What is Tryptic Soy Agar (TSA)?
- Tryptic Soy Agar (TSA) is a widely used laboratory medium that provides a suitable environment for the growth of various microorganisms. It is also known as soybean casein digest medium (SCDM) or Soybean-Casein Digest Agar Medium. TSA is a general-purpose, non-selective growth medium that supports the growth of both Gram-negative and non-fastidious Gram-positive bacteria, as well as many yeasts and molds.
- The composition of TSA includes tryptone, soy peptone, glucose, sodium chloride, and agar. Tryptone and soy peptone provide nitrogen, carbon, long-chain amino acids, vitamins, and minerals, which are essential for microbial growth. Glucose serves as the carbohydrate source, while sodium chloride helps maintain the osmotic balance. Agar is added as a solidifying agent, allowing the medium to solidify into agar plates or slants.
- TSA serves multiple purposes in the laboratory. It is commonly used as an initial growth medium to observe colony morphology and obtain pure cultures of microorganisms. The medium provides sufficient nutrients for a wide variety of microorganisms to grow, allowing for their isolation and identification. It is also used for further biochemical testing of microorganisms and as a storage medium for bacterial cultures. TSA slants are particularly useful for storing and shipping bacterial cultures.
- The versatility of TSA makes it suitable for various applications in microbiology research and diagnostics. It supports the growth of both fastidious and non-fastidious microorganisms, including bacteria like Salmonella, Neisseria, Listeria, and Brucella. TSA can be used for the cultivation, storage, maintenance, and transportation of pure cultures of microorganisms.
- It’s important to note that different manufacturers produce TSA under different brand names, such as Trypticase™ Soy Agar (BBL product of BD Diagnostic Systems) and Tryptone Soy Agar (produced by Oxoid), but they have identical compositions.
- In summary, Tryptic Soy Agar (TSA) is a widely used, non-selective growth medium that supports the growth of a wide variety of microorganisms. It provides the necessary nutrients and conditions for the isolation, cultivation, and storage of pure cultures, making it an essential tool in microbiology laboratories.
Principle of Tryptic Soy Agar (TSA)
The principle of Tryptic Soy Agar (TSA) lies in its composition, which provides the necessary nutrients and conditions for the growth of a wide variety of microorganisms. The pancreatic digest of casein and papaic digest of soybean meal present in TSA supply nitrogen, vitamins, and minerals, which are essential for microbial growth. This combination makes TSA suitable for the cultivation of both fastidious and non-fastidious microorganisms.
Glucose serves as a carbohydrate source in TSA, providing energy for microbial metabolism. Phosphate acts as a buffer, helping to maintain the pH of the medium within an optimal range for microbial growth. Sodium chloride is added to TSA to maintain the osmotic balance, creating an environment that supports microbial growth.
Agar, the solidifying agent, is included in TSA to allow the medium to solidify into plates or slants, providing a solid surface for microbial colonies to grow.
While TSA is a non-selective medium, it can be modified or supplemented to meet specific requirements. For example, blood may be added to facilitate the growth of more fastidious bacteria, as some microorganisms require additional nutrients found in blood. Additionally, antimicrobial agents can be incorporated into TSA to selectively promote the growth of specific microbial groups or inhibit the growth of others.
It’s important to note that minor adjustments can be made to the composition of TSA to suit specific circumstances or research needs. However, the fundamental principle remains the same – TSA provides a nutrient-rich and supportive environment for the growth of a wide range of microorganisms.
Composition of Tryptic Soy Agar (TSA)
| Ingredients | Gms/liter |
| Pancreatic digest of casein | 17.000 |
| Papaic digest of soybean meal | 3.000 |
| Sodium chloride | 5.000 |
| Dextrose (Glucose) | 2.500 |
| Dipotassium hydrogen phosphate | 2.500 |
| Agar | 15.000 |
Final pH (at 25°C): 7.3±0.2
Preparation of Tryptic Soy Agar (TSA)
To prepare Tryptic Soy Agar (TSA), the following steps can be followed:
- Suspend 45 grams of TSA powder in 1000 ml of distilled water.
- Heat the mixture while stirring until it reaches boiling point, ensuring that the medium is completely dissolved.
- Sterilize the TSA medium by autoclaving at 15 pounds of pressure (121°C) for 15 minutes.
- Allow the medium to cool down to a temperature of 45-50°C.
- Thoroughly mix the medium to ensure uniform distribution of components.
- Pour the TSA medium into sterile Petri plates, ensuring a sufficient volume to cover the bottom of each plate.
- Prior to inoculating, warm the plates to room temperature and ensure that the agar surface is dry.
- Inoculate and streak the specimen onto the TSA medium as soon as possible after collection. If the specimen is on a swab, roll the swab over a small area of the agar surface.
- Perform streaking for isolation using a sterile loop to obtain well-separated colonies.
- Incubate the TSA plates aerobically at a temperature of 35-37°C for 18-24 hours. Note that some organisms may require longer incubation periods for visible growth to appear.
- After the incubation period, examine the plates for colonial morphology, which includes observing the size, shape, color, and other characteristics of the colonies.
It’s important to maintain aseptic techniques throughout the preparation and use of TSA to prevent contamination. Additionally, specific variations in the preparation or method of use may be necessary depending on the specific requirements of the experiment or research being conducted.
Recommended Procedure
The recommended procedure for working with Tryptic Soy Agar (TSA) involves the following steps:
- Allow the TSA medium to adjust to room temperature before proceeding with inoculation. This ensures that the medium is at the appropriate temperature for microbial growth.
- Inoculate the TSA medium by performing a four-quadrant streak on plated media. The purpose of streaking is to obtain well-isolated colonies. For tubed media, streak the surface of the medium in a fishtail motion from bottom to top.
- If using TSA with blood, make several stabs into the medium during inoculation. This step enhances the detection of beta-hemolysis reactions.
- Incubate the inoculated TSA plates or tubes aerobically or in a CO2-rich environment at a temperature of 35°C. It is recommended to invert the plates during incubation.
- Examine the TSA plates and tubes after 18 to 24 hours of incubation and again at 48 hours. This allows for the observation of microbial growth and the assessment of colony characteristics.
In addition, there is a specific procedure called the CAMP procedure that can be performed using TSA. Here are the steps for the CAMP procedure:
- Allow the TSA medium to adjust to room temperature, and ensure that the surface of the plate is dry before inoculation.
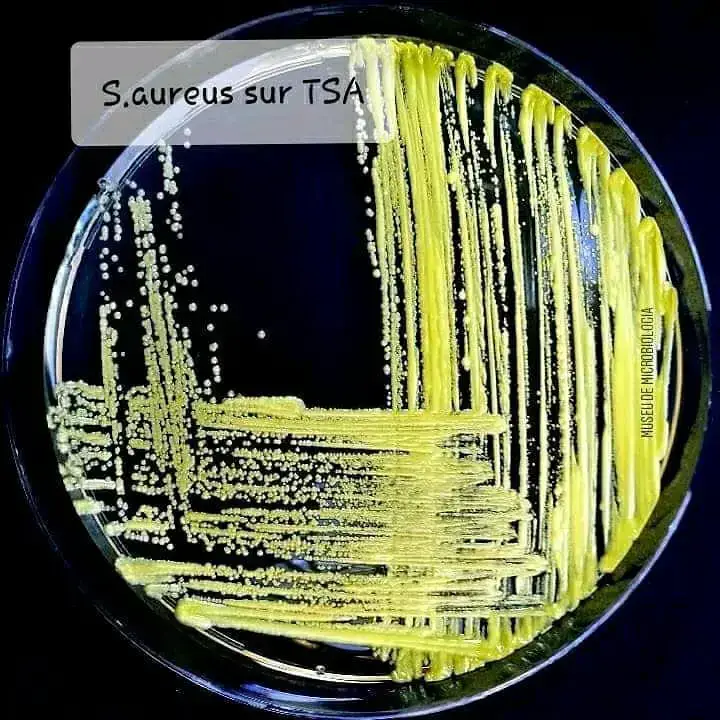
- Obtain a pure overnight culture of Staphylococcus aureus ATCC 25923 or 33862. Using an inoculating needle or the edge of a loop, streak Staphylococcus in a straight line across the center of the plate.
- Streak the test organism in a straight line, approximately 2 to 3 cm long, perpendicular to the Staphylococcus streak. The line should come close (approximately 3 mm) but should not touch the Staphylococcus streak. Multiple test streaks can be performed on each plate, including one known positive control (e.g., Streptococcus agalactiae ATCC 12386).
- Label the streaks on the bottom of the plate, indicating the media side.
- Incubate the plates aerobically in an inverted position at 35°C.
- Examine the plates after 18 to 24 hours of incubation. This allows for the observation of any characteristic reactions, such as CAMP reactions.
By following these recommended procedures, researchers can effectively work with TSA, observe microbial growth, and perform specific tests like the CAMP procedure to obtain valuable information for their studies or diagnostic purposes.
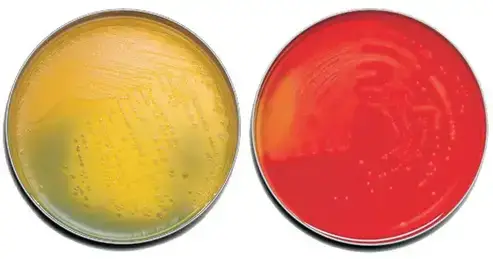
Interpretation of Results on Tryptic Soy Agar (TSA)
The interpretation of results obtained from Tryptic Soy Agar (TSA) and related tests involves the following considerations:
- Primary isolation and colony characterization: TSA with 5% Defibrinated Sheep Blood is commonly used as a primary plating medium for isolating organisms from a specimen. It allows for the separation and isolation of different organisms present. When examining primary plates, it is important to note the different types of colony morphology and the number of each morphotype present. This can provide valuable information about the diversity of microorganisms present in the sample.
- Hemolysis types: Hemolysis is a useful differential characteristic observed on TSA with blood. Different types of hemolysis can be described:
- Alpha-hemolysis (α): Partial hemolysis resulting in a greenish discoloration around the colony.
- Beta-hemolysis (β): Complete lysis of red blood cells resulting in a clear zone around the colony.
- Gamma-hemolysis (γ): No hemolysis, resulting in no change in the medium.
- Alpha-prime-hemolysis (α′): A small zone of complete hydrolysis surrounded by an area of partial hemolysis.
- Additional observations: In addition to hemolysis, other factors such as pigment production and odor should be recorded when examining colonies on TSA. These observations can provide additional clues for the identification of specific microorganisms.
- CAMP test: The CAMP test can be used to presumptively identify group B streptococci (S. agalactiae). A positive CAMP reaction is indicated by the production of a distinct arrowhead zone of complete hemolysis at the point of intersection between the test streak and the streak of Staphylococcus aureus. The hemolysis reaction must extend throughout the depth of the agar plate. A negative CAMP reaction is the absence of an arrowhead phenomenon or a slight increase in the zone of hemolysis without arrowhead formation. It’s important to note that some other organisms may also show a positive CAMP reaction.
- Further tests for identification: The results obtained from TSA and related tests are preliminary, and additional tests should be performed on isolated colonies from pure culture for complete identification. These additional tests may include biochemical, immunological, or molecular tests to further characterize the microorganisms.
By carefully interpreting the results obtained from TSA and related tests, researchers can gain insights into the types of microorganisms present, their hemolytic characteristics, and potentially identify specific species or groups of bacteria.
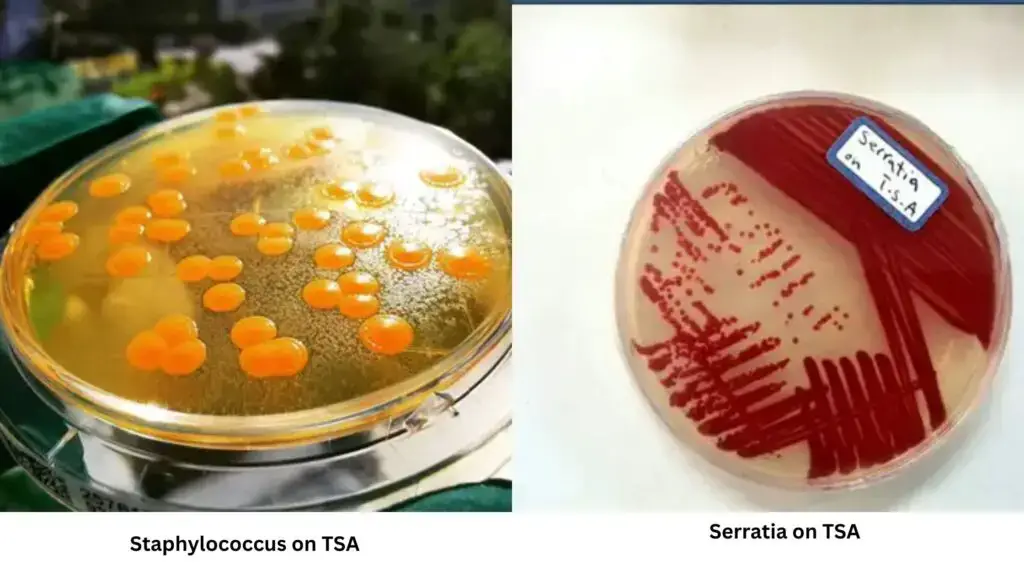
| Organisms | Growth |
| Aspergillus brasiliensis | Positive |
| Candida albicans | Positive |
| Staphylococcus aureus | Positive; pigmented shiny round colonies |
| Staphylococcus epidermidis | Positive |
| Bacillus subtilis | Positive; flat, large irregular colonies |
| Pseudomonas aeruginosa | Positive |
| Escherichia coli | Positive; shiny round colonies |
| Salmonella Typhimurium | Positive |
Quality control
Quality control of Tryptic Soy Agar (TSA) involves assessing various parameters to ensure its performance and reliability. The following aspects are typically evaluated during quality control:
- Appearance: The TSA powder should have a cream to yellow homogeneous free-flowing appearance, indicating that it is well-mixed and without clumps.
- Gelling: The gelling property of TSA is evaluated by comparing it to a 1.5% agar gel. It should form a firm gel that is comparable in consistency.
- Color and clarity of prepared medium: The prepared TSA medium should have a light yellow color and appear clear to slightly opalescent, indicating proper dissolution and absence of particulate matter.
- pH: The pH of a 4.5% w/v aqueous solution of TSA at 25°C should be within the range of 7.3 ± 0.2. This ensures that the pH of the medium is suitable for microbial growth.
- Cultural response: TSA’s ability to support microbial growth is evaluated by observing the cultural characteristics of organisms after incubation. Typically, the medium is inoculated with specific microorganisms, and the growth and recovery rates are assessed.
- Organism Inoculum (CFU): The recommended range of colony-forming units (CFU) for specific organisms, such as Salmonella Typhi ATCC and Salmonella Typhimurium, is 50-100 CFU. This indicates that the medium can support the growth of these organisms.
- Growth Recovery: The percentage of growth recovery is assessed by comparing the observed growth with the expected growth. For example, for Salmonella Typhi ATCC and Salmonella Typhimurium, the recovery should be equal to or greater than 70% of the inoculated CFU.
By evaluating these parameters, quality control ensures that the TSA medium meets the required specifications for consistent and reliable performance. It allows for the detection of any variations or deviations in the product, ensuring that the medium provides optimal conditions for microbial growth and isolation.
Uses of Tryptic Soy Agar (TSA)
Tryptic Soy Agar (TSA) has a wide range of uses in microbiology, making it a versatile and commonly employed medium. Some of its main applications include:
- Culture storage: TSA is frequently used as a medium for storing and preserving bacterial cultures. It provides the necessary nutrients and conditions to maintain the viability of microorganisms over an extended period.
- Enumeration of cells: TSA can be used for cell counting, allowing researchers to determine the number of viable microorganisms present in a sample. This is useful for various applications, such as monitoring microbial populations or assessing the effectiveness of antimicrobial agents.
- Isolation of pure cultures: TSA supports the growth of a broad spectrum of microorganisms, both fastidious and non-fastidious. It is commonly used for the isolation of pure cultures from mixed samples, allowing researchers to obtain individual microbial strains for further study.
- General culture: TSA serves as a general-purpose medium for the cultivation of microorganisms. Its nutrient-rich composition enables the growth of a wide variety of organisms, making it suitable for routine laboratory work and general microbial culture.
- Specific applications: TSA can be modified or supplemented for specific purposes. For example, Tryptone Soya Broth with added dextrose, sodium chloride, and agar is recommended for the cultivation of Salmonella Typhi. Additionally, TSA can serve as a base medium for other agar plate types, such as blood agar plates (BAP) enriched with sheep blood or chocolate agar plates.
- Factor determination: TSA can be used to determine specific factors in certain microorganisms. For example, strips containing X, V, and XV factors can be placed on TSA plates inoculated with Hemophilus species to determine their X, V, and XV factor requirements.
- Halotolerance testing: By adding salt to TSA, the medium can be used to assess the halotolerance level of microorganisms, providing insights into their ability to survive and grow in saline or high-salt environments.
- Environmental testing: TSA finds application in the environmental testing of various industries, including pharmaceuticals, water, cosmetics, and food and beverage industries. It allows for the detection and enumeration of microorganisms present in these samples.
- Maintenance and subculturing of reference strains: TSA can be used as a medium for maintaining and subculturing reference strains, such as those belonging to the Enterobacteriaceae family and staphylococci. This ensures the long-term viability and availability of these strains for research and quality control purposes.
Overall, the versatility of TSA makes it an essential medium in microbiology laboratories, serving various purposes in research, diagnostics, and quality control across different industries.
Limitations of Tryptic Soy Agar
While Tryptic Soy Agar (TSA) is a widely used and versatile medium, it does have certain limitations. These limitations include:
- Limited identification capabilities: While TSA supports the growth of a wide range of microorganisms, it does not provide sufficient information for complete identification. To achieve complete identification, additional biochemical, immunological, molecular, or mass spectrometry testing is required on colonies obtained from pure culture grown on TSA.
- Limited growth of fastidious bacteria: TSA may not promote the growth of certain types of fastidious bacteria on its own. Fastidious bacteria have specific nutritional requirements that may not be fully met by TSA alone. Therefore, additional supplements or specialized media may be needed to support their growth and facilitate their isolation.
- Inability to isolate pathogens from clinical specimens: While TSA can support the growth of various microorganisms, it is not selective for pathogens. It does not contain specific inhibitors or selective agents that target pathogenic bacteria, making it unsuitable for the direct isolation of pathogens from clinical specimens. Selective media with specific inhibitors or enrichment techniques may be necessary for the isolation and identification of pathogenic microorganisms.
It’s important to note that despite these limitations, TSA remains a valuable general-purpose medium for routine laboratory work, culture maintenance, and the cultivation of a wide range of microorganisms. However, researchers and microbiologists should be aware of these limitations and choose appropriate alternative media or techniques when necessary to address specific research or diagnostic needs.
FAQ
What is Tryptic Soy Agar (TSA)?
Tryptic Soy Agar is a commonly used growth medium in microbiology. It provides essential nutrients and conditions for the growth of a wide range of microorganisms.
What is the composition of TSA?
TSA is composed of pancreatic digest of casein, papaic digest of soybean meal, glucose, sodium chloride, phosphate buffers, and agar as a solidifying agent.
What is the purpose of TSA?
TSA serves multiple purposes, including observation of colony morphology, development of pure cultures, sufficient growth for further testing, storage and transport of bacterial cultures, and general cultivation and isolation of microorganisms.
Can TSA support the growth of both fastidious and non-fastidious microorganisms?
Yes, TSA is suitable for the growth of both fastidious and non-fastidious microorganisms, making it a versatile medium in microbiology.
Can TSA be used for pathogen isolation from clinical specimens?
TSA is a non-selective medium and may not be ideal for direct isolation of pathogens from clinical specimens. Selective media or specific enrichment techniques are often employed for pathogen isolation.
How is TSA prepared?
TSA is prepared by suspending 45 grams of TSA powder in 1000 ml of distilled water, followed by boiling to dissolve the medium completely. It is then sterilized by autoclaving at 121°C and poured into sterile Petri plates or tubes.
What are the different types of hemolysis observed on TSA with blood?
The different types of hemolysis observed on TSA with blood are alpha-hemolysis (partial hemolysis), beta-hemolysis (complete lysis), gamma-hemolysis (no hemolysis), and alpha-prime-hemolysis (small zone of complete hydrolysis surrounded by partial hemolysis).
Can TSA be used for environmental testing?
Yes, TSA is commonly used for environmental testing in various industries, including pharmaceuticals, water, cosmetics, food, and beverages.
What is the CAMP test, and how is it performed using TSA?
The CAMP test is a procedure used to identify group B streptococci. It involves streaking a test organism perpendicular to a streak of Staphylococcus aureus on TSA and observing for the presence of a distinct arrowhead-shaped zone of hemolysis.
What are the limitations of TSA?
Some limitations of TSA include its inability to selectively isolate pathogens from clinical specimens, the need for additional tests for complete identification of microorganisms, and its limited ability to support the growth of some fastidious bacteria without supplements or specialized media.
References
- https://www.himedialabs.com/eu/coasdstds/index/download/id/M1968/source/tds/lang/EN
- https://microbeonline.com/tryptic-soy-agar-tsa-composition-preparation-uses/
- https://microbiologie-clinique.com/trypticase-soy-agar-principle-interpretation.html
- https://legacy.bd.com/europe/regulatory/Assets/IFU/HB/CE/BA/BA-256665.pdf
- https://www.dalynn.com/dyn/ck_assets/files/tech/PB81.pdf
- http://emp.com.ge/culture-media/tryptic-soy-agar/
- https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/product/documents/122/065/22091dat.pdf
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.