- Legumes, a diverse group of plants within the Fabaceae family, are cultivated and consumed globally in various forms, including fresh green vegetables, shelled seeds, and dried seeds. The dried seeds, commonly referred to as pulses, serve as a staple in many diets due to their impressive nutritional profile. This article aims to explore the significance of legumes through specimens and microchemical tests, emphasizing their functions and biochemical properties.
- Legumes are recognized for their high nutritional value. They provide a robust source of protein, with concentrations ranging from 20% to 45%, depending on the species. Pulses typically contain between 17% and 30% protein, making them a critical component of plant-based diets, particularly for individuals seeking alternatives to animal proteins. Among legumes, soybeans stand out as the richest protein source, exceeding 40%. This high protein content is complemented by significant levels of carbohydrates, primarily in the form of starch, which constitutes approximately 60% of their composition. Additionally, legumes are rich in dietary fiber, contributing between 5% and 37% to their overall makeup, which aids in digestion and promotes gut health.
- Moreover, legumes are packed with essential vitamins and minerals that play crucial roles in human health. They provide important nutrients such as calcium, iron, niacin, thiamine, and riboflavin. These components not only support various metabolic processes but also enhance overall physiological functions. For instance, calcium is vital for bone health, while iron is essential for the formation of hemoglobin and prevention of anemia.
- To effectively study legumes, specimens and microchemical tests are employed to investigate their biochemical properties. Specimens are typically collected from diverse species to provide a comprehensive understanding of their nutritional and functional characteristics. These specimens can be analyzed for protein content, carbohydrate composition, and the presence of essential vitamins and minerals.
- Microchemical tests further enhance the understanding of legumes’ biochemical makeup. For instance, tests such as the Biuret test can be utilized to detect proteins, while Benedict’s test can identify reducing sugars, enabling a detailed analysis of the carbohydrate content. These tests are crucial for assessing the nutritional value and quality of legumes, contributing to food science and nutrition studies.
Materials required
The study of legumes, particularly in understanding their biochemical composition and properties, requires specific materials to conduct experiments effectively. The following items are essential for analyzing legumes such as Glycine max (soybean) and Cicer arietinum (gram). Each material plays a critical role in the experimental process, ensuring accurate results and insights into the nutritional value of these important crops.
- Glycine max (Soybean) Herbarium Specimen: This specimen is crucial for botanical and morphological studies, providing a reference for identification and analysis of soybean characteristics.
- Cicer arietinum (Gram) Herbarium Specimen: Similar to the soybean specimen, this herbarium specimen serves as a reference point for studying the structural and functional attributes of gram, contributing to the understanding of its botanical identity.
- Soybean Seeds: These seeds are essential for various biochemical tests, allowing researchers to analyze the nutrient content, particularly protein levels, carbohydrates, and vitamins present in soybeans.
- Gram Seeds: Like soybean seeds, gram seeds are used in experiments to assess their nutritional composition. They provide valuable data on protein content and other essential nutrients.
- Test Tubes: These glass containers are necessary for holding liquids and conducting reactions. They facilitate controlled experiments, allowing for accurate measurements and observations.
- Test Tube Holder: This tool is used to securely hold test tubes during experiments, preventing spillage and ensuring safety while handling hot or reactive materials.
- Test Tube Stand: This stand provides stability for multiple test tubes, enabling researchers to conduct several experiments simultaneously without the risk of tipping.
- Dropper: A dropper is essential for adding precise amounts of reagents during experiments, ensuring that concentrations remain consistent for accurate analysis.
- Burner: A laboratory burner is required for heating substances, allowing for reactions that necessitate elevated temperatures or for sterilization of materials.
- Concentrated HNO3 (Nitric Acid): This strong acid is used in various chemical tests to analyze the composition of legumes. It can assist in breaking down compounds for further study.
- Ammonia Solution: This solution plays a role in certain reactions, particularly in protein testing, and is utilized to precipitate specific proteins from legume samples.
- 1% CuSO4 (Copper Sulphate Solution): Copper sulfate solution is often employed in biochemical tests, particularly for detecting proteins through colorimetric methods.
- 40% NaOH Solution (Sodium Hydroxide): This strong base is used to create an alkaline environment necessary for certain reactions, especially those involving proteins and amino acids.
- Biuret Reagent: This reagent is critical for testing the presence of proteins in legume samples. The Biuret test, which results in a color change, helps quantify protein concentration effectively.
Procedure
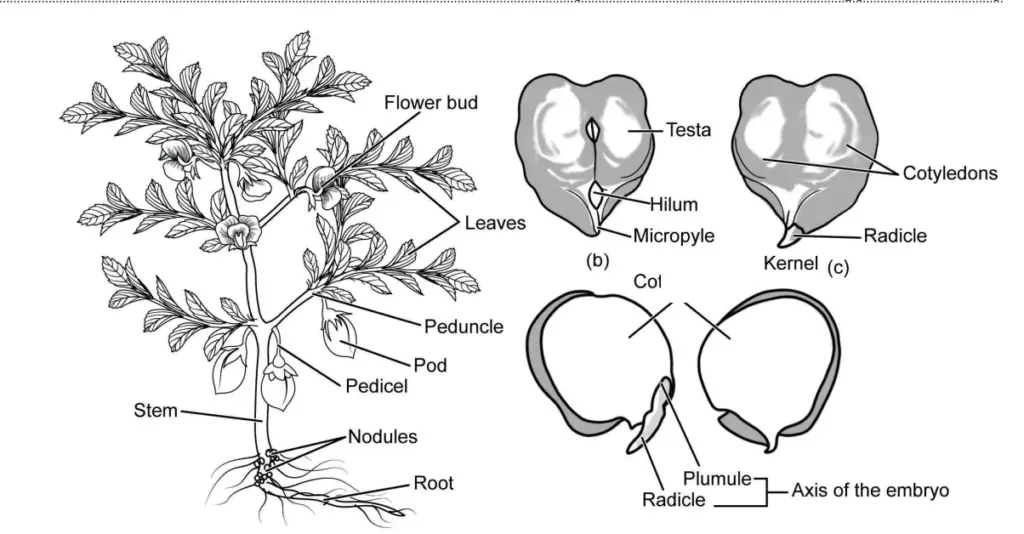
The procedure for studying the herbarium specimens of legumes, specifically Glycine max (soybean) and Cicer arietinum (black gram), involves a systematic approach to observe and document their morphological features. This process provides valuable insights into the structural characteristics of these plants. The following steps outline the detailed procedure to ensure thorough analysis and accurate recording.
- Acquisition of Specimens: Begin by obtaining the herbarium specimens of Glycine max (soybean) from the Leguminosae family and Cicer arietinum (black gram) from the Poaceae family. These specimens serve as the primary material for morphological study.
- Observation of Morphological Features: Carefully examine each specimen, focusing on distinct morphological features. Take note of the following components:
- Roots: Observe the root structure, including the type (taproot or fibrous), arrangement, and any notable characteristics.
- Stems: Investigate the stem for attributes such as thickness, texture, and branching patterns.
- Leaves: Analyze the leaves for their shape, size, venation pattern, and arrangement on the stem.
- Inflorescence: Document the structure of the inflorescence, including the arrangement of flowers and any distinctive features.
- Recording Structural Features of Grains: Examine the grains of both soybean and black gram. Note their size, shape, color, and surface texture. This analysis helps understand the nutritional and agricultural significance of these seeds.
- Diagram Creation: As you observe the specimens, create well-labeled diagrams to illustrate the morphological features of each component. These diagrams should be drawn in your notebook or practical copy, providing a visual reference to complement your notes. Ensure that labels are clear and accurately represent the features being depicted.
Observation
Observations of the herbarium specimens of Glycine max (soybean) and Cicer arietinum (gram) reveal a wealth of morphological features that underscore the diversity and structural characteristics of these legumes. The following detailed observations elucidate the key components of each plant, offering insights into their biology and functionality.
- Soybean (Glycine max):
- The soybean plant is recognized as an erect, branched, annual herb, typically ranging from 50 cm to 180 cm in height under natural growth conditions. Its upright growth habit is a significant characteristic that supports its overall development and productivity.
- The root system features a well-developed taproot, which enhances stability and nutrient absorption. Additionally, small spherical nodules are present on the roots, indicative of nitrogen-fixing symbiotic relationships with rhizobia bacteria, crucial for soil fertility.
- The stem is erect, providing structural support, and facilitates the plant’s ability to capture sunlight effectively for photosynthesis.
- Soybean leaves are notable for their large size and hairy texture. They are arranged alternately on the stem, with stipules present and long petioles connecting each leaf to the stem. The compound leaves are typically trifoliate, consisting of three leaflets that may be either 5- or 9-foliate. The leaflets are characterized by their ovate to lanceolate shape, enhancing the plant’s surface area for light absorption.
- Inflorescences are short, axillary racemes that typically contain 3 to 15 flowers. The flowers are small and exhibit a color range from nearly white to deep purple, contributing to the plant’s reproductive success through pollinator attraction.
- The pods of the soybean plant arise in clusters, possessing a hairy surface. Each pod is usually compressed and slightly curved, providing protection for the seeds during development.
- Seeds of Glycine max are small and globose, characterized by a small hilum. Typically, each pod contains 2 to 3 seeds, though variations allow for the presence of 1 to 5 seeds per pod. The seeds exhibit a color spectrum ranging from creamy white to yellow, reflecting genetic diversity within the species.
- Gram (Cicer arietinum):
- The gram plant is also an erect, branched, annual herb but is shorter than the soybean, generally reaching heights of 20 to 25 cm in nature. This stature allows it to thrive in various agricultural environments.
- Its leaves are imparipinnate, featuring 9 to 15 pairs of ovate, elliptic, or obovate leaflets with serrate margins. This leaf structure maximizes light interception while providing structural integrity to the plant.
- The flowers of the gram plant vary in color from white to pink, adding to its visual diversity and aiding in attracting pollinators.
- The pods are small and inflated, containing one or more seeds. Each seed is angular, exhibiting a prominent beak and a small hilum at the anterior end, which is essential for seed attachment and dispersal.
- Notably, the seeds of Cicer arietinum are rich in proteins, carbohydrates, and fiber, making them a valuable component of human and animal diets.
Microchemical test (proteins)
The microchemical tests for proteins, specifically the Xanthoproteic test and the Biuret test, serve as effective methodologies for detecting the presence of proteins in seeds such as Glycine max (soybean) and Cicer arietinum (gram). These tests provide valuable insights into the protein content of legumes, which are significant sources of dietary protein.
- Preparation of Biuret Reagent:
- The Biuret reagent is essential for the Biuret test. To prepare it, 1.5 g of copper sulfate (CuSO4) is mixed with 6 g of sodium potassium tartrate. This mixture is dissolved in 500 ml of distilled water.
- Next, 375 ml of 2M sodium hydroxide (NaOH) is measured. Both solutions are combined in a volumetric flask, and distilled water is added to achieve a final volume of 1000 ml. This reagent is pivotal for detecting proteins through the formation of a colored complex.
- Preparation of Ammonia Solution (30%):
- This solution is prepared by dissolving 30 g of ammonium hydroxide (NH4OH) in 100 ml of distilled water. It is used in the Xanthoproteic test to induce color changes that signify the presence of proteins.
- Preparation of Sodium Hydroxide Solution (30%):
- To prepare this solution, 30 g of sodium hydroxide (NaOH) is dissolved in 100 ml of distilled water. It also plays a role in the Xanthoproteic test by reacting with the protein solutions.
- Xanthoproteic Test:
- Begin by crushing the seeds of either gram or soybean to create a fine powder.
- Transfer the powdered seeds into a test tube and add 5 drops of concentrated nitric acid (HNO3).
- Boil the sample over a burner for 2 minutes, ensuring a firm grip on the test tube with a holder. A yellow precipitate will form, indicating a reaction between the nitric acid and the proteins.
- Introduce a small quantity of either ammonia solution or sodium hydroxide solution using a dropper.
- Shake the test tube thoroughly to mix the solution well.
- Observe any color changes in the precipitate, which signify the presence of proteins.
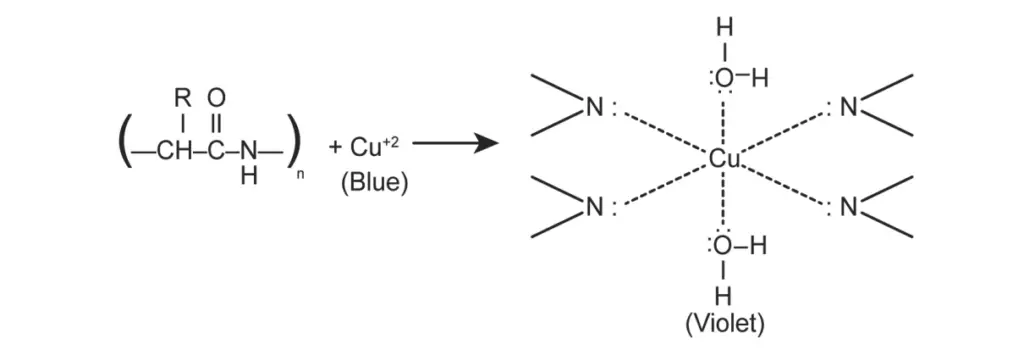
- Biuret Test:
- For this test, take a small amount of gram or soybean seeds and grind them into a fine powder.
- Place the powdered seeds in a test tube and add 10 drops of water, followed by a thorough shake of the mixture.
- Using a dropper, add two drops of copper sulfate solution.
- Subsequently, add 10 drops of sodium hydroxide solution with a dropper and shake the test tube again. As an alternative, Biuret reagent can be added directly instead of the two separate solutions.
- Allow the test tube to rest in a test tube stand for several minutes.
- Finally, observe the color of the mixture, which will indicate the presence of proteins.
- Observations:
- In the Xanthoproteic test, the initial yellow precipitate will change to an orange color upon the addition of alkali, confirming the presence of proteins. This reaction occurs due to the nitration of aromatic amino acids, leading to the formation of yellow-colored nitroderivatives.
- In the Biuret test, the appearance of a violet color indicates the presence of proteins. This test detects compounds containing two or more peptide bonds (CO-NH groups), which are characteristic of proteins and peptides. The alkaline copper sulfate reacts with these peptide bonds, forming a violet-colored product due to the coordination complex formed between cupric ions and the nitrogen in the peptide bonds.
Precautions
Precautions are critical when conducting experiments involving microchemical tests on legumes, such as Glycine max (soybean) and Cicer arietinum (gram). Ensuring safety and accuracy during these procedures not only protects individuals but also enhances the reliability of the results obtained. Therefore, following established precautions is essential.
- Attention to Detail: Each aspect of the plant structure must be observed and documented meticulously. Careful notation helps ensure that morphological features, such as root, stem, leaves, and inflorescence, are accurately represented, which is vital for understanding the biological characteristics of the specimens being studied.
- Safe Heating Practices: When heating samples, caution must be exercised to prevent accidents or unintended reactions. Heating should be conducted at a controlled temperature to avoid overheating, which may alter the composition of the sample or lead to the formation of hazardous fumes.
- Proper Test Tube Handling: During the heating process, test tubes must be positioned away from the body to minimize the risk of burns or spills. Holding test tubes with a test tube holder provides an additional layer of safety by ensuring that hands are kept at a safe distance from potential hazards.