Sudan stain is the special group of lysochrome dyes that is used for staining fats and fat droplets in different biological specimens. It is the process where these lipid-soluble dyes get dissolved into the fat molecules and give a distinct coloration.
These dyes are synthetic organic diazo dyes, and it is seen that they are also used commercially for coloring wax, grease, plastic, and other non-polar substances. The main Sudan dyes include Sudan II, Sudan III, Sudan IV, Oil Red O, and Sudan Black B. These are fat-soluble dyes, and the staining mainly appears red in most cases depending on the dye used.
It is used to detect lipids, lipoproteins, and triglycerides from frozen or paraffin sections, and the stain appears as a red powder in dry form. Sudan II is also used industrially for coloring non-polar substances like oils, hydrocarbons and wax.
Sudan III is used widely for staining lipids as it is a lysochrome and diazole dye, and it is the process where lipids are clearly differentiated under the microscope.
Sudan Black B stain is the most commonly used Sudan dye for staining a wide range of lipids including phospholipids, sterols, and neutral triglycerides. It is non-fluorescent and thermally stable, and it stains fats and lipids blue-black instead of red.
This stain is not completely specific for lipids unlike other Sudan dyes, and therefore it can also stain chromosomes, Golgi bodies, and leukocyte granules. It is the dye that has very high affinity for neutral fats, and the staining becomes useful in hematological diagnosis.
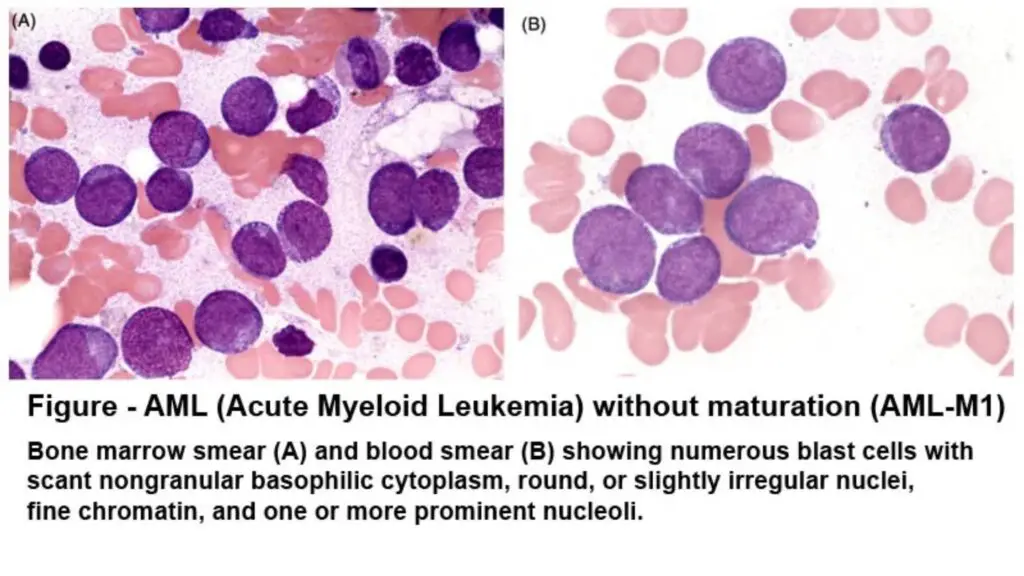
It is referred to as an important stain for differentiation of Acute Myeloid Leukemia (AML) from Acute Lymphoid Leukemia (ALL) because the pattern of Sudan Black B staining is similar to that of myeloperoxidase. The stain can be used even in blood smears that are older than two weeks, and it stains both azurophilic and specific granules in neutrophils whereas myeloperoxidase stains only azurophilic granules. It is also seen that Sudan Black B stain shows very little fading with time and thus gives a stable staining result.
Principle of Sudan Black B Stain
Sudan Black B is a fat-soluble dye, and it is the process where the dye moves out of the solvent phase and becomes dissolved into the lipid components of the specimen. It stains lipids such as sterols, neutral fats, and phospholipids, and these lipid materials are present in the azurophilic and secondary granules of myelocytic cells as well as the lysosomal granules of monocytic cells. During staining, the dye has higher solubility in lipids than in the staining solvent, so it preferentially migrates and gets deposited into the lipid droplets. It is seen that on microscopic examination, different degrees of black coloration appear depending on the amount and nature of the lipid present in the cell structures.
Sudan staining usually employs frozen tissue sections that are fixed in formalin or paraffinized sections. Sudan Black B is the most commonly used dye among the Sudan groups, and it is a slightly basic dye that combines with the acidic groups of lipid compounds. Because of this affinity, it stains phospholipids, lipoproteins, and triglycerides in the specimen. The reaction is referred to as a physical staining process, as there is no chemical bonding, but the coloration occurs due to the dye’s high solubility within the lipid substances.
Control
Use a positive control of a fat smeared slide, and a negative control slide of a paraffin processed tissue, such as lung.
Requirement
- Propylene glycol placed in two Coplin jars
- 85% propylene glycol (85 ml propylene glycol + 15 ml distilled water)
- Hematoxylin solution
- Glycerin jelly for mounting
- Sudan Black B/propylene glycol mixture containing 0.7 g Sudan Black B dissolved in 100 ml propylene glycol
- Heating of the Sudan Black B mixture to 100°C with constant stirring
- Filtering the solution through Whatman No. 2 filter paper, cooling, and refiltering through a frittered glass filter
- Storage of the prepared stain at 60°C, where the solution remains stable for about 1 year
Procedure of Sudan Black B Stain
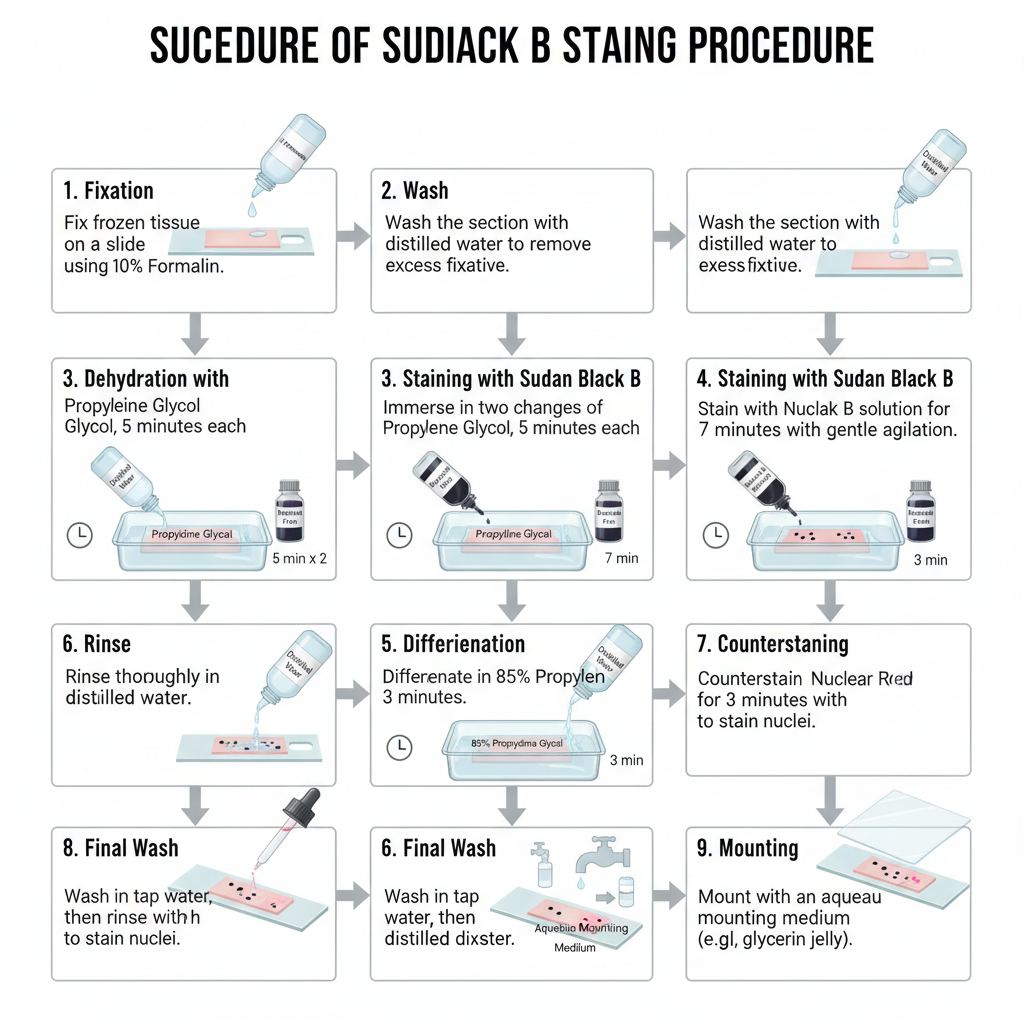
- Fix the frozen tissue sections on a clean glass slide using fresh 10% formalin.
- Wash the fixed section with distilled water and remove excess water.
- Add propylene glycol in two changes, 5 minutes each.
- Apply Sudan Black B staining solution and keep for about 7 minutes with gentle agitation.
- Add 85% propylene glycol and leave the slide for 3 minutes.
- Rinse the stained section in distilled water.
- Add Nuclear Fast Red and keep for 3 minutes.
- Wash in tap water twice and then rinse again in distilled water.
- Mount the section using aqueous mounting jelly (glycerin jelly).
Results and Interpretation
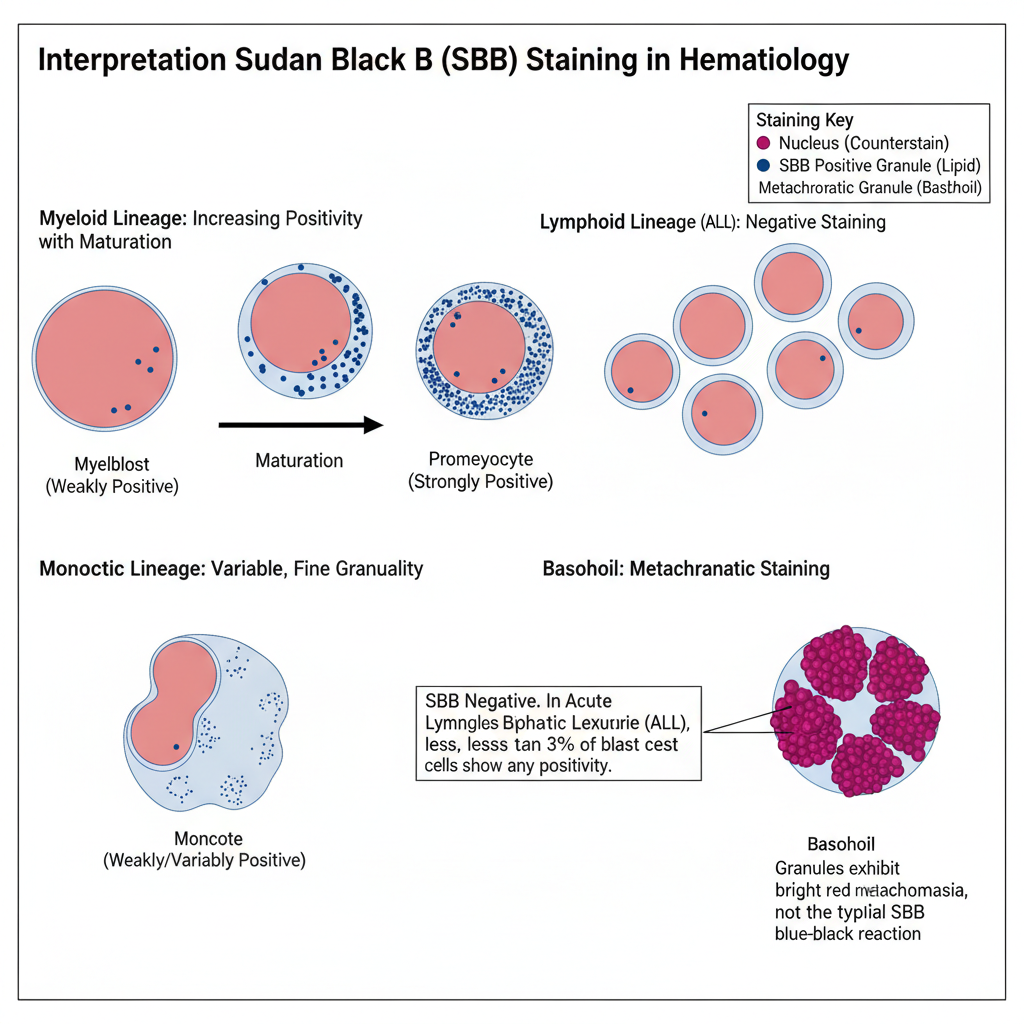
Sudan Black B stains fats, lipids, triglycerides, and lipoproteins as blue-black pigments in the specimen, and the nuclei appear red due to the counterstain. Production of black and granular pigment is taken as a positive reaction indicating the presence of lipid material.
These lipids are present in the azurophilic and secondary granules of myelocytic cells, so these cells are SBB positive, and the staining becomes more intense when the cells mature from myeloblasts to the later forms. Basophils usually do not give a positive reaction, but their granules may sometimes show bright red or purple metachromatic staining.
Lipids are also present in the lysosomal granules of monocytic cells, so monocytic cells show variable reactions which may range from negative to weakly positive. Lymphoid cells are SBB negative, and in the case of ALL, less than 3% of the blast cells show any positive staining.
Uses of Sudan Black B Staining
- It is used to differentiate AML from ALL because myeloblasts show strong positive staining while lymphoblasts are usually negative.
- It helps as a surrogate stain for myeloperoxidase when MPO shows weak or negative reactions.
- It is used for demonstrating Auer rods more clearly than routine Romanowsky stains.
- It helps in subtyping AML, including AML-M3 and leukemias with monocytic components.
- It stains granulocytes by showing both azurophilic and specific granules in neutrophils.
- It is used as a marker of cellular senescence by staining lipofuscin pigment.
- It can detect senescent cells in fresh, cultured, and archival tissues.
- It helps in quenching lipofuscin autofluorescence during immunofluorescence studies.
- It can be used for quantitative fluorescence microscopy due to far-red emission of stained lipofuscin.
- It allows co-staining with antibody-based immunofluorescence markers.
- It is used in general histology to stain neutral fats, phospholipids, sterols, and lipoproteins.
- It is applied to frozen sections for demonstrating structural or reserve lipids in tissues.
- It is used in microbiology for staining fat bodies in bacteria such as Bacillus species and Mycobacterium species.
- It helps in screening bacteria for intracellular biopolymers like PHAs and PHB in bioplastics research.
- It is used in qualitative detection of lipid accumulation in microbial and yeast cells.
- It is used in forensic science for fingerprint enhancement by staining sebaceous lipids.
- It helps in detecting fats contaminated with grease or oil in technical examinations.
Limitations of Sudan Black B Staining
- It cannot chemically distinguish different lipid classes because the staining is based on physical solubility.
- It is not fully lipid specific and may also stain proteins, chromosomes, Golgi bodies, and sometimes nuclear areas.
- Rare ALL cases may show slight Sudan Black B positivity, which can affect diagnostic interpretation.
- It requires frozen sections or fresh smears because paraffin processing dissolves lipids.
- Organic solvents like alcohol, acetone, and xylene cannot be used, as they remove stained lipids and cause false negatives.
- Only aqueous mounting media can be used since resin-based mountants dissolve the dye-lipid complex.
- Air bubbles trapped during mounting may displace stained lipid deposits if pressed out.
- Dye precipitation may occur when using concentrated or old staining solutions and may mimic true lipid granules.
- Sections may swell or detach during washing steps due to solvent interaction with the tissue.
- The dye solution becomes unstable with age because commercial Sudan Black B contains mixed components that degrade over time.
- Sudan Black B is a synthetic azo dye and is suspected of causing genetic defects, requiring careful handling and protective equipment.
Advantages of Sudan Black B Staining
- It stains a wide range of lipids including neutral triglycerides, phospholipids, and sterols, giving broad lipid detection.
- It provides deep blue-black coloration which gives better contrast than older Sudan dyes.
- It stains both azurophilic and specific granules in neutrophils clearly.
- It is thermally stable and non-fluorescent, making routine staining easier.
- It is useful for differentiating AML from ALL because myeloblasts show strong positive staining.
- It is more sensitive than MPO staining in detecting myeloid cells when MPO may show weak or negative reactions.
- It gives better visualization of Auer rods in leukemic cells than Romanowsky stains.
- It can be used for older smears since the stain shows very little fading over time.
- It is cost-effective and useful in laboratories where immunophenotyping is not available.
- It acts as a marker for lipofuscin and is used in studies of cellular senescence and aging.
- It can stain senescent cells in fresh, cultured, or archival tissues.
- It can be used together with immunofluorescence techniques and helps in reducing autofluorescence for clearer imaging.
- It is used in microbiology for detecting fat bodies in bacteria and screening PHA-producing organisms.
- It is used in forensic studies by staining fatty components in fingerprints.
- It is applied in histology to detect structural or reserve lipids in different tissues.
FAQ on Sudan Black B Staining
What is Sudan Black B staining used for?
Sudan Black B staining is used for detecting fats, lipids, phospholipids, and lipoproteins in cells and tissues. It is also used in hematology to identify myeloid cells and to help in the differentiation of AML from ALL.
What is the principle of Sudan Black B staining?
The principle is based on the high solubility of the dye in lipids rather than in the staining solvent. The dye leaves the solvent phase and becomes dissolved in lipid substances, staining them blue-black. It is a slightly basic dye that combines with acidic groups in lipid compounds.
What does Sudan Black B stain?
It stains sterols, neutral fats, phospholipids, and lipoproteins as blue-black pigments. It can also stain azurophilic and secondary granules of myelocytic cells and lysosomal granules of monocytic cells.
How is Sudan Black B staining performed?
It is done by fixing frozen sections or smears, treating them with propylene glycol, adding Sudan Black B solution for staining, washing, counterstaining with Nuclear Fast Red, and mounting with aqueous mounting media such as glycerin jelly.
What are the applications of Sudan Black B staining?
It is used to detect lipids in tissues, confirm myeloid lineage, differentiate AML from ALL, stain older smears, and demonstrate lipid-containing granules in hematological studies. It is also used in research for identifying lipid distribution.
What is the clinical importance of Sudan Black B staining?
Clinically it helps in diagnosing AML by showing strong positivity in myeloblasts. It also supports identification of myeloid maturation and helps exclude lymphoid lineage in suspected leukemia cases.
What is the positive staining reaction of Sudan Black B?
A positive reaction shows blue-black or black granular pigmentation within the cytoplasm, indicating the presence of lipid-containing granules.
Is Sudan Black B lipid specific?
It is mainly a lipid stain but not completely specific. Besides lipids, it may also stain chromosomes, Golgi bodies, and some non-lipid cell structures.
What are the results and interpretation of Sudan Black B staining?
Myeloid cells show positive blue-black staining, with intensity increasing from myeloblast to mature forms. Monocytic cells may show weak or variable staining. Lymphoid cells are negative. Less than 3% blasts may show positivity in ALL.
How does Sudan Black B differentiate AML from ALL?
AML cells (myeloblasts) show strong positive staining due to abundant lipid-containing granules. ALL blasts are SBB negative or show less than 3% positivity, helping in clear differentiation.
What types of cells are stained by Sudan Black B?
Myelocytic cells with azurophilic and secondary granules, monocytic cells with lysosomal lipids, and occasionally non-lipid structures. Basophils may show metachromatic red or purple granule staining.
What fixatives are recommended for Sudan Black B staining?
Fresh 10% formalin for frozen sections and 40% formaldehyde vapor for smears are recommended fixatives. Slides should be fixed immediately to avoid loss of lipids.
How is the Sudan Black B staining solution prepared?
Sudan Black B is dissolved in propylene glycol with heating, filtered twice, and stored warm. Another common preparation uses 0.3 g Sudan Black B dissolved in 100 ml absolute ethanol, later mixed with phenol buffer to make a working solution.
What are the advantages of Sudan Black B over myeloperoxidase staining?
It can stain smears older than two weeks, it stains both azurophilic and specific granules, and the stain has very little fading. It also does not require enzymatic activity, so storage conditions affect it less than MPO.
What are Sudan dyes?
Sudan dyes are lysochrome (fat-soluble) synthetic diazo dyes used to stain lipids in biological specimens. Examples include Sudan II, Sudan III, Sudan IV, Oil Red O, and Sudan Black B. They dissolve into lipid structures and give color depending on the dye used.
- https://www.clinisciences.com/en/buy/cat-sudan-black-b-stain-3954.html
- https://fdocuments.in/document/special-stain-final.html
- https://microbenotes.com/sudan-black-b-staining/
- http://www.bio-diagnostic.com/images/sudan_black__b__stain.pdf
- https://webpath.med.utah.edu/HISTHTML/MANUALS/SUDANF.PDF
- https://en.wikipedia.org/wiki/Sudan_II
- https://en.wikipedia.org/wiki/Sudan_III
- https://en.wikipedia.org/wiki/Oil_Red_O
- https://en.wikipedia.org/wiki/Sudan_stain
- https://laboratorytests.org/sudan-black-b-stain/
how Do i reference your work please
Just Copy the link: Sudan Black B Staining