What is Chromatography?
- Chromatography can define as a technique used for separation of components that are present in a mixture, based on their different movement rates by a medium (like paper, gel, or liquid).
- The principle is mainly depending on differential partition of substances between stationary phase and mobile phase, which are two different phases.
- The stationary phase is the one that stay fixed by the system, while the mobile phase moves by it carrying the sample molecules along.
- The components that interact strongly with stationary phase move slow, but those which have less interaction move fast and come out earlier.
- It is widely used by laboratories for qualitative and quantitative analysis of compounds.
- Chromatography is applied in many types, like Paper chromatography, Thin Layer Chromatography (TLC), Gas Chromatography (GC), and High Performance Liquid Chromatography (HPLC) etc.
- In Paper chromatography, paper acts as stationary phase, and solvent acts as mobile one, it is mostly used for separation of pigments or amino acids.
- In TLC, a thin layer of adsorbent (like silica gel or alumina) is coated on glass/plastic plate, the components migrate differently by capillary action.
- Gas chromatography used gas as mobile phase, and a liquid/solid stationary phase, it is used for volatile compound identification.
- HPLC involves a liquid mobile phase passed by a column under high pressure, it gives more accurate, precise and quick results compared to simple column method.
- The process is affected by several factors like temperature, pressure, solvent polarity and flow rate etc.
- Through chromatography, both purification and identification of compounds are achieved, it plays vital role in biochemistry, pharmaceuticals, and environmental testing.
- Sometimes it’s said as the “heart” of separation science, because it can handle very complex mixtures with good sensitivity.
- However, it’s a bit costly and time-taking method when compared to simpler separations.
- Overall, chromatography refers as essential analytical tool for chemical, biological, and medical fields for separating and studying components in mixture.
Chromatography Definition
Chromatography is a technique used to separate and analyze the components of a mixture based on their properties and interactions with a stationary and mobile phase.
What is a stationary phase?
- The stationary phase can define as the phase which stay fixed or remain immobile by the chromatographic system during the separation process.
- It may be solid or liquid supported by a solid base (like silica, alumina, cellulose etc.) depending upon the type of chromatography used.
- The main function of stationary phase is to retain or adsorb components of mixture temporarily based on their interaction strength.
- The stronger the interaction between the substance and stationary phase, the slower it move during chromatography.
- Separation occurs because each substance has different affinity toward stationary and mobile phases.
- In paper chromatography, the stationary phase is the water molecules adsorbed on the cellulose fibers of paper.
- In TLC (Thin Layer Chromatography), it is thin layer of silica gel / alumina coated on a glass or plastic plate.
- In Gas Chromatography (GC), the stationary phase is usually a liquid film coated on solid support or column wall.
- For HPLC, stationary phase generally consists of small particles packed tightly in column, made of materials like silica with chemically bonded groups.
- The nature of stationary phase largely determines how well the separation will be done, it controls retention time and selectivity of solutes.
- Its polarity, surface area, and particle size are important factors that influence migration rate of analytes.
- Sometimes stationary phase is modified chemically to improve resolution or to make it specific for certain compounds.
- It may be polar or nonpolar type, based on which compound interacts more (polar stationary phase used for polar analytes).
- During process, mobile phase moves over stationary one carrying different solutes at different rates, leading to distinct zones or bands.
- Therefore stationary phase refers as heart of chromatographic separation because without it, the selective distribution of molecules cannot prevail.
What is the mobile phase?
- The mobile phase can define as the phase which moves by or through the stationary phase, carrying the mixture components with it during the chromatographic process.
- It may exist in form of liquid or gas depending on the type of chromatography being used.
- The main role of mobile phase is to transport analytes through the stationary medium so that separation based on interactions can be achieved.
- The rate of movement of each component is decided by their solubility in the mobile phase and their attraction toward stationary phase.
- In Paper chromatography, the mobile phase is a solvent or mixture of solvents which travel up the paper by capillary action.
- In TLC (Thin Layer Chromatography), a suitable solvent or solvent system acts as mobile phase that move along the plate surface.
- In Gas Chromatography (GC), the mobile phase is an inert gas like helium, nitrogen or hydrogen which carry vaporized samples through column.
- In HPLC (High Performance Liquid Chromatography), a liquid under high pressure is used as mobile phase, often consisting of buffers or organic solvents.
- The polarity of mobile phase plays major role in determining separation efficiency and retention time of analytes.
- Solvent strength, flow rate, viscosity and composition are parameters which affect performance of mobile phase during separation.
- It may be polar or nonpolar depending upon the stationary phase used, for achieving desired resolution.
- The movement of mobile phase causes different solutes to migrate at different speeds, forming distinct bands or peaks on chromatogram.
- Sometimes gradient elution is used where mobile phase composition gradually changed to improve separation of complex samples.
- The purity and stability of mobile phase are important because impurities may cause baseline noise or distorted results.
- Hence, mobile phase refers as the “moving carrier” of chromatography which makes the whole separation phenomenon possible.
Types of Chromatography
Here is the Types of Chromatography;
- Affinity chromatography
- Column chromatography
- Anion exchange chromatography
- Cation exchange chromatography
- Flash chromatography
- Gas chromatography
- Gel filtration chromatography/Size exclusion chromatography
- High-performance liquid chromatography (HPLC)
- Hydrophobic interaction chromatography
- Ion exchange chromatography
- Liquid chromatography
- Paper chromatography
- Reverse-phase chromatography
- Thin-layer chromatography (TLC)
| Type of Chromatography | Description |
|---|---|
| Gas Chromatography (GC) | Separates volatile compounds using a gaseous mobile phase. Commonly used for analyzing volatile organic compounds (VOCs) and gases. |
| Liquid Chromatography (LC) | Separates compounds using a liquid mobile phase. Includes techniques such as high-performance liquid chromatography (HPLC), where high-pressure pumps are used for improved separation efficiency. |
| Thin-Layer Chromatography (TLC) | Involves a thin layer of stationary phase applied to a solid support. Components of a mixture interact with the mobile phase and the stationary phase to achieve separation. Commonly used for qualitative analysis and identification of compounds. |
| High-Performance Liquid Chromatography (HPLC) | A type of liquid chromatography that uses high-pressure pumps for improved separation efficiency and speed. Widely used for the analysis of compounds in pharmaceuticals, environmental analysis, and food science. |
| Ion Exchange Chromatography | Separates charged molecules based on their interactions with an ion-exchange resin. Involves the exchange of ions between the mobile phase and the resin for separation. Used for purifying proteins, separating ions, and analyzing biomolecules. |
| Reverse-Phase Chromatography | Relies on hydrophobic interactions between the stationary phase and analytes in the mobile phase. Suitable for separating nonpolar and moderately polar compounds. Commonly used in pharmaceutical analysis and compound purification. |
| Affinity Chromatography | Separates and purifies biomolecules based on specific interactions with immobilized ligands or affinity tags on the stationary phase. Used for isolating proteins, enzymes, and antibodies. |
| Size Exclusion Chromatography (SEC) or Gel Filtration Chromatography | Separates molecules based on their size or molecular weight using a porous stationary phase. Larger molecules elute first while smaller molecules penetrate the pores and elute later. Often employed for the purification and analysis of proteins and polymers. |
| Chiral Chromatography | Separates enantiomers (mirror-image isomers) based on their interaction with a chiral stationary phase. Used for the separation of chiral compounds in pharmaceutical analysis, drug development, and research. |
| Supercritical Fluid Chromatography (SFC) | Utilizes supercritical fluids (e.g., carbon dioxide) as the mobile phase, offering unique solubility and selectivity properties. Often employed for separating thermally labile and nonvolatile compounds. |
| Paper Chromatography | Involves a specialized paper as the stationary phase, with separation occurring based on differential adsorption or partitioning of components. Widely used for qualitative analysis, such as separating ink components or detecting impurities. |
| Hydrophobic Interaction Chromatography (HIC) | Utilizes hydrophobic interactions between the stationary phase and hydrophobic regions of analytes. Used for separating proteins and biomolecules based on their hydrophobicity. |
| Expanded Bed Chromatography | Involves a stationary phase composed of rigid, porous particles that can accommodate a fluidized bed of sample and mobile phase. Enables purification of biomolecules directly from crude samples without the need for extensive sample preparation. |
| Flash Chromatography | A preparative chromatography technique used for fast purification of compounds in larger quantities. Typically employs a column packed with silica gel or other stationary phases, allowing rapid separation and purification. |
1. Affinity chromatography

Principle of Affinity chromatography
Affinity chromatography can define as a type of liquid chromatography based on specific biological interaction between two molecules – one immobilized on the stationary phase and the other present in the mobile phase.
It works by binding the target molecule (protein, enzyme, antibody etc.) to a ligand which has high and specific affinity toward that molecule.
The stationary phase usually consists of a solid support like agarose, sepharose, or cellulose beads to which ligand molecules are covalently attached.
The mobile phase carries the sample solution by the column, allowing desired molecule to bind with ligand while others pass unretained.
After unwanted materials washed away, the bound molecule is eluted by changing pH, ionic strength or by using competitive ligand solution.
This technique depends mainly upon biochemical specificity, so it provides very high selectivity and purity during separation.
Ligands used may include substrates, cofactors, inhibitors, antibodies, or nucleic acids, depending on target molecule type.
Example – enzyme purification by immobilized substrate, antibody purification by Protein A / G affinity, or nucleic acid separation by poly-T columns.
The process usually involves three main steps – adsorption, washing, and elution, each optimized for the particular molecule.
It is widely used in biotechnology, immunology, clinical diagnostics, and biochemical research for purification and isolation of biomolecules.
The elution step must be handled carefully since too harsh conditions can denature sensitive biomolecules like enzymes or antibodies.
Affinity chromatography allows even a single-step purification of highly specific compounds which otherwise require multiple steps.
The column can be reused many times if the ligand remains active and not degraded during repeated runs.
The method sometimes prevail issues like ligand leakage, column fouling, or slow flow rate when large proteins used.
Therefore, affinity chromatography refers as a powerful and highly selective separation method that relies on biological recognition principles for purification of complex biomolecular mixtures.
Steps of Affinity chromatography
- The Preparation of Affinity Matrix is done first. In this step, a suitable matrix (like agarose or cellulose) is selected and activated chemically so ligand can attach on it covalently. Sometimes matrix are pre-activated already for convenience.
- Then Immobilization of Ligand is carried out. The ligand which has specific binding ability with target molecule is attached with the matrix by covalent bonds. After coupling, unreacted sites on matrix are blocked to avoid non-specific binding, this process also called as “blocking step”.
- Column Packing step comes next. The ligand-bound matrix is poured inside a glass or plastic column carefully, avoiding formation of air bubbles, because bubbles disturb flow rate and resolution.
- After that, the Equilibration of Column is performed with a suitable buffer (like phosphate buffer, Tris-HCl etc.) to maintain pH and ionic strength similar with the sample condition. This step helps to prepare column for sample loading.
- Application of Sample – the crude mixture or sample solution containing target molecule is added to the top of column. During this step, molecules pass through matrix, only those which have affinity with ligand will bind, rest others will flow out.
- Washing Step – unbound and weakly bound molecules are washed out by same or slightly different buffer to remove impurities. The washing continues till baseline absorbance achieved in elution profile.
- Elution of Bound Molecules is next important step. Bound molecules are released by changing pH, ionic strength, or adding a competitive ligand. This breaks ligand-target interaction. Elution can be done in stepwise or gradient mode.
- Then, Regeneration of Column is done by washing it with strong buffer or salt solution, which remove remaining molecules, making column ready for reuse.
- At last, Storage of Column – after regeneration, column is stored at 4°C (or room temp sometimes) in buffer containing preservative (like sodium azide) to prevent microbial growth.
Uses of Affinity chromatography
- Affinity chromatography used mainly for protein purification, where proteins with high specificity for a ligand are separated from complex mixtures.
- It is applied for enzyme isolation, enzymes are captured by substrate or inhibitor attached on the stationary phase.
- Antibody and antigen purification often performed by this method, because binding between them is very strong and specific.
- In research field, it is used for receptor–ligand interaction studies, the technique helps to understand binding sites and strength of interaction.
- Purification of nucleic acids (DNA/RNA) is carried out by using complementary sequences or specific binding proteins on the column.
- Used for removal of contaminants or unwanted proteins that do not show affinity, hence sample become more pure and concentrated.
- Hormones and vitamins isolation from biological samples is also done by this technique, since they bind with their respective receptors or carriers.
- The method used in biotechnology industries for purification of recombinant proteins expressed in E. coli or yeast systems.
- Immunoassays preparation sometimes depend by affinity chromatography to obtain high-purity antibodies for diagnostic uses.
- It applied for protein–protein interaction studies to identify binding partners and signaling components etc.
- In clinical laboratories, it used to isolate plasma proteins and biomarkers for disease investigation.
- The method also utilized for lectin–carbohydrate binding analysis, where glycoproteins are separated from mixture.
- Some researchers used it for purification of fusion proteins which contain affinity tags like His-tag or GST-tag for easy recovery.
- It can used for enzyme immobilization, where enzyme is bound with matrix for reuse in industrial catalysis.
- Affinity chromatography thus has sturdy and hardy application range in biochemistry, molecular biology, medicine and industrial biotech fields.
Examples of Affinity chromatography
1. Protein A – Affinity chromatography
This type used for purification of antibodies (IgG). Protein A ligand bind specifically with Fc region of immunoglobulin, so it is widely used by biopharma industries.
2. Ni²⁺ – NTA Affinity chromatography
Here nickel–nitrilotriacetic acid (Ni–NTA) matrix used for purification of His-tagged recombinant proteins, binding occur between histidine residues and Ni²⁺ ions.
3. Lectin – Affinity chromatography
Lectins like Concanavalin A (Con A) used for separation of glycoproteins, they interact specifically with carbohydrate moieties on proteins.
4. Avidin / Biotin – Affinity chromatography
Based on strong avidin–biotin interaction, it is used in biochemical assays and purification where very high binding affinity required.
5. Enzyme–Substrate analog – Affinity chromatography
Used for enzyme purification, where immobilized substrate analog attract enzyme molecules having complementary active site.
6. Immunoaffinity chromatography –
In this type, antibody–antigen interaction is used. For example, purification of hormones, viral proteins, toxins etc.
7. Dye–Ligand Affinity chromatography
Synthetic dyes like Cibacron Blue F3GA used as ligands, for separating dehydrogenases / kinases / serum albumin etc.
8. Metal Chelate Affinity chromatography –
Based on interaction between metal ions (Cu²⁺, Zn²⁺, Co²⁺) and electron-donor groups on protein surface. Often used for purification of metalloproteins.
9. Glutathione–Sepharose Affinity chromatography
Used to purify GST-tagged proteins by using glutathione immobilized on agarose beads, elution done with reduced glutathione.
10. Heparin–Affinity chromatography –
Heparin binds with many proteins like growth factors, lipoprotein lipase, hence applied for their isolation in lab and industrial works.
2. Column chromatography
Column chromatography can define as a separation technique which used for separating compounds from mixture, based on their differential adsorption on stationary phase and movement by mobile phase.
The method used both for analytical and preparative purpose, but mostly it used in laboratories for purification of chemical substances.
In this technique, a column (usually glass or plastic) is packed with adsorbent like silica gel / alumina, which act as stationary phase.
The mixture to be separated is placed on top of column and then a solvent (mobile phase) is passed by it, so different compounds move with different speed.
Compounds which have higher affinity to stationary phase move slow, and others which have more solubility in mobile phase move faster, causing separation.
The process depend by many factors like polarity of solvent, particle size, column length etc.
Fractions eluted from column are collected separately and analyzed for required compound, sometimes by spectrophotometer or TLC (thin layer chromatography).
This method used widely in biochemistry, organic chemistry, pharmaceutical science for isolation of pure compounds.
Column chromatography is simple, inexpensive, and effective method, though sometimes it take long time and large solvent volume.
Overall it provides sturdy and handy way for purification of both small molecules and biomolecules in laboratory work.

Principle of Column Chromatography
The principle of column chromatography based on the differential adsorption or partition of components between two phases – stationary and mobile.
In this method, one phase (the stationary phase) remain fixed inside the column, while another (mobile phase) flow by it carrying the mixture components.
Each compound in the mixture has different affinity or attraction toward stationary and mobile phase, which cause their separation during movement.
The components that have more affinity with stationary phase get adsorbed strongly and move slower down the column.
Whereas the molecules having higher solubility in mobile phase get eluted faster, hence they separated earlier.
The separation depend on adsorption coefficient, polarity, molecular weight, and nature of solvent used.
The process can work either on adsorption principle (like silica or alumina) or on partition principle where liquid stationary phase used.
During elution, components form distinct bands or zones which can be collected separately for analysis.
The overall principle rely on repeated adsorption–desorption between two phases, causing gradual separation of each compound.
So, column chromatography mainly works on the balance between stationary–mobile phase interactions, which decide how fast or slow each component prevail in movement.
Steps of Column Chromatography
- The first step involve preparation of the column, where the glass tube is cleaned, dried and fixed vertically, sometimes with cotton plug at bottom to hold adsorbent.
- Then packing of stationary phase is done by adding silica gel or alumina with solvent to form a uniform bed, care taken to avoid air bubbles which disturb separation.
- Equilibration of column carried out next, solvent (mobile phase) passed slowly till proper wetting and uniform flow achieved, this help to stabilize adsorbent layer.
- The sample mixture prepared in suitable solvent is then carefully loaded over surface of column, so the sample form narrow and even band at top.
- After sample addition, elution process started where mobile phase (eluent) continuously added at top and allowed to flow down by gravity or low pressure.
- Different compounds travel by different rate according to their affinity by stationary and mobile phase, hence they separate gradually along the column.
- During development of column, colored or colorless bands appear which move downward slowly as elution proceeds, sometimes their progress is observed visually.
- The fractions are collected separately from outlet at fixed intervals, each fraction contain particular compound depending on elution order.
- Every fraction is then analyzed by TLC or UV spectrophotometry to identify and confirm the compound of interest etc.
- After process completion, column sometimes washed and regenerated for reuse if the adsorbent still active and not contaminated.
Uses of Column Chromatography
- Column chromatography used mainly for purification of compounds, both organic and inorganic, from complex mixtures based on their adsorption difference.
- It applied for separation of plant pigments, like chlorophyll, xanthophyll and carotenoids from leaf extracts, which move with different speed in column.
- The method used for isolation of natural products like alkaloids, flavonoids, glycosides etc., from plant materials in phytochemical studies.
- It used for purification of proteins, enzymes, amino acids, and other biomolecules in biochemical laboratories.
- Pharmaceutical industries use column chromatography for purification of drugs and removal of impurities during formulation development.
- It often applied for analytical purpose, to check purity of compounds and identification of unknown components.
- The technique used in preparative chemistry, where large amount of pure compound needed for further reactions or synthesis.
- In research laboratories, it help to separate metabolites or intermediates from reaction mixtures.
- It also used to remove colored impurities from solutions, especially during synthesis of organic compounds.
- Column chromatography thus serve as simple, versatile and economical technique used widely in chemistry, biochemistry, pharmacy and industrial research fields etc.
Examples of Column Chromatography
1. Adsorption chromatography –
In this type, the stationary phase is solid (like silica gel or alumina) and separation occur by adsorption on its surface. For example, separation of plant pigments from leaf extract.
2. Partition chromatography –
Here both stationary and mobile phases are liquid. The compounds distributed themselves between two liquid layers, like separation of amino acids or small organic molecules.
3. Ion exchange chromatography –
Based on electrostatic attraction between ions in sample and oppositely charged groups on stationary phase. Example, separation of proteins / amino acids using DEAE-cellulose or CM-cellulose column.
4. Gel filtration chromatography –
Also called size exclusion chromatography, here molecules are separated according to size or molecular weight. Large molecules elute first, for example separation of polysaccharides and proteins.
5. Affinity chromatography –
Used when specific biological interaction occur between ligand and target molecule, like enzyme–substrate or antigen–antibody binding. Example purification of antibodies, enzymes etc.
6. Metal chelate chromatography –
In this method, metal ions like Ni²⁺, Cu²⁺, Zn²⁺ attached to matrix and used for purification of His-tagged recombinant proteins.
7. Reverse phase chromatography –
In this type, stationary phase is nonpolar and mobile phase polar, so nonpolar compounds retain longer. It used for lipids, steroids, and hydrophobic molecules separation.
8. Dye-ligand chromatography –
Here synthetic dyes (like Cibacron Blue F3GA) act as ligand for purification of dehydrogenase or kinases.
9. Immunoaffinity chromatography –
This method used for isolation of antigens / hormones / toxins by specific antibody interaction.
10. Gel permeation chromatography –
It’s commonly used in polymer chemistry for determination of molecular weight distribution of synthetic polymers.
3. Anion exchange chromatography

- Anion exchange chromatography can define as a type of ion-exchange chromatography where negatively charged ions (anions) are separated based on their interaction with a positively charged stationary phase.
- The stationary phase contains positively charged functional groups (like quaternary ammonium), which attract and hold negatively charged molecules from the sample solution.
- The mobile phase usually consist of a buffer solution which maintain pH and ionic strength during separation.
- Molecules are separated depending on their charge density and affinity toward the charged groups of stationary phase.
- When buffer of increasing salt concentration or changing pH is passed by the column, the bound anions are eluted in order of their binding strength.
- The technique is widely used for purification of proteins, nucleic acids (DNA/RNA) and enzymes that carry net negative charge under specific pH.
- Commonly used matrices are DEAE-cellulose (Diethylaminoethyl) and Q-Sepharose, both act as weak and strong anion exchangers respectively.
- The process depend mainly by electrostatic interactions, so it is affected by pH, ionic strength and composition of buffer system.
- Elution usually performed in gradient way to get precise separation of molecules with similar charges.
- Therefore, anion exchange chromatography serve as very important and handy technique for separation of biomolecules based on their surface charge property.
Principle of Anion Exchange Chromatography
- The principle of anion exchange chromatography based on electrostatic attraction between negatively charged molecules (anions) and positively charged groups on stationary phase.
- The stationary phase usually contain functional groups like –NH₃⁺ or quaternary ammonium, which carry permanent positive charge capable of binding anions from sample.
- When sample solution pass by the column, negatively charged molecules interact and attach to the positive sites, while neutral or less charged species move freely with mobile phase.
- The strength of this binding depend on net charge, pH, ionic strength, and molecular structure of analyte.
- The mobile phase (buffer) help to maintain pH conditions so that target molecules stay ionized during adsorption.
- Elution of bound molecules is achieved by increasing salt concentration or altering pH, which reduce electrostatic interactions and cause release of anions from column.
- The molecules having weak charge or low affinity elute first, while strongly charged ones elute later when ionic competition increases.
- The principle thus rely mainly on charge-based separation, where opposite charges attract and similar charges repel during chromatographic process.
- For example, proteins, nucleotides, and enzymes with negative charge at given pH are separated efficiently using anion exchange resin.
- So overall, the technique function by controlled binding and elution of anions depending on their electrostatic interactions with oppositely charged stationary phase.
Steps of Anion Exchange Chromatography
- The first step involve equilibration of column, where the anion exchange resin is washed with suitable buffer to achieve desired pH and ionic condition before sample loading.
- Then sample preparation done by adjusting its pH so that target molecule carries negative charge and can interact with positively charged groups on resin surface.
- Sample application carried out by slowly adding the prepared solution to the column, allowing anionic molecules to bind electrostatically with the stationary phase.
- During washing step, unbound and weakly bound impurities are removed by passing buffer of same composition, so only desired anionic molecules remain attached.
- After that elution process started, where salt concentration or pH of buffer gradually increased to disturb ionic interaction and release the bound anions.
- The molecules having weak electrostatic forces elute first, while strongly bound ones elute later when ionic strength become higher.
- The eluted fractions are collected separately in tubes, and each fraction can be analyzed for protein or nucleic acid content by absorbance at 280 nm or other method.
- After elution completed, regeneration of resin done by washing with high salt or basic solution to remove remaining impurities and restore its binding capacity.
- Finally, the column is re-equilibrated again with starting buffer if reuse intended, ensuring that ionic exchange sites are properly conditioned.
- So these steps followed systematically provide accurate and repeatable separation of anionic biomolecules by anion exchange chromatography technique.
Uses of Anion Exchange Chromatography
- Anion exchange chromatography used mainly for separation and purification of negatively charged biomolecules like DNA, RNA, and acidic proteins.
- It applied for protein purification where proteins having negative charge at working pH are bound to positively charged resin and eluted under controlled ionic conditions.
- The method used in enzyme purification, especially for those having acidic isoelectric point (pI) values.
- It help to remove contaminants or unwanted ions from biological or chemical samples by selective adsorption.
- The technique widely used in biopharmaceutical industries for purification of therapeutic proteins, monoclonal antibodies, and vaccines.
- In water treatment systems, it applied for removal of anions like chloride, sulfate, nitrate, and phosphate from water samples.
- It used in nucleic acid purification, especially plasmid DNA isolation and separation of oligonucleotides.
- The method also used for charge-based characterization of biomolecules, determining isoelectric points and charge variants.
- It often used as intermediate purification step in multi-step chromatography process to increase product purity.
- Thus, anion exchange chromatography act as very versatile and essential tool used in research, clinical diagnostics, environmental, and industrial applications etc.
Examples of Anion Exchange Chromatography
1. DEAE–Cellulose chromatography –
It is one of the most common examples where diethylaminoethyl (DEAE) group act as weak anion exchanger. Used for purification of proteins and enzymes having negative charge at pH above their isoelectric point.
2. Q–Sepharose chromatography –
This use quaternary ammonium groups as strong anion exchanger. It’s used for high-resolution separation of nucleic acids, plasma proteins, and other biomolecules.
3. Mono Q column –
It’s a prepacked column used in FPLC (Fast Protein Liquid Chromatography) system for separation of recombinant proteins / antibodies.
4. DEAE–Sephadex chromatography –
A gel-based anion exchanger, used for purification of enzymes and serum proteins, especially when mild binding conditions are needed.
5. QAE–Sephadex chromatography –
Contain quaternary aminoethyl functional group and work as strong anion exchanger, widely used for DNA fragments and peptides purification.
6. DEAE–Agarose chromatography –
Applied for large biomolecule separation, especially viruses, plasmid DNA, and high-molecular-weight proteins.
7. HiTrap Q HP column –
Commonly used in biopharmaceutical laboratories for purification of monoclonal antibodies and therapeutic proteins.
8. Sepharose Q Fast Flow –
It’s used for large-scale purification of immunoglobulins and vaccine components under industrial conditions.
9. DEAE–Toyopearl chromatography –
It’s applied for enzyme purification and also used in downstream processing of biopharmaceuticals.
10. Q–HyperD column –
Used for separation of negatively charged oligonucleotides or peptides where strong anion exchange is required.
4. Cation exchange chromatograph
Cation exchange chromatography can define as a type of ion-exchange chromatography where positively charged ions (cations) are separated based on their interaction with negatively charged stationary phase.
The stationary phase contains negatively charged functional groups, like sulfonate (-SO₃⁻) or carboxylate (-COO⁻) which attract and bind positively charged molecules from the sample.
The mobile phase usually consist of buffer solution that control pH and ionic strength, so molecules remain charged properly during separation.
The principle work by electrostatic attraction where cations in sample are retained on column until displaced by other ions or by change in buffer conditions.
Molecules with higher positive charge or stronger affinity toward stationary phase elute later than weakly charged ones.
Common resins used are CM-cellulose (carboxymethyl cellulose) as weak cation exchanger and SP-Sepharose (sulfopropyl) as strong cation exchanger.
This method mostly used for separation and purification of basic proteins, peptides, and other biomolecules carrying net positive charge at given pH.
The technique depend on factors like pH, salt concentration, and composition of mobile phase which influence the charge and binding strength.
Elution achieved by increasing ionic strength (salt gradient) or altering pH that neutralize charge and cause molecules to detach from resin.
Thus, cation exchange chromatography is important for purification of proteins, enzymes and biological macromolecules based on their positive charge behavior.
Principle of Cation Exchange Chromatography
- The principle of cation exchange chromatography based on electrostatic attraction between positively charged molecules (cations) and negatively charged groups present on stationary phase.
- The stationary phase is composed of resins or matrices having acidic functional groups like –COO⁻ (carboxyl) or –SO₃⁻ (sulfonate) which hold permanent negative charge.
- When a mixture of molecules passed by the column, the positively charged molecules are attracted and bound to these negative sites, while neutral or negatively charged ones move freely.
- The extent of binding depend by several factors like pH of buffer, ionic strength, and charge density of the molecule.
- At suitable pH condition, molecules with higher positive charge interact more strongly and retained longer on column.
- The bound cations are then eluted by gradually increasing salt concentration or by altering pH, which reduce electrostatic interactions and displace molecules.
- The principle rely on reversible ion-exchange mechanism, where positively charged analytes exchange with cations of mobile phase.
- The separation thus occur according to difference in net positive charge and affinity of molecules toward stationary phase.
- For example, proteins with basic isoelectric point (pI) are retained strongly under low pH conditions and eluted by ionic gradient.
- Hence, cation exchange chromatography function on simple charge-based attraction between opposite ions for effective biomolecule separation.
Steps of Cation Exchange Chromatography
- In first step, the column preparation is done where cation exchange resin packed tightly inside column, and equilibrated with buffer to maintain required pH and ionic condition.
- The equilibration buffer normally used with low ionic strength so the resin surface fully charged with negative groups like –COO⁻ or –SO₃⁻.
- The sample solution containing mixture of proteins or other positively charged molecules is then loaded into the column carefully, so they interact properly with stationary phase.
- During loading, cations from sample bind with negatively charged groups on resin due to electrostatic attraction while neutral or negative molecules pass unretained.
- Then washing step is performed by same equilibration buffer to remove unbound or weakly attached molecules, ensuring that only desired cations remain attached on matrix.
- After washing, elution process is carried out where bound molecules are released by gradually increasing salt concentration or by changing pH of buffer solution.
- Increasing ionic strength introduce competing cations (Na⁺, K⁺ etc.) which replace bound analytes and cause them to elute from column.
- Alternatively, change in pH alter charge of biomolecules and weaken attraction between molecule and resin surface resulting in elution.
- The eluted fractions are collected in separate tubes and then analyzed for purity and concentration by spectrophotometric or electrophoretic methods.
- At last, column is regenerated and re-equilibrated for reuse by washing with strong salt or buffer to remove remaining residues.
Uses of Cation Exchange Chromatography
- Cation exchange chromatography used for purification of basic proteins, which carry positive charge at certain pH condition.
- It widely applied in biotechnology and pharmaceutical industry for isolation of therapeutic proteins, enzymes, and antibodies.
- The technique help in separation of peptides and amino acids, especially those having different isoelectric points (pI).
- It used in purification of enzymes like lysozyme, ribonuclease, and hemoglobin, which possess net positive charge in acidic buffer.
- The method also used for water softening process, where cations like Ca²⁺, Mg²⁺ are replaced with Na⁺ ions on resin.
- It help in removal of metal ions or charged contaminants from biological or chemical solutions by binding them to resin surface.
- The technique used for protein fractionation, to separate closely related protein variants based on surface charge difference.
- It is commonly used in quality control of biopharmaceutical products to check purity and charge heterogeneity.
- In laboratory research, it used for purification of nucleotides, small cationic molecules, and other biological macromolecules.
Examples of Cation Exchange Chromatography
- CM-cellulose (Carboxymethyl cellulose)– used as weak cation exchanger for purification of positively charged proteins like lysozyme, cytochrome c, etc.
- SP-Sepharose (Sulfopropyl Sepharose)– act as strong cation exchanger commonly used in separation of enzymes and basic peptides.
- Dowex 50 resin– employed for purification of amino acids and small cationic molecules from complex mixtures.
- Mono S column– used for high-resolution separation of basic proteins under FPLC (Fast Protein Liquid Chromatography) system.
- CM-Sephadex C-50– suitable for isolation of cationic proteins from cell extracts, often used in laboratory protein purification.
- Amberlite IR-120– a strong acid cation exchange resin used for water treatment and ion purification processes.
- Bio-Rex 70 resin– used for purification of nucleotides and metal cations in biochemical and analytical research.
- CM-Sepharose Fast Flow– applied for purification of recombinant proteins expressed in bacterial or yeast systems.
- AG50W-X8 resin– used for separation of inorganic cations and trace metal analysis in environmental samples.
- S-Sepharose resin– frequently utilized for large-scale purification of positively charged therapeutic proteins and enzymes etc.
5. Flash chromatography
Flash chromatography can define as a type of liquid chromatography technique used for rapid and efficient separation of compounds based on their polarity difference.
The method developed as an improvement over traditional column chromatography to reduce time and increase resolution.
In this technique, compressed gas pressure (usually air or nitrogen) is applied to push the mobile phase through the stationary phase at higher flow rate.
The stationary phase generally consist of silica gel packed in a column, while the mobile phase is a suitable solvent or mixture of solvents.
The compounds in sample are separated due to different interactions with stationary and mobile phase, where less polar compounds elute faster and more polar elute later.
Flash chromatography operate under moderate pressure (5–15 psi) which improve speed and efficiency of separation compared to gravity flow column.
The method often used in organic chemistry laboratories for purification of synthetic compounds or reaction mixtures.
It’s also applicable for isolation of natural products, peptides, and small organic molecules from complex mixtures.
The process monitored by UV detector or thin-layer chromatography (TLC) to track compound elution and fraction collection.
Therefore, flash chromatography considered as a fast, reproducible and convenient technique for compound purification in analytical and preparative scale work.
Steps of Flash Chromatography
- Column preparation done by packing the column tightly with silica gel or other suitable stationary phase, which is usually equilibrated with solvent before sample loading.
- Sample loading carried out by dissolving the crude mixture in small amount of solvent and then carefully applied on top of stationary phase.
- The column surface usually covered with thin layer of sand or cotton to protect disturbance during solvent flow.
- Selection of mobile phase is done according to polarity of compounds, commonly using solvents like hexane, ethyl acetate, methanol, etc.
- The elution process initiated by applying air or nitrogen pressure (about 5–15 psi) to force mobile phase move faster through column.
- Compounds get separated as they interact differently with stationary phase, depending upon their polarity and adsorption strength.
- Fractions are collected at regular intervals in separate tubes during the elution process.
- The progress of separation usually monitored by Thin Layer Chromatography (TLC) to check compound movement in solvent system.
- The required compound fractions then combined and solvent removed by rotary evaporator or vacuum drying.
- Finally, purified compound obtained which can further analyzed by spectroscopic or chromatographic techniques for confirmation.
Uses of Flash Chromatography
- Flash chromatography used mainly for purification of organic compounds obtained from synthesis reactions.
- It helps to isolate target compound from impurities, by exploiting polarity differences among mixture components.
- The technique applied in pharmaceutical and chemical industries for purification of intermediates or active drug molecules.
- It often used by researchers in natural product isolation from plant or microbial extracts for compound identification.
- Flash chromatography applied in peptide and amino acid purification, especially during synthesis or modification experiments.
- It’s also used for screening of reaction products to determine yield and purity in organic synthesis labs.
- The method allows quick separation of compounds prior to analysis by NMR, IR, or Mass spectrometry, making characterization easier.
- In biotechnology, it used for purification of metabolites, dyes, and small biomolecules etc.
- Flash chromatography commonly employed in analytical chemistry for fractionation of mixtures in preparative scale.
- Therefore, it’s considered as fast, efficient and low-cost purification technique suitable for both research and industrial purposes.
Examples of Flash Chromatography
- Silica gel column – commonly used for separation of organic compounds based on polarity differences in solvent system.
- Reverse phase flash chromatography – performed using C18 bonded silica for purification of non-polar or moderately polar compounds.
- Alumina-based flash chromatography – utilized when sample components are sensitive to acidic silica and require milder stationary phase.
- Normal phase flash system – used with solvent gradients like hexane/ethyl acetate for separating reaction mixtures in organic synthesis.
- Automated flash chromatography (Biotage / Isolera system) – used for high-throughput purification with real-time UV monitoring.
- Ion exchange flash chromatography – applied for purification of ionic compounds like amino acids or small peptides.
- Chiral flash chromatography – employed for resolution of racemic mixtures using chiral stationary phase.
- Gradient flash chromatography – where polarity of mobile phase gradually increased for better resolution of complex mixtures.
- Disposable cartridge flash columns – used in research labs for small-scale, fast purification of organic intermediates.
- Flash chromatography using reversed-phase media – helpful for purification of bioactive molecules, dyes, or pharmaceutical compounds etc.
6. Gas chromatography

Gas chromatography (GC) can define as a technique used for separation and analysis of volatile compounds present in a mixture.
In this method, the mobile phase is an inert gas like helium, nitrogen or hydrogen, which carries vaporized sample through the column.
The stationary phase is either a solid adsorbent or a liquid coated on solid support inside the column, where separation occur based on interaction of compounds with it.
The sample is injected in vapor form into the heated injection port where it immediately vaporizes and carried by gas flow.
Separation is achieved because different compounds travel through the column at different rates depending on their boiling point, polarity, and adsorption affinity.
The separated components detected by various detectors like Flame Ionization Detector (FID), Thermal Conductivity Detector (TCD), or Mass Spectrometer (MS).
The resulting signal is recorded as chromatogram, which shows peaks representing different compounds in mixture.
It is widely used in chemical, biochemical, food and environmental laboratories for identification and quantification of organic substances.
Gas chromatography considered as a precise, fast and sensitive technique for analyzing trace level volatile materials etc.
Principle of Gas Chromatography
- The principle of gas chromatography (GC) based on differential partitioning of components between a stationary phase and a mobile phase which is an inert carrier gas.
- The sample mixture is first vaporized and then carried by gas stream through the column containing stationary phase where separation occurs.
- The compounds in mixture distributed between two phases according to their volatility and affinity toward the stationary phase.
- Components with lower boiling points or weaker interaction with stationary phase elute faster, while high boiling compounds retained longer in column.
- The separation efficiency depend on factors like temperature of column, flow rate of carrier gas, type of stationary phase, and column length.
- The retention time (tᵣ) of each compound is specific under given condition and used for qualitative identification.
- Quantitative analysis performed by comparing peak areas on chromatogram proportional to concentration of analyte.
- Hence the principle rely on partition equilibrium between gaseous and stationary phase, which result in effective separation of volatile substances.
Steps of Gas Chromatography
- In first step, sample preparation is done where the mixture converted into gaseous state usually by heating or dissolving in volatile solvent.
- Then sample injection carried out by inserting small volume (1–2 µL) into injection port using micro syringe under high temperature to ensure complete vaporization.
- The carrier gas like helium, nitrogen or hydrogen used to carry vaporized sample through the chromatographic column.
- Inside the column, separation of components occur based on their distribution between stationary phase and mobile gas phase.
- Each compound travel at different speed because of difference in their boiling point, adsorption affinity, and molecular weight.
- The separated components come out of column at different time intervals called retention time (tᵣ).
- These eluted compounds detected by specific detectors like FID, TCD, or MS which generate electrical signal proportional to concentration.
- The signals recorded as chromatogram, displaying peaks that correspond to different compounds in the sample.
- The identification and quantification of compounds done by comparing retention time and peak area with those of standard compounds.
- After completion, column is purged and reconditioned with carrier gas to remove residues and prepare for next analysis.
Uses of Gas Chromatography
- Gas Chromatography (GC) widely used for separation and identification of volatile organic compounds present in mixtures.
- It applied in pharmaceutical industries for checking purity of drugs and detection of impurities during production.
- GC used in environmental analysis for detecting pollutants, pesticides, and hydrocarbons in air, soil and water samples.
- It used for food and flavor analysis to determine aroma compounds, preservatives, and additives in food products.
- In forensic science, GC applied for detection of alcohol, drugs, toxins or explosives in biological specimens or materials.
- It used in petroleum industry for characterization of hydrocarbons and monitoring of fuel composition.
- GC also used for clinical diagnosis, like determination of fatty acids, blood alcohol level, and metabolic disorders in biological fluids.
- It used in research laboratories for studying chemical reactions, product purity and structure elucidation of compounds.
- The technique applied in quality control and quality assurance of industrial processes for maintaining standardization.
- Hence, gas chromatography used as a reliable and sensitive analytical tool across chemical, biological, and environmental sciences etc.
Examples of Gas Chromatography
- Separation of hydrocarbons – used for analysis of gasoline and petroleum fractions for determining composition of alkanes, alkenes etc.
- Detection of blood alcohol – applied in forensic toxicology for quantitative measurement of ethanol in blood or breath sample.
- Food flavor analysis – used to identify volatile aroma compounds in fruits, coffee, spices and dairy products.
- Pesticide residue analysis – performed in agricultural and environmental samples to detect organophosphates and chlorinated hydrocarbons.
- Pharmaceutical purity testing – used to check impurities or degradation products in drug formulations.
- Gas mixture analysis – applied for determination of air composition like oxygen, nitrogen, CO₂, CH₄ and other gases.
- Essential oil profiling – used in cosmetic and herbal industries for characterizing terpenes and aromatic compounds.
- Metabolite study – used in clinical biochemistry for estimation of fatty acids, steroids and organic acids in body fluids.
- Explosive and fire residue identification – performed in forensic labs for tracing accelerants or volatile residues.
- Environmental pollutant monitoring – used for detecting volatile organic compounds (VOCs) in water and atmospheric samples etc.
7. Gel filtration chromatography/Size exclusion chromatography
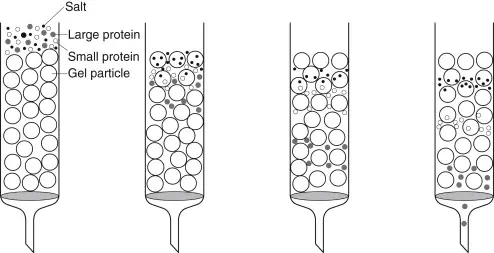
Gel filtration chromatography, also termed as Size exclusion chromatography (SEC), can define as a separation technique based on molecular size and shape of biomolecules in solution.
The method used mainly for purification of proteins, polysaccharides, enzymes and nucleic acids by difference in their molecular dimensions.
The column packed with porous beads made of materials like Sephadex, Sepharose, or Bio-Gel, which act as stationary phase.
When a sample mixture passed through column, the smaller molecules enter into the pores of beads and hence travel slowly, while large molecules excluded from pores move faster and elute first.
The separation therefore depend on the degree of penetration into pores, which is directly related to size of molecules in solution.
The mobile phase usually an aqueous buffer which help to maintain native structure of macromolecules during separation.
No chemical interaction occur between solute and stationary phase, so the process considered as gentle and non-destructive for biological molecules.
Gel filtration chromatography widely used for desalting, molecular weight determination, and removal of aggregates from protein samples.
Hence, it works on the simple principle of exclusion by size where molecular sieving effect used for effective separation of components in a mixture.
Principle of Gel Filtration Chromatography
- The principle of Gel Filtration Chromatography based on the separation of molecules according to their size and molecular weight while passing through a porous matrix.
- The column contains gel beads having pores of specific size range that act as a molecular sieve for separation of molecules in solution.
- When a mixture of molecules is passed through the column, large molecules are excluded from the pores and move faster, while smaller ones enter into the pores and move slowly through the matrix.
- Therefore, the molecules get separated according to their hydrodynamic volume, not by charge or affinity interaction.
- The elution order always follows from larger to smaller molecules since large molecules have shorter path and elute earlier.
- The mobile phase (usually buffer) helps to carry molecules without any binding or chemical interaction with stationary phase.
- The retention time of each molecule depends on its ability to enter the pores, which determined by molecular size relative to pore diameter.
- Hence, the basic principle rely on molecular sieving or exclusion effect, where only the physical dimension of molecules decides their separation in the column.
Steps of Gel Filtration Chromatography
- In the first step, column preparation is done where the column packed with suitable gel material like Sephadex, Sepharose, or Bio-Gel and equilibrated with buffer solution.
- The sample preparation performed by dissolving or diluting the biomolecules in same buffer used for equilibration to maintain uniform conditions.
- The prepared sample mixture carefully applied on top of the column without disturbing gel surface to avoid uneven flow.
- Then the elution process begins by passing buffer (mobile phase) through column, which help in movement of molecules through gel matrix.
- The molecules start separating depending on their molecular size, larger ones elute first while smaller molecules enter the pores and move slowly.
- Fractions collected sequentially from the outlet in test tubes or fraction collector as elution continues.
- Each fraction monitored by measuring absorbance at specific wavelength (like 280 nm) or by biochemical assay to identify the presence of target compound.
- The elution profile or chromatogram plotted showing absorbance versus elution volume to represent the separation pattern.
- The desired fraction pooled and concentrated if required for further analysis or purification.
- Finally, the column is washed and stored properly with preservative buffer to maintain stability of gel for next use.
Uses of Gel Filtration Chromatography
- Gel filtration chromatography used for separation of proteins mainly according to their molecular size and shape, larger molecules elute first while smaller ones penetrate deep into gel pores.
- It used for determining molecular weight of unknown proteins / polysaccharides, by comparing with standard molecules that already known.
- In purification process, enzyme mixtures or complex protein solutions are separated by this method for getting a single active form which is very useful in biochemical study.
- This technique often applied for desalting and buffer exchange, because small salts and buffer components get separated from the big protein molecules effectively.
- It used in removal of impurities or aggregates from biological samples, so a high purity sample can be obtained before analysis like electrophoresis or spectroscopy.
- Gel filtration also help to study protein conformational changes or aggregation behavior, since elution volume changes when molecules change shape or form complex.
- The method used in isolation of biomolecules like polysaccharides, nucleic acids (DNA, RNA), enzymes, hormones etc., depending upon their size exclusion properties.
- For fractionation of serum proteins and other body fluids, it used in clinical and diagnostic laboratories to understand disease related protein profile.
- In biotechnology industry, gel filtration employed for purification of antibodies, hormones, growth factors, etc., at various step of production.
- Sometimes it used as final polishing step after affinity or ion exchange chromatography to achieve more refined / homogeneous product.
- A very important use is removal of low molecular weight reagents (like phenol, ethanol, salts) after chemical modification of proteins, which otherwise disturb the assay.
- It also utilized in analysis of polymer distribution in synthetic polymer research, where molecular weight distribution of polymers are studied.
- Such as, in food industries gel filtration used to separate carbohydrates (mono-, di-, and oligosaccharides), vitamins, and pigments from crude extracts etc.
- Because no harsh chemical used, it very suitable for sensitive biomolecules which might be denatured by other separation techniques.
- In some cases, it applied for refolding of denatured proteins, because the gradual removal of denaturants during gel filtration helps proper folding.
Examples of Gel Filtration Chromatography
- Separation of two proteins (mixture of Albumin (~67 kDa) and Cytochrome C (~12.4 kDa)) was performed by gel filtration, the larger albumin eluted first and the smaller cytochrome later.
- Purification of recombinant human interferon-γ (IFN-γ) from inclusion bodies was achieved by a size‐exclusion column (urea-gradient gel filtration) in which aggregates were removed and monomeric protein collected.
- Use of gel filtration to remove salts / small molecules from a protein sample (desalting or buffer exchange) so that the protein sample could be transferred into a new buffer with minimal dilution.
- Separation of nucleic acid species (for example plasmid DNA, or phage DNA from RNA) by gel filtration on e.g., a Sephacryl S-1000 column; large DNA eluted ahead of smaller nucleic acid fragments.
- Isolation of virus particles (for instance influenza virus or recombinant viral antigen) from contaminating proteins using size‐exclusion chromatography (gel filtration) medium like Sephacryl S-1000 SF.
- Fractionation of polymer distribution in synthetic polymers: using size‐exclusion (gel permeation) related technique where gel filtration matrix separates high molecular weight vs low molecular weight polymer chains.
8. High-performance liquid chromatography (HPLC)

- HPLC can define as an analytical technique used to separate, identify, and quantify compounds in a liquid mixture.
- A liquid sample is injected into a stream of solvent (mobile phase) which flows through a column packed with solid particles (stationary phase) and the components are separated.
- The mobile phase is driven under high pressure so that separation is faster and resolution is higher compared with classical liquid chromatography, they improved the speed and efficiency.
- When the sample components travel through the column they interact differently with the stationary phase and mobile phase, so they emerge (elute) at different times (retention times) and are detected.
- The detector records a chromatogram, which shows peaks (each peak corresponds to a component) and the area of peak gives quantity roughly.
- Many types of stationary phases, mobile phases, and detectors are possible so HPLC is very versatile for analysing many kinds of compounds (organic, biological, ionic) etc.
- One active sentence: I have used HPLC for checking drug purity in my lab and it gave good separation of isomers.
- The technique it is widely used in pharmaceutical, environmental, food, clinical labs because non-volatile or thermo-sensitive molecules can be handled (unlike GC) etc.
- In HPLC method development the selection of mobile phase composition, flow rate, column length/diameter, particle size of stationary phase all matter and many trade-offs exist (speed vs resolution vs cost).
Principle of HPLC
- HPLC can define as a technique where a mobile phase (liquid solvent) is forced under high pressure through a column containing a stationary phase, and separation of components is effected by their different interactions with those phases.
- In this method the sample mixture is injected into mobile phase and flows into column where components are retarded by stationary phase depending on affinity, size, charge, polarity etc., so they elute at different times (retention times).
- The stationary phase is generally made of small porous particles (for example silica or polymer beads) which provide a large surface area and thus many interactions with sample molecules.
- The mobile phase composition and flow rate are controlled carefully because they influence how strongly components interact with stationary phase; thus resolution and speed of separation are affected.
- Separation mechanisms that operate in HPLC include adsorption, partitioning, ion-exchange, size exclusion (gel filtration) etc., depending on the column packing and mobile phase.
- Larger or less interactive molecules travel faster (elute earlier) while smaller or more strongly interacting molecules are retained longer, so peaks are recorded by detector as they exit column.
- A chromatogram is produced by detector, where each peak corresponds to one component; the area under peak relates to amount (quantity) of that component.
- Because high pressure is applied to push mobile phase and enable fine particle stationary phase, HPLC achieves higher resolution, faster run times, and better separation compared with traditional liquid chromatography.
Steps of HPLC
- Sample preparation is carried out (filtration, dilution, removal of particulates) so that the sample is compatible with system and column.
- Mobile phase (solvent mixture) is prepared and degassed / filtered, because any bubbles or particles can disturb the flow or detection.
- Stationary phase / column is chosen and conditioned (equilibrated) with mobile phase at the proper flow rate until a stable baseline is achieved.
- The sample is injected (manually or via autosampler) into the mobile phase stream so that it enters the column under high pressure and begins separation.
- Separation in the column occurs: components interact differently with stationary phase & mobile phase, causing different retention times.
- Detection / data collection is performed: when component elutes the detector records a signal (peak) and a chromatogram is produced.
- Data analysis is carried out: retention times are used for identification, peak areas/ heights are used for quantification.
Uses of HPLC
- In pharmaceutical industry HPLC is used for quality-control of drugs, checking the purity, the amount of active ingredient, and impurities.
- In clinical / medical labs HPLC is used to determine drug levels, detect vitamins (for example vitamin D in blood serum), monitor biomarkers and support diagnosis of disease.
- In food & beverage industry HPLC is applied for food safety, detecting additives, preservatives, pollutants, measuring nutrient composition and verifying authenticity of food / drink.
- In environmental monitoring HPLC is used for analysis of pollutants, water quality, trace-chemicals in samples from soil, air, water etc.
- In biotechnology and life-sciences HPLC is used for characterisation of proteins, peptides, nucleic acids, separation of biomolecules and research in bioanalytical fields.
- In chemical / polymer industry HPLC is used for analysis of synthetic polymers, molecular weight distribution, materials composition, and refining of chemical products.
- In forensic science HPLC is used for detection of illicit drugs, toxic substances, substances in body fluids for legal or medical-legal purposes.
Example of HPLC
- A pharmaceutical lab method was developed for a drug substance where HPLC was used to separate the active pharmaceutical ingredient (API) and its degradation products, and the method validation data were reported to show good resolution and accuracy.
- In an environmental study a sample-mixture of water contaminants was analysed by HPLC, the mobile phase and column conditions were selected so that low level pollutants were detected and quantified, the technique allowed sensitive measurement of trace compounds.
- A food-industry application was illustrated where HPLC was used for testing the purity and composition of beverage additives, the sample was injected and peaks corresponding to each additive were recorded, identification and quantification were done.
- In a biochemical research project HPLC was used to separate peptide isomers (or structural variants) and the researcher reported that HPLC gave sufficient resolution where other simpler methods failed, the system was optimized for that mixture.
9. Hydrophobic interaction chromatography
HIC can define as a chromatographic technique where molecules are separated on basis of their hydrophobicity (how “water-hating” their surfaces are) rather than only size or charge.
In this method a stationary phase that has hydrophobic ligands (for example phenyl, octyl, butyl groups) attached to a solid support (like silica or agarose) is used.
The sample is loaded onto the column under high salt (high ionic strength) conditions which cause proteins’ hydrophobic patches to be more exposed and thus they interact strongly with the hydrophobic ligands.
Elution is achieved by reducing the salt concentration (or changing buffer conditions) so that hydrophobic interactions are weakened and molecules come off the column in order of increasing hydrophobicity.
Because mild, mostly aqueous conditions are used, the biomolecules (like proteins) can retain their native structure and activity, so HIC is considered non-denaturing compared to many other hydrophobic separation techniques.
The retention strength (how long a molecule stays bound) depends on factors like salt type/concentration, ligand hydrophobicity, surface hydrophobic patches of molecule, temperature, and pH (though pH effect may be less).
HIC is particularly valuable for separating molecules which are difficult to separate by charge or size alone, for example variants of therapeutic antibodies where hydrophobic differences exist.
Principle of Hydrophobic Interaction Chromatography
- HIC can define as a chromatographic method where molecules are separated by their hydrophobicity (how “water-fearing” their surfaces are) rather than only by size or charge.
- A stationary phase is used which has hydrophobic ligands (like alkyl or aryl groups) attached to a solid support (for example silica or agarose) so that hydrophobic regions of molecules can bind.
- The sample is loaded onto the column under high salt (high ionic strength) conditions which cause hydrophobic patches on proteins or biomolecules to become more exposed, they interact with the hydrophobic ligands and thus retention occurs.
- Elution is achieved by reducing the salt concentration (or changing buffer conditions) so that hydrophobic interactions are weakened and molecules are released; molecules with less hydrophobicity elute earlier while more hydrophobic molecules elute later.
- Because mild aqueous conditions are used (versus harsh organic solvents), the biomolecules (especially proteins) can retain their native structure and activity, so HIC is considered non-denaturing.
- The strength of retention (how strongly a molecule sticks) depends on factors like salt type & concentration, ligand hydrophobicity, surface hydrophobic patches of molecule, temperature, and pH (though pH effect is smaller).
Steps of Hydrophobic Interaction Chromatography
- Column and media are prepared (equilibrated) with a high-salt buffer so that the stationary phase is ready for hydrophobic interactions.
- Sample is loaded into column under the high salt conditions so that hydrophobic patches of molecules are exposed and binding to hydrophobic ligands occurs.
- Unbound / weakly-bound species are washed out while stronger hydrophobic ones remain retained on the column.
- Elution step is carried out by lowering salt concentration (or modifying buffer) so that the hydrophobic interactions weaken and molecules elute in order of increasing hydrophobicity.
- Final wash (sometimes with very low salt or added mild organic modifier / detergent) is done to remove very tightly bound molecules and re-condition column for next run.
Uses of Hydrophobic Interaction Chromatography
- In biopharmaceutical production HIC is used for purification of proteins (for example monoclonal antibodies, mAbs) under mild and non-denaturing conditions so that biological activity is preserved.
- HIC is used for removal of aggregates / product variants in complex mixtures, because the more hydrophobic impurities or aggregates bind stronger, thus the target molecule can be separated.
- In analytical settings HIC is applied for characterisation of subtle protein variants (like different drug-to-antibody ratio, DAR, species in antibody-drug conjugates, or oxidative variants) by ranking them by hydrophobicity.
- In downstream processing HIC is used as a polishing step after other separations (size-exclusion, ion-exchange) because it gives orthogonal separation based on hydrophobicity and helps improve purity.
- HIC has also been used in virus clearance or removal in biologics manufacturing because viruses often have hydrophobic surfaces and can bind to HIC media differently from the desired molecule.
- In research work HIC is used for studying protein hydrophobicity / surface properties (for example to assess effect of mutation on hydrophobic patches, or to separate isoforms) since variation in hydrophobic surface influences retention.
10. Ion exchange chromatography
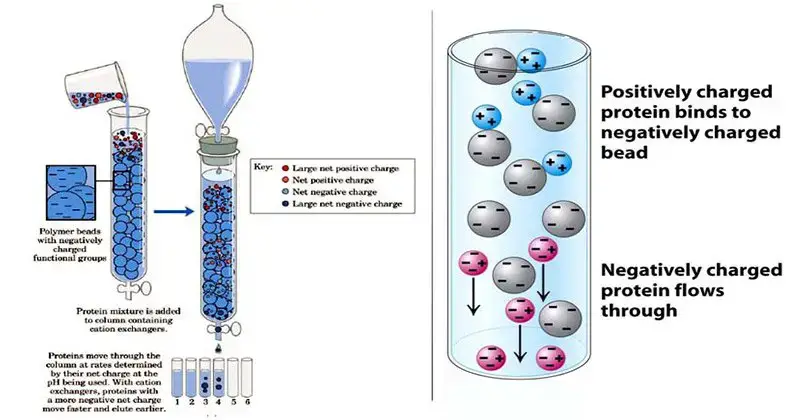
- Ion exchange chromatography refers as one of the most important techniques for separation of charged molecules / ions from mixtures.
- In this method, separation is done by the reversible exchange of ions between a solid stationary phase and a liquid mobile phase.
- The stationary phase mostly consist of ion-exchange resins, which are insoluble matrix (like polystyrene or cellulose) containing charged functional groups.
- Molecules are separated mainly based on their charge type and strength of interaction with the resin surface.
- There are two main kinds – Cation exchange and Anion exchange chromatography, they both work on same principle but opposite charges.
- In cation exchange, negatively charged resin attract positively charged molecules (cations), while in anion exchange, positively charged resin attract anions.
- The sample is usually loaded into the column, ions in sample get adsorbed on the resin, depending on their affinity and ionic strength.
- Then the molecules are eluted by changing salt concentration or pH of mobile phase – the stronger bound molecules elute later.
- It’s widely used for separation of proteins, peptides, amino acids, and nucleotides, etc.
- The technique is very sensitive, and separation can done even when concentration differences are small.
- Binding capacity depend by the type of resin, pH, ionic strength, and buffer conditions.
- Sometimes buffers like Tris-HCl, phosphate buffer, or acetate buffer are used (depending on the nature of analyte).
- The process is often followed by detection using UV spectrophotometer for absorbance measurement at 280 nm.
- The method is economical and easy to operate, but sometimes time-consuming and require careful control of pH / ionic strength.
- It’s applied in purification of enzymes, antibodies, and also in water softening / desalination process.
- In analytical biochemistry, ion-exchange chromatography plays a sturdy and hardy role in study of protein charge heterogeneity.
- The technique sometimes also called as ion-chromatography (IC) though it’s a broader version for small ions analysis.
- Such as, in water quality testing, IC used to determine Cl⁻, NO₃⁻, SO₄²⁻ etc.
- Through exchange of ions, charged species get separated, that’s how the purity of biomolecule prevail from impurities.
- Overall, it’s a charge-based separation method, reliable, precise (but little tedious sometimes).
Principle of Ion Exchange Chromatography
- The technique is based on reversible exchange of ions between a mobile liquid phase and a charged stationary phase (resin).
- A stationary phase is prepared that carries fixed charged functional groups (for example, negatively-charged groups for cation-exchange, positively-charged groups for anion-exchange).
- When sample containing charged molecules (ions or ionizable biomolecules) passes through the column, those molecules with opposite charge to the resin groups are adsorbed/retained on the resin by electrostatic forces.
- Molecules that are neutral or carry same charge as the resin will pass through more quickly (elute earlier) because they have weaker or no binding.
- The strength of interaction (hence retention) depends on several factors: the magnitude of charge of analyte, ionic strength of mobile phase (salt concentration), pH of buffer (which affects ionisation state) etc.
- Elution of bound molecules is achieved by one or both of: increasing ionic strength (introducing more competing ions) or altering pH so analyte charge changes and loses affinity to resin.
- In effect, separation is achieved because different molecules will have different charges (or different strength of binding) and thus will elute at different times under the elution conditions.
- Two major types are defined: cation-exchange where the resin is negatively charged and binds cations, and anion-exchange where resin is positively charged and binds anions.
- For proteins (for example), the net charge at given pH (relative to their isoelectric point, pI) will determine whether they bind and hence the principle is exploited for protein purification.
Steps of Ion Exchange Chromatography
- Column packing is done first, where the resin (ion-exchange resin) is placed into a column bed and the bed is settled, buffer flow is started.
- Equilibration of the column is carried out by passing the equilibration buffer so that the resin environment is stabilised, and baseline is achieved (Such as, 5-10 column volumes).
- The sample is prepared (filtered/centrifuged) and the buffer pH/ionic strength is adjusted to match binding conditions, then the sample is loaded onto the column in the state matching the binding buffer.
- Unbound or weakly bound molecules are removed by washing the column with the same buffer or a low-salt buffer to purge non-desired entities.
- Bound molecules are eluted by changing mobile phase conditions — either increasing salt concentration (ionic strength) or altering pH – a gradient may be used to make separation finer.
- Fractions are collected as the eluate emerges and the fractions are analysed to determine which ones contain the target molecule.
- After elution the column is often re-equilibrated (washed with equilibration buffer) to prepare for next run.
Uses of Ion Exchange Chromatography
- IEX is widely used for purification of biomolecules, especially proteins, peptides, nucleic acids, etc., based on their charge properties.
- In biotechnology/bioprocessing the technique is applied to isolate enzymes, antibodies, recombinant growth-factors, and other charged molecules, it offers high capacity and scalability.
- In pharmaceutical industry the method is employed for separation and analysis of active pharmaceutical ingredients (APIs), impurities, excipients and for quality control of drug formulations.
- Environmental/analytical uses are found in water and soil testing: small ions (anions/cations) are separated and quantified (for example Cl⁻, NO₃⁻, SO₄²⁻ etc) in drinking water or industrial effluents.
- In food & beverage industry IEX is used for analysing charged species (salts, acids, sugars) and monitoring quality of raw materials or products.
- Industrial chemical processes exploit IEX for metal ion separation/concentration, removing unwanted ions, for water softening / demineralisation and large-scale purification of ionic substances.
- In research labs the method is used for resolving heterogeneity of biomolecules (like charge variants of a protein) and for preparative scale separation when other methods fail to give enough resolution.
Examples of Ion Exchange Chromatography
- In protein purification, a strong cation-exchanger resin was used to isolate human C-peptide from ballast proteins and peptides, where the analyte was purified by IEX then further analysed by LC-MS/MS.
- In water quality testing IEX was applied to separate small anions (like Cl⁻, NO₃⁻, SO₄²⁻) and cations from drinking water or effluents for monitoring purposes.
- In pharmaceutical industry IEX was used for separation and purification of active pharmaceutical ingredients (APIs), intermediates and impurities in drug production.
- In food & beverage industry IEX (or ion-chromatography broadly) was used to analyse charged species (acids, sugars, anions/cations) in beverages, raw materials etc.
- In biotechnology downstream-processing IEX was used to isolate enzymes, antibodies, recombinant growth-factors and other charged biomolecules from complicated mixtures.
11. Liquid chromatography
- Liquid Chromatography (LC) refers as a technique where mixture components are separated by passing a liquid mobile phase through a stationary phase.
- A sample solution is introduced into the mobile phase and it moves by or through a column or bed which holds the stationary phase.
- The stationary phase is often a solid material (for example silica particles) or a solid support with bonded groups, and the mobile phase is a solvent or solvent-mixture that carries the analytes.
- Separation is achieved because different analytes interact differently with the stationary vs mobile phase — those with stronger affinity to stationary phase will elute later, while those with weaker affinity elute earlier.
- The technique is widely used for identification, quantification, and purification of components in complex mixtures (for example in pharmaceuticals, environmental, food sciences).
- Variants of LC include high performance liquid chromatography (HPLC) where higher pressures / finer particles are used to get better resolution.
- The term “liquid” means the mobile phase is a liquid (not gas). So retention times, solvent composition, flow rates etc matter in separation.
- The process is controlled by parameters like flow-rate of mobile phase, composition of mobile phase (solvent mixture, pH, ionic strength), temperature etc which influence how strongly components bind to stationary phase.
Principle of Liquid Chromatography
- Liquid chromatography can define as a separation technique where mixture components are carried by a liquid mobile phase and are separated by differential interactions with a stationary phase.
- The mobile phase flows through or over the stationary phase (for example in a column) and components of the mixture are retained or moved at different rates depending on their affinity to each phase.
- Components that have stronger interaction (adsorption or partitioning) with the stationary phase will elute later, while those having weaker interaction move faster and elute earlier.
- The separation is influenced by factors like polarity, charge, size of analytes, and by the nature of mobile phase (solvent composition, pH) and stationary phase (material, particle size) and flow-rate.
- In more advanced forms like High‑Performance Liquid Chromatography (HPLC) high pressure is applied so that finer particles and better resolution are achieved (which means sharper separation and shorter time) .
- The principle therefore comes down to difference in retention time of different components because of their varying interaction with mobile vs stationary phase, which leads to their separation.
Steps of Liquid Chromatography
- Sample preparation is done, where the sample is filtered or diluted (to remove particulates) and then dissolved in a suitable solvent.
- Mobile phase (the solvent or solvent-mixture) is prepared and degassed if needed to remove bubbles, then the system is equilibrated with that mobile phase.
- The stationary phase is packed or selected (for example a column with solid support) and the mobile phase is made to flow through it so that equilibrium conditions are reached.
- The prepared sample is injected into the mobile phase stream (via injector or autosampler) and carried into the column.
- Separation is achieved while the mobile phase passes through the column, where different components interact differently with the stationary phase and thus elute at different times (retention times).
- As the components exit the column they are detected by a detector (for example UV-vis detector) and the data is collected (chromatogram is generated).
- After the run the column (and system) is cleaned or re-equilibrated so that next sample run can be done reliably.
Uses of Liquid Chromatography
- It is used for pharmaceutical industry to check purity of drugs and to monitor active ingredients in formulations, quality control is carried out by this method.
- In environmental analysis the technique is applied to analyse water/soil/air samples for pollutants, pesticide residues, herbicides etc.
- Food & beverage industry uses LC for verifying ingredient contents and detecting unwanted substances (pesticides, additives) in raw materials and finished products.
- In forensic science LC is used for detection of illicit drugs, metabolites in biological fluids (blood/urine) because it’s sensitive and reliable.
- Biotechnology and life-science research uses the method for analysing biomolecules like peptides, proteins, nucleic acids, metabolites in complex mixtures.
- Industrial/chemical manufacturing uses LC for process monitoring, polymer/additives analysis, checking of petrochemical products etc.
Examples of Liquid Chromatography
- In pharmaceutical development a drug formulation was analysed by LC to separate and quantify active ingredient, metabolite and impurity peaks.
- In environmental monitoring water sample was processed by LC for detection of pollutants, pesticide residues and other small organic molecules in trace amounts.
- In food safety testing LC was used for detection of veterinary drug residues in meat or pesticide in vegetables, or for checking additives in beverages.
- In forensic toxicology LC coupled with mass spectrometry (LC-MS) was used to detect illicit drugs, metabolites in blood/urine samples for doping or crime investigations.
- In petrochemical / polymer industry LC was applied for polymer / copolymer analysis, surfactant/additive quantification and process monitoring of chemical products.
12. Paper chromatography

- Paper chromatography can define as a method where a sample mixture is separated on a sheet of paper (stationary phase) by a moving liquid (mobile phase) that carries components at different rates.
- A strip or sheet of filter paper (often cellulose) is used, and the paper is sometimes humid so that a layer of water is the actual stationary phase, they act as partition medium.
- The mobile solvent is allowed to travel by capillary action up (or down) the paper carrying sample components; different components move different distances because they differ in solubility / affinity to paper vs solvent.
- The separation is based on partitioning (or adsorption) between the stationary water/paper and the mobile solvent, so that more soluble components in the solvent will travel further while strongly retained ones will lag behind.
- A small drop or “spot” of sample is applied near one edge of the paper, then the paper is placed so that edge dips in the solvent – the solvent front moves and a chromatogram of separated spots is formed.
- The method is inexpensive and uses very small amounts of material, and it’s often used for qualitative analysis (for example inks, dyes, pigments) though it has been largely replaced by TLC for many lab uses.
- The measurement of retention is often done by calculating Rₓ (R_f) value: distance moved by substance divided by distance moved by solvent front.
Principle of Paper Chromatography
- Paper chromatography can define as a technique where a mixture is separated by differential movement of its components by a mobile phase through a stationary phase made of paper.
- The stationary phase is the paper (often made of cellulose) which holds water or solvent-film in its pores and the mobile phase is a suitable solvent or mixture that moves over / through the paper by capillary action.
- Separation is achieved because different components of the mixture have different affinities for the stationary phase (paper/water film) and the mobile phase (solvent) and thus they migrate at different rates.
- In many cases the underlying mechanism is partition chromatography (components distribute between stationary liquid film and mobile liquid) or adsorption chromatography (components stick differently onto the paper surface), sometimes both are involved.
- The distance travelled by each component relative to the distance travelled by solvent front gives a value called RfR_fRf (retardation factor) which helps identification of substances.
- Because the mobile phase moves by capillary action in paper the solvent front travels up (or down) the paper and components that are more soluble in mobile phase / less retained by stationary phase will move further.
- The principle therefore depends on the balance of solubility, adsorption/affinity, and capillary movement – components that are highly soluble and low affinity to paper go fast, others go slow and separation results.
Steps of Paper Chromatography
- A piece of filter paper (or chromatography paper) is cut to suitable size and a base-line is drawn in pencil about 1-2 cm from one end.
- The sample mixture is spotted (a small dot/line) on the base-line using a capillary tube or micropipette, and it’s allowed to dry.
- A suitable mobile phase (solvent or solvent-mixture) is prepared in the container, ensuring the solvent level is below the base-line so the spot is not submerged.
- The paper is placed (usually vertically or folded-cylinder) such that the base end touches the solvent and the spot remains above the solvent line, then the solvent is allowed to move by capillary action up the paper.
- The solvent front is allowed to travel until a suitable height (for example 2/3 of paper) and then the paper is removed from the solvent and allowed to dry.
- The separated spots (components) are visualised (if needed by spraying reagent) and their distances from base-line and solvent front are measured to calculate RfR_fRf values for identification.
- The paper may be preserved or analysed further for documentation or quantification of the separated components.
Uses of Paper Chromatography
- It is used for qualitative analysis of mixture components, where the very small amounts of sample are required and separation of dyes, pigments, amino acids etc is possible.
- In food & beverage industry the method is employed to check food-colours, additives and contaminants (for example in sweets, jams, drinks) for quality/control purposes.
- In pharmaceutical field it is applied to check purity of drugs, detect impurities, monitor reaction mixtures and early-stage drug development.
- Forensic science uses the technique to analyse inks, dyes, trace compounds from crime scenes, and link evidence by comparing separated spots.
- In environmental monitoring the method is used for detection of pollutants, heavy metals, contaminants in water / soil samples, where other more complex methods might not be needed.
- In biochemistry and life-sciences the technique is used to separate biomolecules like amino acids, sugars, etc from mixture for identification or study of metabolic pathways.
Examples of Paper Chromatography
- A food dye mixture from sweets was analysed by paper chromatography and separate coloured spots were visualised, and it’s used to check allowed vs non-permitted colourants.
- A sample of amino acids from a hydrolysate was separated on paper (cellulose paper) and the individual amino acid spots were identified by their RfR_fRf values.
- Ink from a pen was subjected to paper chromatography in forensic analysis and the different dye components were matched to known inks for evidence linking.
- A water sample from lake was processed by paper chromatography to check for organic acid / contaminant components in the environment.
- A pharmaceutical reaction mixture was checked by paper chromatography to identify impurity peaks and confirm presence of active compound, early stage QC.
13. Reverse-phase chromatography
- Reverse-phase chromatography (RPC) can define as a mode of liquid chromatography where a non-polar (hydrophobic) stationary phase and a polar mobile phase are used for separation of compounds.
- In this technique the mobile phase is usually water or aqueous buffer mixed with organic solvent (like methanol or acetonitrile) and the stationary phase is often silica particles bonded with long hydrocarbon chains (for example C18, C8) so hydrophobic interactions dominate.
- The basis of separation is that more hydrophobic analytes (molecules) are retained longer because they interact strongly with the hydrophobic stationary phase, while more polar molecules remain in the mobile phase and elute earlier.
- The retention strength is influenced by factors like mobile phase composition (ratio of water : organic solvent), pH of buffer (which affect ionisation of analyte), and stationary phase ligand length/density (for example C18 vs C8) which control hydrophobic binding.
- The method is basically the “reverse” of normal-phase chromatography where the stationary phase is polar and mobile phase is nonpolar; here polarity roles are inverted so the term “reverse-phase” is used.
- Because of its robustness and versatility, RPC is widely used in analytical chemistry for separation of organic compounds, peptides/proteins, pharmaceuticals etc., and they often use gradient elution (changing mobile phase composition over time) to improve resolution.
Principle of Reverse-Phase Chromatography
- Reverse-phase chromatography can define as a type of liquid chromatography where a non-polar (hydrophobic) stationary phase is used together with a polar mobile phase, and separation is achieved by hydrophobic interactions.
- In this mode the stationary phase is typically silica particles bonded to long alkyl chains (for example C18, C8) making it hydrophobic, and the mobile phase is a mixture of water (or aqueous buffer) + organic solvent (like methanol or acetonitrile) — so the system is inverted compared with normal-phase chromatography.
- The separation principle rests on the fact that more hydrophobic analytes have stronger affinity to the hydrophobic stationary phase, thus they are retained longer, while more polar analytes stay in the mobile phase more and elute earlier.
- The retention time (time an analyte stays on the column) is influenced by mobile phase composition (higher organic solvent content usually decreases retention of hydrophobic analytes), stationary phase ligand length/density, and analyte hydrophobicity/ionisation.
- Gradient elution is often used (where mobile phase organic solvent proportion is increased during run) to help elute strongly retained hydrophobic compounds and improve resolution of complex mixtures.
Steps of Reverse-Phase Chromatography
- A stationary phase (often silica particles bonded with hydrophobic ligands like C18 or C8) is packed into a column and the column is conditioned.
- The mobile phase (a polar solvent mixture, for example water + organic solvent like acetonitrile or methanol) is prepared (including degassing/filtration) and the system is equilibrated with this mobile phase.
- The sample mixture is injected into the mobile phase flow and it is carried into the column under controlled flow/pressure.
- While the mobile phase passes, analytes interact with the hydrophobic stationary phase: more hydrophobic compounds are retained strongly, polar ones pass through faster.
- Elution is done by altering the mobile phase composition (often by increasing organic solvent concentration i.e., a gradient) so that the retained compounds are released in order of hydrophobicity.
- The eluted compounds are detected (for example by UV-vis detector or mass spectrometer) and the data (chromatogram) is collected.
- After the run the column is cleaned (flushed with strong solvent) and re-equilibrated with initial mobile phase so that next run can be done reliably.
Uses of Reverse-Phase Chromatography
- It is used in pharmaceutical analysis where drug compounds, metabolites and impurities are separated with good resolution and reliability.
- In biotechnology / proteomics the technique is applied for separation of peptides, proteins and biomolecules from complex mixtures, by exploiting hydrophobic interactions.
- For environmental testing RPC is used to analyse organic pollutants, small molecules in water / soil / air samples because non-polar compounds are well retained and separated.
- In food & beverage chemistry the method is used for checking additives, contaminants, vitamins, flavour molecules, etc. in complex food matrices.
- In clinical / forensic science the method is used for detection of trace drugs, metabolites, biomarkers in biological fluids (blood/urine) where high sensitivity and specificity are required.
- In industrial / chemical manufacturing RPC is used for process monitoring, purity check of chemical intermediates, and quality control of non-polar compounds in production streams.
Examples of Reverse-Phase Chromatography
- In pharmaceutical analysis a drug mixture was separated by RPC (using a C18 column and water/acetonitrile mobile phase) to identify active compound, metabolite and impurity peaks.
- In food & beverage chemistry RPC was employed to separate flavour compounds in wine/coffee and to differentiate natural vs synthetic additives in dairy etc.
- In environmental monitoring RPC was used for detection of organic pollutants in water / soil samples, especially hydrophobic contaminants that are retained strongly.
- In biotechnology / proteomics RPC was applied for peptides / proteins separation by their hydrophobicity using water + organic solvent gradients for elution.
- In chemical / industrial manufacturing RPC was used for process monitoring of non-polar chemical intermediates and quality control by separating compounds with different hydrophobic characters.
14. Thin-layer chromatography (TLC)
- Thin-layer chromatography can define as a separation technique where a mixture is applied onto a flat support (glass/metal/plastic) coated with a thin layer of solid adsorbent (such as silica gel or alumina) and a solvent (mobile phase) is allowed to move by capillary action over that layer.
- The stationary phase is the thin layer of adsorbent and the mobile phase is a liquid solvent or mixture of solvents that moves over the layer; differences in how much each component of the mixture sticks to the stationary phase vs dissolves in the mobile phase cause separation.
- During development the compounds in the mixture move at different speeds: those with greater affinity for mobile phase travel further, while those with stronger adsorption to stationary phase remain nearer the origin, and thus they are separated on the plate.
- After the run the distances travelled by the compounds are measured and a retention-factor (Rₓ or R_f) value is calculated (distance travelled by component ÷ distance travelled by solvent front) which helps in identification.
- Because TLC is quick, low cost and simple it is widely used for checking mixture composition, monitoring reaction progress and early stage separations of small amounts of material.
Principle of Thin-Layer Chromatography (TLC)
- Thin-Layer Chromatography can define as a method where a stationary phase (thin layer of adsorbent like silica gel / alumina) is coated on a plate and a mobile phase (liquid solvent or mixture) moves over it, causing separation of mixture components.
- The separation is achieved because different compounds have different affinities for the stationary phase vs the mobile phase and thus they travel at different rates up the plate by capillary action.
- The compounds that are more strongly adsorbed by the stationary phase move slowly (stay nearer the origin) while those more soluble in the mobile phase move further (towards solvent front) thus giving distinct spots.
- A retention factor (R_f) value is used to quantify the movement: R_f = (distance travelled by component) ÷ (distance travelled by solvent front) and this helps in identification of compounds under same conditions.
- The process is fundamentally based on adsorption / partition phenomena (or a mix of both) depending on nature of adsorbent, solvent and analyte — so the stationary phase and mobile phase competition determine separation.
Steps of Thin-Layer Chromatography (TLC)
- A TLC plate (glass, plastic or metal sheet coated with a thin adsorbent layer) is selected and a baseline is drawn about 0.5-1 cm from the bottom using pencil.
- The sample solution is prepared (for example ~1 mg in 1 mL volatile solvent) and small spots are applied on the baseline using a capillary tube, and allowed to dry.
- A TLC development chamber is prepared by pouring few cm depth of mobile phase solvent into a beaker or jar and lining with filter paper to saturate the vapour phase.
- The spotted plate is placed vertically in the chamber so that the baseline is above the solvent level and the mobile phase rises up the plate by capillary action.
- The solvent front is allowed to move until a suitable distance (e.g., 2/3 or until near top) then the plate is removed and the solvent front is marked immediately.
- The plate is dried and the separated spots are visualised (for example under UV-light or by staining reagent) and the R_f values are calculated (distance travelled by component ÷ distance travelled by solvent front).
- The results are interpreted for number of components, purity of sample or reaction progress.
Uses of Thin-Layer Chromatography (TLC)
- It is used for monitoring reaction progress, for example to check if starting material has disappeared or product formed, by comparing spots on a plate.
- It is employed for identifying compounds present in a mixture, by comparing their Rₓ or R₍f₎ values with known standards.
- It is used for determining purity of a substance, because a single spot suggests high purity though multiple spots indicate impurities or mixture.
- In pharmaceutical industry it is applied for detecting impurities in drugs or formulations and for separation of multicomponent formulations.
- In food & beverage / cosmetic industry it is used for checking colours, preservatives, sweetening agents and quality of products by separating and identifying additives etc.
- In environmental & forensic analysis the technique is used for detection of pollutants / insecticides / dyes from water, soil, plants or forensic samples.
Examples of Thin-Layer Chromatography (TLC)
- A mixture of analgesics (for example Ibuprofen, Aspirin, Acetaminophen) was separated on a TLC plate (silica gel) under UV light and distinct spots were observed with different Rₓ or R_f values.
- A plant extract (for example from leaves) was analysed by TLC to detect the presence of antioxidants (by spraying with DPPH reagent which changes colour) and differentiation of metabolites was obtained.
- In food testing a sample containing dyes/preservatives was run by TLC and the separated spots were used to identify which additives were present and their relative mobility.
- In environmental/forensic work a water or soil extract was processed by TLC to check for pesticide residues or organic pollutants and the separated spots helped in screening the contaminant presence.
- In biochemical separation a lipid mixture (for example fatty acids / ceramides) was separated by TLC (thin layer of adsorbent) and the different lipids were visualised by appropriate staining, thus isolation of components was achieved.
FAQ
What is chromatography?
Chromatography is a separation technique used to separate and analyze the components of a mixture based on their differential interactions with a mobile phase and a stationary phase.
What are the different types of chromatography?
There are several types of chromatography, including:
Gas chromatography (GC)
Liquid chromatography (LC)
Thin-layer chromatography (TLC)
High-performance liquid chromatography (HPLC)
Ion exchange chromatography
Reverse-phase chromatography
Affinity chromatography
Size exclusion chromatography
Chiral chromatography
What is the difference between gas chromatography and liquid chromatography?
Gas chromatography involves the separation of volatile compounds using a gaseous mobile phase, while liquid chromatography separates compounds using a liquid mobile phase.
What is thin-layer chromatography (TLC)?
Thin-layer chromatography is a type of chromatography where a thin layer of stationary phase is applied to a solid support (e.g., a plate), and separation occurs through differential interactions between the components of a mixture and the mobile phase.
What is high-performance liquid chromatography (HPLC)?
High-performance liquid chromatography is a liquid chromatography technique that utilizes high-pressure pumps to improve separation efficiency and speed. It is commonly used for the analysis of various compounds in fields such as pharmaceuticals, environmental analysis, and food science.
What is ion exchange chromatography?
Ion exchange chromatography is a technique that separates charged molecules based on their interactions with an ion-exchange resin. It involves the exchange of ions between the mobile phase and the resin to achieve separation.
What is reverse-phase chromatography?
Reverse-phase chromatography is a liquid chromatography technique that relies on hydrophobic interactions between the stationary phase (typically a hydrophobic material) and the analytes in the mobile phase. It is commonly used for the separation of nonpolar and moderately polar compounds.
What is affinity chromatography?
Affinity chromatography is a technique used to separate and purify biomolecules based on their specific interactions with ligands or affinity tags immobilized on the stationary phase. It is often employed for the isolation of proteins, enzymes, and antibodies.
What is size exclusion chromatography?
Size exclusion chromatography, also known as gel filtration chromatography, separates molecules based on their size or molecular weight. It utilizes a porous stationary phase through which smaller molecules can penetrate more easily, resulting in differential elution times.
What are the applications of chromatography?
Chromatography has diverse applications across various fields, including pharmaceutical analysis, environmental monitoring, food analysis, forensic science, biochemistry, and research in areas such as proteomics and metabolomics. It is used for compound identification, quantification, purification, and separation of complex mixtures.
- Wilson, K., Walker, J. (2018). Principles and Techniques of Biochemistry and Molecular Biology (8 eds.). Cambridge University Press: New York.
- Ó’Fágáin, C., Cummins, P. M., & O’Connor, B. F. (2017). Gel-Filtration Chromatography. Methods in molecular biology (Clifton, N.J.), 1485, 15–25. https://doi.org/10.1007/978-1-4939-6412-3_2
https://microbenotes.com/types-of-chromatography/