What is Two-Dimensional (Crossed) lmmunoelectrophoresis?
- The technique called two-dimensional (crossed) immunoelectrophoresis (2-D IEP) was developed for the analysis and quantitation of mixtures of proteins (or antigens) in a complex sample.
- In the first dimension a protein mixture is loaded onto an agarose gel and electrophoresed so that the proteins are separated by their mobility/charge (and to some extent size) under native conditions.
- After the first run the gel strip (or the separated proteins) is then placed onto or into a second agarose gel layer that contains a predetermined amount of antibody (antisera) against the proteins/antigens.
- In the second dimension electrophoresis is applied at right-angles (perpendicular) to the first dimension, so that the separated antigen bands migrate into the antibody-containing gel and precipitin arcs (or “bell-shaped” precipitates) form where antigen-antibody reaction occurs.
- The result is a 2-D pattern (often called an immunoelectropherogram) in which each arc corresponds to a particular antigen in the mixture, the position and size of the arc being related to the mobility of the antigen and the amount present (and antibody concentration) in the gel.
- Because the proteins were separated first and then immunologically reacted, this method gives higher resolution than simple one-dimensional immunoelectrophoresis (or diffusion methods) and allows relative quantitation of antigens in one assay.
- The technique is especially useful for studying antigen heterogeneity, isoforms, fragments, complexes (association/dissociation phenomena), and polymorphisms in protein samples like serum, tissue extracts, microbial homogenates, etc.
- It is, however, somewhat more labour-intensive than simpler immunoassays and requires good antisera, carefully controlled electrophoresis and interpretation of the precipitation patterns (which may be complex).
Principle of Two-Dimensional lmmunoelectrophoresis
The principle of two-dimensional (crossed) immunoelectrophoresis (2-D IEP) involves a two-step electrophoretic separation that permits antigen mixtures to be analysed and quantitated.
In the first dimension the antigen (protein) mixture is loaded on an agarose gel and it is separated by electrophoresis by charge and mobility (and sometimes size) under nearly native conditions.
After that separation a strip of the gel (or the separated bands) is transferred/placed into a second gel layer which already contains a known amount of antibody (antisera) distributed in the agarose.
The second dimension electrophoresis is applied at right-angles (90°) to the first separation so that the separated antigens migrate into the antibody-containing gel and precipitation of antigen-antibody complexes occurs while they move.
The precipitates often appear as characteristic “bell-shaped” or arc-shaped zones whose position is a function of the antigen’s first-dimension mobility and second-dimension migration as well as the amount of antigen vs antibody.
Because of that combination of separations the method gives high resolution of antigenic species (including isoforms, fragments, complexes) and allows semi-quantitative or quantitative estimation of components in the mixture.
The method thus relies on electrophoresis of proteins, followed by immuno-precipitation under electrophoretic drive (not just diffusion), enabling sharper discrimination and measurement of multiple antigens in a single assay.
first dimension separation → second dimension movement into antibody gel → formation of precipitin zones whose geometry/size reflect antigen amount and identity.
Reagents and Equipment
Reagents–
- A suitable electrophoresis buffer (for example 0.03 M barbitone buffer, pH ~8.4) is required for the separation.
- A molten agarose gel (for example 1% agarose dissolved in electrophoresis buffer) is needed for gel casting.
- The gel quality reagent (low‐electroendosmosis agarose) is preferred, as proteins migration is influenced by gel matrix.
- A reference sample (for example bovine serum) and test antigen mixture are required for loading in the first dimension.
- A defined antiserum (antibody mixture) must be incorporated in the second‐dimension gel or supplied to the assay to react with separated antigens.
- A protein stain (for example 0.1% Coomassie Brilliant Blue in 50% methanol + 10% acetic acid) is used to visualise the precipitin arcs
Equipment–
- A flat‐bed electrophoresis unit with cooling plate is required for the gel runs.
- Glass plates (for example 5 x 5 cm size, thickness 1.0‐2.0 mm) are needed to cast the agarose gel.
- Wicks or filter‐paper soaked in buffer to act as electrode contacts (electrode wicks) are used in the apparatus.
- A microsyringe or pipette for sample application and a levelling plate for gel casting to ensure uniform thickness are needed.
- Gel well‐puncher / well cutter to make sample wells in the agarose is required.
- Additional accessories like buffer reservoirs, power supply, temperature control (for example maintaining gel at ~55 °C while pouring) may be used.
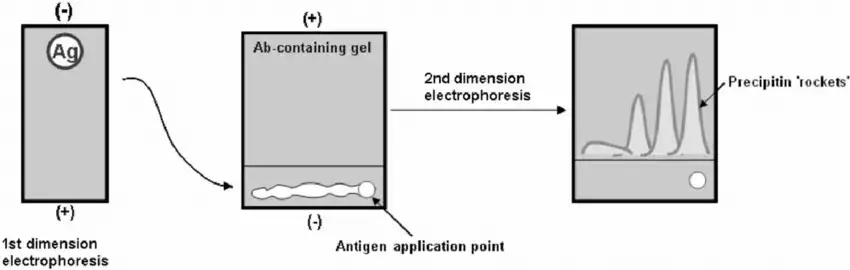
Protocol/Procedure of Two-Dimensional lmmunoelectrophoresis
- Prepare agarose gel (for first dimension) by dissolving say 1% agarose in electrophoresis buffer (for example barbitone buffer pH ~8.4) and pour between glass plates, allow to solidify.
- Sample (protein/antigen mixture) is applied in a trough or wells in the first-dimension gel, a reference sample may also be loaded.
- First-dimension electrophoresis is run under native conditions (for example at a given voltage for a set time) so that the mixture is separated by charge and mobility.
- After first run a strip of the gel containing the separated proteins is cut out carefully (or the gel plate is prepared for transfer) to move to second dimension.
- Meanwhile a second agarose gel layer (or same plate prepared) is made which contains a known amount of specific antiserum (antibody) embedded in the gel matrix.
- The gel‐strip (from first dimension) is placed on the antibody‐gel such that the direction of migration will be at right angles (90°) to the first dimension.
- Second dimension electrophoresis is applied, the separated antigens migrate into the antibody gel and precipitation of antigen-antibody complexes is allowed/induced while electrophoresis proceeds.
- After electrophoresis completes the gel is washed, dried and stained (for example Coomassie or another protein stain) so that the precipitin arcs or “bell-shaped” zones are visualised.
- The positions, shapes and sizes of these precipitation arcs are interpreted: each arc corresponds to an antigen in the mixture, the size relates to amount of antigen and antibody concentration, mobility relates to first and second dimension separation.
- Quantitation or semi-quantitation is done by comparing the arcs of sample to those of reference antigens or standards.
- Record results, analyse heterogeneity or isoforms of antigens, note any extra arcs (fragments/complexes) which show up due to high resolution of this method.
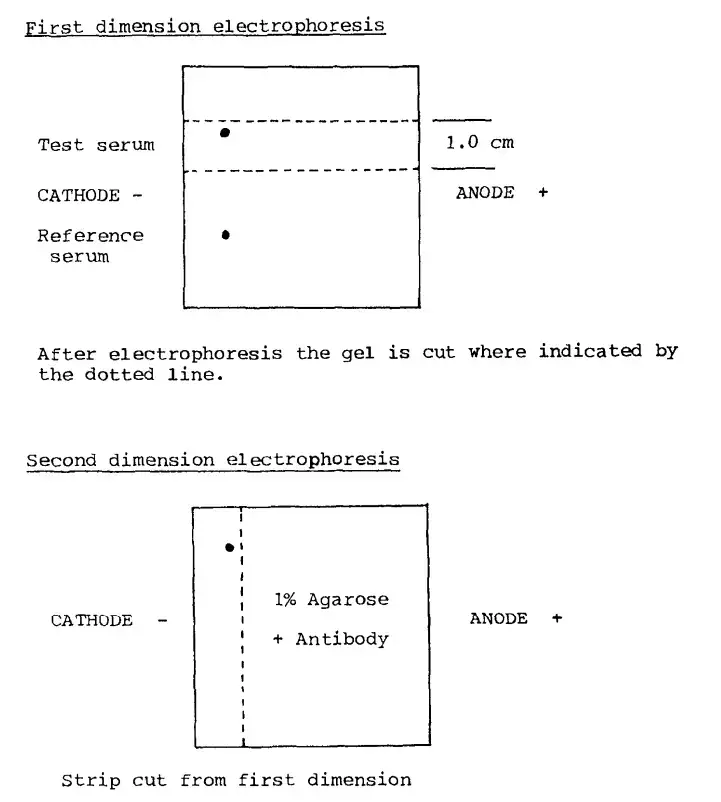
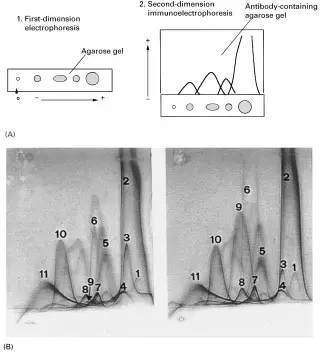
Applications of Two-Dimensional lmmunoelectrophoresis
- The method has been used for analysis of complex mixtures of proteins (antigens) in fluids like serum, tissue extracts, cell homogenates etc.
- In clinical / diagnostic work the technique is applied for detection of abnormal plasma proteins and for assessment of zones of immunoglobulins or complement systems (for example the Complement C1 complex) in disease states.
- It has been employed for studying heterogeneity of antigen molecules — for example isoforms, fragments, complexes — because each antigen yields a distinct precipitin arc, so differences are resolvable.
- In research on microbial extracts / bacterial antigens the technique is used for characterising antigenic components, verifying purity or identity of antigens, checking cross-reactivity etc.
- In food- or plant-protein work it has been applied for comparing and quantitating storage proteins, seed proteins and checking for polymorphisms in crops (for example rice proteins).
- The method is valued when high resolution and relatively quantitative discrimination among many antigenic species is required, especially when newer high-throughput techniques are unavailable or where antisera are available.
- It can assist in quality control of biochemical or pharmaceutical protein preparations (checking for impurities / verifying composition) because the precipitation arcs reflect concentration vs antibody amounts.
Advantages of Two-Dimensional lmmunoelectrophoresis
- High resolution is achieved because the sample is separated in one dimension and then immuno‐precipitated in a second dimension, which allows very fine discrimination of antigenic components.
- Complex mixtures of proteins/antigens can be analysed simultaneously, so many components are mapped in one assay rather than doing many separate tests.
- Quantitative or semi-quantitative measurement is allowed by the geometry/size of the precipitation arcs, so the amount of antigen can be estimated relative to standards.
- The specificity is high because the antigen-antibody interactions are used after a good separation, so cross-reacting species can be distinguished more easily.
- The method is relatively flexible — for example different antisera may be embedded, the separation conditions are adjustable, so heterogeneity (isoforms, fragments, complexes) of antigens can be detected.
- It is well suited for mixture analysis where you need to know composition of a complex sample (for example sera, extracts) rather than just identify a single component.
Limitations of Two-Dimensional lmmunoelectrophoresis
- The method is relatively slow and time-consuming, with extended electrophoresis plus incubation steps, so rapid throughput is limited.
- Sensitivity is lower than many modern immunoassays (for example immunofixation or mass-spec based), and small/low‐abundance antigens may fail to give clear precipitin arcs.
- Interpretation of the precipitation patterns is challenging because overlapping arcs or diffuse zones may occur, and skilled reading is required; this reduces reproducibility for less experienced users.
- Dependence on good quality specific antisera (antibodies) is strong: if antibody is weak or cross-reactive the results are compromised, and only antigens for which suitable antisera exist can be analysed.
- The technique is less suited for very complex samples with extremely large number of antigen species or very low abundance components, because resolution and quantitation degrade under those circumstances.
- Equipment and gel preparation require care (e.g., gel uniformity, electrophoresis control, transfer to antibody gel) so the method is more labor-intensive than simpler assays and may suffer variation if conditions are not optimized.
- Quantitation is approximate rather than extremely precise (semi-quantitative) in many setups, because the size/area of precipitin zones depends on multiple variables (antibody concentration, gel conditions, antigen diffusion) which may vary between runs.
FAQ
What is Two-Dimensional Immunoelectrophoresis (2D-IEP)?
Two-Dimensional Immunoelectrophoresis is a technique used to separate and analyze proteins based on their charge and molecular weight. It involves the combination of two-dimensional gel electrophoresis and immunoprecipitation using specific antibodies.
How does 2D-IEP work?
In the first dimension, proteins are separated based on their charge using isoelectric focusing (IEF) in a gel. The gel is then subjected to a second dimension separation based on molecular weight using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The separated proteins can then be probed with specific antibodies to identify and characterize target proteins.
What are the advantages of using 2D-IEP?
The advantages of 2D-IEP include high resolution, specificity, quantitative analysis, comprehensive protein profiling, and its utility in biomarker discovery studies.
What are the limitations of 2D-IEP?
Some limitations of 2D-IEP include its labor-intensive nature, limited sample throughput, subjectivity in interpretation, sensitivity limitations for low-abundance proteins, and technical challenges in optimization.
What types of samples can be analyzed using 2D-IEP?
2D-IEP can be used to analyze various types of samples, including cell lysates, tissue extracts, serum or plasma samples, and purified protein samples.
Can 2D-IEP be used for quantitative analysis?
Yes, 2D-IEP can be used for quantitative analysis by comparing the intensities of protein spots or bands. It enables the determination of relative protein concentrations and the identification of differentially expressed proteins.
What is the role of antibodies in 2D-IEP?
Antibodies are used in 2D-IEP to specifically detect and characterize target proteins. They bind to their respective antigens in the gel, allowing for the identification of antigen-antibody interactions and the analysis of antigenic properties.
How long does a typical 2D-IEP experiment take?
The duration of a 2D-IEP experiment can vary depending on several factors, including the number of samples, the complexity of the proteins being analyzed, and the specific protocol being used. Typically, it can range from several hours to overnight.
Are there alternative techniques to 2D-IEP?
Yes, there are alternative techniques for protein analysis, such as one-dimensional gel electrophoresis, two-dimensional gel electrophoresis without immunoprecipitation, and mass spectrometry-based proteomics approaches. The choice of technique depends on the research objectives and the specific requirements of the study.
What are some common applications of 2D-IEP?
2D-IEP has various applications, including protein characterization, biomarker discovery, immunological research, disease diagnosis, and monitoring treatment response. It has been used in studies related to cancer, autoimmune diseases, infectious diseases, and protein expression profiling.
- Sanchez-Perez, Angeles & Villanueva, J & Villa, Tom. (1983). Effect of tunicamycin on exo-1,3-beta-D-glucanase synthesis and secretion by cells and protoplasts of Saccharomyces cerevisiae.. Journal of general microbiology. 128. 3051-60.
- Stockley, R & Burnett, David & Afford, S. (1981). The immunological measurement of ‘free’ secretory piece and its relationship to local IgA production. Clinical and experimental immunology. 45. 124-30.
- Fornaguera, Cristina & Solans, Conxita. (2017). Methods for the In Vitro Characterization of Nanomedicines—Biological Component Interaction. Journal of Personalized Medicine. 7. 2. 10.3390/jpm7010002.
- Walker, J. M. (n.d.). Two-Dimensional (Crossed) Immunoelectrophoresis. New Protein Techniques, 299–310. doi:10.1385/0-89603-126-8:299
- https://www.abbexa.com/2d-immunoelectrophoresis