, but these are far less severe than the actual disease.
Do vaccines have long-term side effects?
Vaccines are thoroughly tested for both short-term and long-term side effects. Long-term side effects are extremely rare, and the benefits of vaccination in preventing serious diseases far outweigh any potential risks.
Are vaccine-preventable diseases still a threat?
Vaccine-preventable diseases can still pose a threat, especially in communities with low vaccination rates. Outbreaks can occur when the population immunity decreases, making it important to maintain high vaccination coverage.
Can vaccines be given to pregnant women?
Some vaccines are recommended for pregnant women to protect both the mother and the baby. These include vaccines for influenza and pertussis (whooping cough). Pregnant women should consult their healthcare providers for specific recommendations.
How long does vaccine protection last?
Vaccine protection can vary depending on the specific vaccine. Some vaccines provide lifelong immunity, while others may require booster doses to maintain protection. Ongoing research helps determine the duration of vaccine effectiveness.
References
- Kuby Immunology 7th Edition
- Microbiology by Prescott
- Microbiology and Immunology 2nd Edition by Shubash Chandra Parija
- https://medium.com/who/how-are-vaccines-developed-81de4863b95d
- https://medium.com/who/how-do-vaccines-work-4eb2a63fb60a
- https://www.hindawi.com/journals/jvac/
- https://www.cdc.gov/vaccines/index.html
- https://www.who.int/immunization/en/
- https://www.nih.gov/topics/vaccines-immunization
- https://vaers.hhs.gov/
- https://www.ema.europa.eu/en/human-regulatory/overview/vaccines
- https://www.journals.elsevier.com/vaccine
Best source to understand and learn about basics of Vaccinology