Wastewater treatment or Sewage Treatment
- The purpose of wastewater treatment is to remove impurities from wastewater and transform it into effluent that may be reintroduced to the water cycle.
- Once restored to the water cycle, wastewater has a minimal influence on the environment or is reused for a variety of applications (called water reclamation).
- A wastewater treatment facility is the location of the treatment process. There are numerous types of wastewater, each of which is treated in a specific type of wastewater treatment facility.
- The treatment plant for domestic wastewater (also known as municipal wastewater or sewage) is known as a sewage treatment plant.
- For industrial wastewater, either a separate industrial wastewater treatment facility or a sewage treatment plant is used for treatment (usually after some form of pre-treatment).
- Agricultural wastewater treatment plants and leachate treatment plants are other types of wastewater treatment facilities.
- Typical wastewater treatment techniques include phase separation (e.g., sedimentation), biological and chemical processes (e.g., oxidation), and polishing.
- The principal byproduct of wastewater treatment facilities is a type of sludge that is often processed in the same or a different wastewater treatment plant:
- Ch.14 Another byproduct of anaerobic treatment procedures is biogas.
- Reclaimed water can be produced from treated wastewater. The primary objective of wastewater treatment is to render treated wastewater safe for disposal or reuse.
- However, before wastewater is treated, choices for disposal or reuse must be evaluated so that the appropriate treatment method may be used.
- Frequently, “wastewater treatment” refers to “sewage treatment.”
Characteristics of Wastewater
Chemical Characteristics
- Approximately 99.9 percent of domestic wastewater or sewage comprises of water, 0.02 to 0.03 percent suspended particles, and other soluble organic and inorganic components.
- On a percentage basis, the amount of solids appears to be little; yet, the enormous volume of material handled daily by a major municipal plant (e.g., hundreds of millions of gallons) contains up to 100 tonnes of solids.
- The chemical ingredients, albeit present in low amounts, are quite significant and sensitive to variation, both within and within communities, and even from hour to hour. Inorganic chemicals initially present in the water supply will also be present in the sewage; human faeces and other home wastes provide organic compounds, while industrial wastes contribute both organic and inorganic components.
- Examples include slaughterhouses, sugar plants, paper mills, and creameries; mines and metal businesses provide acids and salts of metals and other inorganic wastes.
- The organic molecules in sewage are categorised as either nitrogenous or nonnitrogenous. The most important nitrogenous chemicals are urea, proteins, amines, and amino acids; nonnitrogenous compounds include carbohydrates, lipids, and soaps.
- Modern technologies may result in major changes to the nature of sewage.
- Utilization of domestic waste disposal machines has increased the overall organic load.
- Some synthetic detergents that have replaced soaps are resistant to microbial breakdown.
Biochemical Oxygen Demand (BOD)
- The biochemical oxygen demand (Boo) is a measurement of the quantity of oxygen consumed in the respiratory processes of bacteria to oxidise organic materials in sewage and for the subsequent metabolism (oxidation) of cellular components generated from wastes.
- One of the fundamental reasons for treating wastewater before returning it to a body of water (e.g., a stream or lake) is to lessen the demand on the dissolved oxygen supply of the receiving body of water.
- The magnitude of the BOlD is proportional to the amount of oxidizable organic material in the wastewater; that is, the higher the BOO, the greater the amount of oxidizable organic material.
- BOD level is used to quantify the “strength” of wastewater.
Microbiological Character
- Since the composition of wastewater varies, it is reasonable to anticipate that the types and numbers of organisms will vary. Fungi, protozoa, algae, bacteria, and viruses are found.
- Raw sewage may contain millions of bacteria per millilitre, such as coliforms, streptococci, anaerobic spore-forming bacilli, the Proteus group, and other species originating from the human gastrointestinal tract.
- Additionally, faeces may contain harmful protozoa, bacteria, and viruses. Sewage may include the pathogens that cause dysentery, cholera, and typhoid fever.
- The poliovirus, infectious hepatitis virus, and coxsackie viruses are expelled in the faeces of infected people and may therefore be found in sewage.
- Certain viruses can be easily isolated from sewage.
- In the process of sewage digestion, the predominant physiological kinds of bacteria may change.
- Initially, facultative kinds (Enterobacter, Alcaligenes, Escherichia, Pseudomonas, etc.) prevail in an anaerobic digester.
- This is followed by stringent anaerobes that create methane, such as Methanobacterium, Methanosarcina, and Methanococcus.
- The organic acids generated by facultative bacteria are digested by methane-forming bacteria, resulting in methane and carbon dioxide.
- These gases are produced in large quantities by anaerobic digesters. Significant shifts in the major species of organisms occur as a result of the numerous procedures involved in wastewater treatment.
- The relevance of these alterations will be examined in the future.
Wastewater Treatment Process
Diverse wastewater treatment technologies exist. We shall explain the treatment methods as they pertain to two distinct circumstances: (1) a single home or unit structure, and (2) a community or municipality.
1. Single Dwelling Unit
Anaerobic digestion and/or aerobic metabolic processes can be used to treat and dispose of wastewater and sewage from single-family homes and other unit structures (e.g., some motels and shopping centres). The septic tank, an anaerobic digestion system, is one of the more frequent installations used to do this.
a. Septic Tank
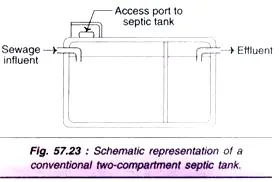
- A septic tank is a sewage-settling tank designed to contain the solids of the sewage entering the tank for a sufficient amount of time to allow for adequate sludge breakdown.
- Thus, the unit performs two processes: sedimentation and biological sludge degradation.
- As sewage enters this sort of tank, sedimentation happens from the upper portion, allowing the tank to discharge a liquid with fewer suspended particulates.
- The sedimented particles are degraded by anaerobic bacteria; hence, the final products are extremely unstable, i.e., high in BOD and pungent.
- Through a disposal field, the effluent from the septic tank is spread beneath the soil’s surface.
- In the absence of public sewers, septic tanks are the most effective way to dispose of sewage from small installations, particularly single-family homes and rural businesses in remote areas.
- However, they cannot be depended upon to eradicate pathogenic germs in sewage. Therefore, it is essential to prevent the tank’s drainage from compromising the drinking water supply.
Septic Tank Mechanism
- Septic tanks are advised for single-family homes, small villages, and institutions with a population of less than 300.
- The operation of septic tanks is based on anaerobic digestion. As the sewage solids settle to the bottom of the tank, this occurs.
- Biodegradable organic matter is transformed to gases (CH4, CO2, H2S, etc.) and liquid compounds in anaerobic conditions. This results in a significant reduction in sludge volume.
- On the surface of a septic tank, a thick film of scum maintains anaerobic conditions. There are some organic materials and pathogens in the effluent from the septic tank.
- Therefore, the disposal of septic tank effluent must be handled with extreme care. The septic tanks are removed and cleaned on a regular basis, often once every two to five years (depending on the tank size and its use).
Construction and operation of septic tanks
- Typically, septic tanks are constructed with brick or stone masonry. In recent years, thick-walled polyethylene and fibre glass tanks have also been utilised.
- Regardless of the building material employed, the septic tank must be watertight and functional.
- The tank’s capacity varies according to the number of users. For a family of five, a tank of 1.5 m in length and 0.75 m in width is recommended. The recommended cleaning interval for such a tank is 1-2 years.
- The first compartment of a traditional septic tank is double the size of the second compartment. For proper sedimentation of solids, the tank must be built to prevent short circuits at the tank’s top and bottom.
- In addition, the location of the inlet and outflow should be such that the septic tank’s contents are not disturbed as sewage enters or as effluent exits.
- The septic tank should have a ventilation line with a mosquito-proof wire mesh covering its top.
- As sewage enters the septic tank, solids sink to the bottom, while oil and other light substances float to the surface and form scum.
- The organic material that has settled to the bottom undergoes facultative and anaerobic decomposition to produce more stable chemicals and gases (CH4, CO2, and H2S).
- In this manner, the amount of solid sewage entering the septic tank decreases continuously. Nonetheless, sludge collects near the tank’s base.
- Periodically, septic tank de-sludge must be performed (once in 2-5 years).
b. Outdoor Toilets
- Where plumbing installations cannot be completed for any reason, outdoor toilets or water closets may be installed.
- While this arrangement is being implemented, care could be made to ensure that flies do not have access to these and that alterations in drainage from these to water sources are eliminated.
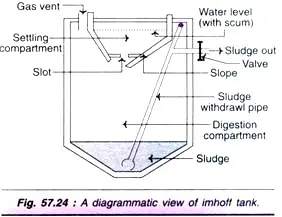
c. Imhoff Tank
- This is a variation of a septic tank and is typically used to treat sewage from larger communities.
- There are two chambers, one atop the other. The upper chamber is filled with sewage, while the heavier particles settle into the bottom chamber and decay slowly under anaerobic conditions.
- The released gas (mostly methane) can be extracted through a tunnel and used as fuel.
- The sewage effluent (remaining sewage water) is either discharged into a bigger body of water or decomposed aerobically.
- Periodically, the sludge is removed, aerated, and utilised as fertiliser.
2. Municipal Treatment Processes
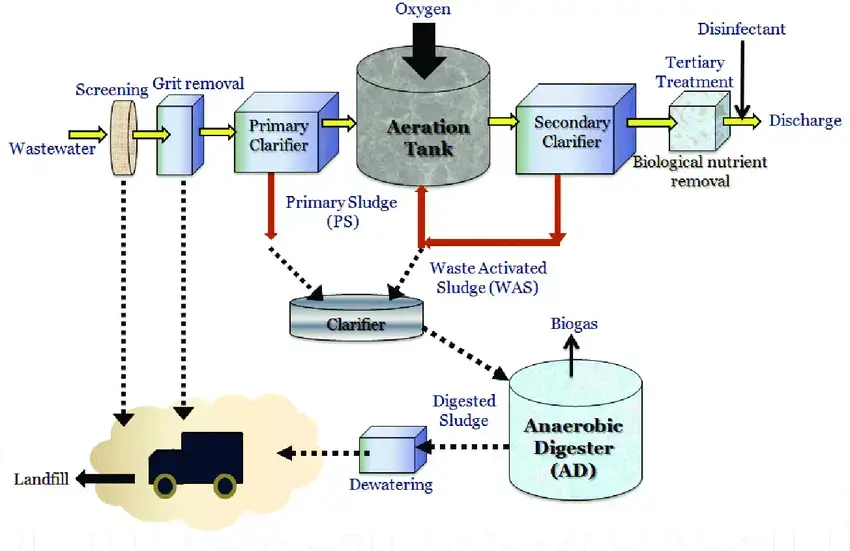
The municipal wastewater-treatment process can be summed up as follows.
- Preliminary Treatment: The purpose of preliminary treatment is to remove huge debris and heavy inorganic material from the wastewater flow.
- Primary treatment: Primary treatment consists of removing coarse materials and removing settleable solids.
- Secondary treatment (biological): To adsorb and subsequently oxidise organic wastewater elements, i.e., to reduce BOD.
- Advanced treatment: To remove other unwanted components to further lower BOD; removal of nutrients such as phosphate and nitrogen is included.
- Final treatment: Final treatment consists of disinfecting and removing liquid effluent.
- Solids processing: Solids processing entails stabilising solids extracted from liquid processes, dehydrating solids, and finally disposing of solids (land application, landfill, incineration).
1. Preliminary Treatment
- Wood, rags, plastics, and other waste are present in the influent of treatment plants. In addition to organic matter from residential, industrial, commercial, and institutional water consumption, sand, eggshells, and other coarse inorganic particles are present in the flow.
- The purpose of preliminary treatment is to remove huge debris and heavy inorganic material from the wastewater flow.
- Screening the incoming wastewater flow is one of the initial steps in the treatment process.
- This debris is removed using screens composed of parallel bars or stepped plates set at an angle in the course of the wastewater flow.
- Mechanical rakes remove debris from the bars, after which the screens are cleansed, compressed to remove excess water, and buried in a landfill.
- The removal of these materials safeguards the treatment plant’s pipelines and equipment from obstruction and/or damage.
Screening
- After screening, wastewater flows through aerated pipes designed to reduce the flow velocity to 0.3 metres per second.
- Here, heavy inorganic elements separate and settle from the effluent. The deposited inorganic debris is known as grit.
- The accumulated grit is periodically scraped from the channels, cleaned, and subsequently disposed of via burial in a landfill.
- Due to the abrasive nature of grit, its removal early in the treatment process reduces pump and other equipment wear.
- Otherwise, this inorganic waste would eventually settle in other process zones and consume treatment capacity or volume.
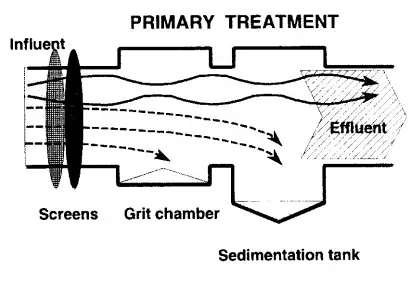
2. Primary Treatment
- A range of mechanical processes, including screening, grinding, and grit chambers, are utilised to remove coarse solids from incoming wastewater in a wastewater treatment facility. The wastewater is next treated to eliminate settleable solids.
- Primary sewage treatment eliminates 60% of suspended particles, 30% of COD, 35% of BOD, 10% of phosphorus, and 20% of total nitrogen.
Primary Treatment Process
It consists of the following procedures:
- Sedimentation: Under quiet conditions, around 50% of suspended solids can be removed via gravity settling.
- Mechanical Flocculation and Coagulation: Fine suspended solids and colloidal particles are eliminated by passing waste water through a clariflocculator and employing coagulants such as alum and polyelectrolytes.
- Neutralization: Extremely acidic and alkaline waste fluids are neutralised using lime slurry, NaOH, and H2SO4 or carbon dioxide, respectively.
Benefits of primary treatment
Advantages of basic wastewater treatment include:
- Controlling suspended solids
- Decrease in BOD
- Reduction in waste activated sludge volume
- Elimination of floating debris
- Lastly, a partial balancing of flow rates and organic load.
3. Secondary (Biological) Treatment
- This is an entirely biological treatment of sewage that has been mechanically treated, and it involves microbial activity that biodegrades organic substrates and oxidizable inorganic chemicals.
- This treatment achieves two crucial phases, namely the aerobic phase and the anaerobic phase.
- The aerobic phase consists of the aerobic digestion of sludge using various filters (such as trickling filters), oxidation ponds, and the activated sludge process, whereas the anaerobic phase consists of the anaerobic digestion of sludge.
I. Aerobic Phase of Secondary Treatment
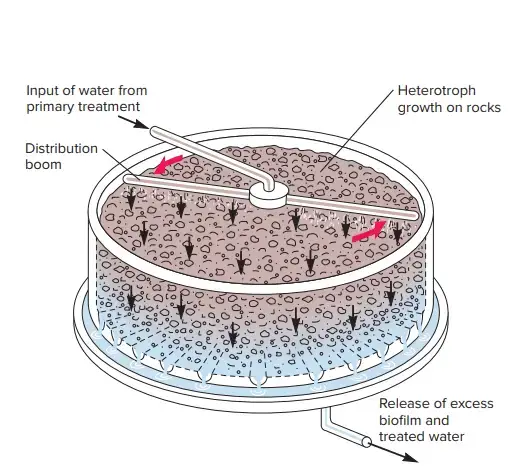
A. The Trickling Filter
- The bed of crushed stone, gravel, slag, or synthetic material with drains at the bottom of the tank constitutes the trickling filter.
- A trickling filter is described as “a pile of rocks over which sewage or organic wastes drip gently.”
- Either a revolving arm or nozzles are utilised to spray the liquid sewage across the surface of the bed. The spraying of the liquid saturates it with oxygen.
- The intermittent application of sewage enables the bed to maintain aerobic conditions.
- The filtering media of the tank becomes coated with the zoogloeal film, a microbial flora composed of bacteria, fungus, protozoa, and algae.
- As sewage percolates over these surfaces, bacteria absorb the organic elements and convert them into more stable end products.
- This process can be viewed as a stationary microbial colony (on the stones) receiving a steady supply of nutrients (organic constituents of the sewage).
- A newly created bed need the zoogloeal film in order to function well. This must be accomplished over the course of several weeks.
- The upper portion of the trickling filter is conducive to the growth of algae, which can sometimes become so extensive as to hinder the filter’s operation.
- Numerous protozoan and fungal species populate the filter; their abundance is determined by the availability of oxygen and nutrients.
- In such a varied environment, the microbial activities and interactions are evidently extraordinarily complex.
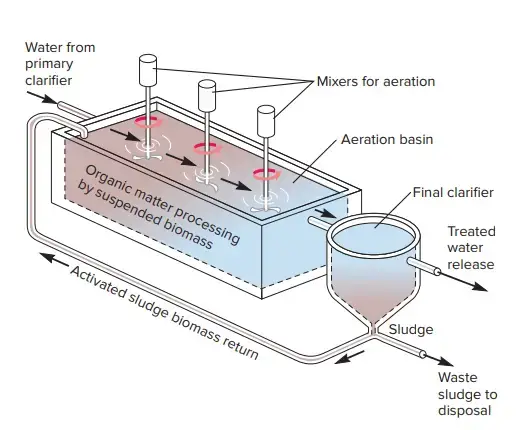
B. The Activated-Sludge Process
- Sewage subjected to vigorous aeration forms floc; the finely suspended and colloidal materials of sewage forms aggregates known as floccules.
- If this floc is allowed to settle and then reintroduced to new sewage that has been rapidly aerated, flocculation occurs more quickly than previously.
- By repeating this process, i.e., addition of sedimented floc to fresh sewage, aeration, sedimentation, addition of sediment to fresh sewage, aeration, etc., a stage is achieved in which complete flocculation of the fresh sewage happens in a few hours.
- These floc particles, also known as “activated sludge,” contain a huge number of bacteria that are actively metabolising, in addition to yeasts, moulds, and protozoa.
- This combination of microbial growth is extraordinarily powerful at oxidising organic molecules.
- A poor settlement of activated sludge flocs has a negative impact on the functioning of a sewage treatment facility. The sludge grows increasingly dense and harder to manage.
- The proliferation of filamentous bacteria is the leading cause of poor settling (bulking) of activated sludge.
- In this situation, numerous kinds of bacteria have been identified from sludge.
- The utilisation of activated sludge is crucial for wastewater treatment. The mixture is then pumped to a sedimentation tank after an aeration time of 4 to 8 hours.
- The effluent from these tanks represents secondary-level treated wastewater; there is a significant reduction in suspended particles and BOD.
- This technique of treatment is advantageous in that it requires a little amount of land and the finished effluent does not require a significant degree of dilution before disposal.
C. Oxidation Ponds (Lagoon)
- Oxidation ponds (also known as lagoons or stabilisation ponds) are shallow ponds (two to four inches in depth) designed to promote algae development in wastewater effluent.
- Utilization of oxidation ponds should follow initial treatment.
- Oxygen for biochemical oxidation of nutrients is given by air, while Chlorella pyrenoidosa’s release of O2 during photosynthesis provides an additional significant supply of oxygen.
II. Anaerobic Phase of Secondary Treatment (Anaerobic Digestion of Sludge)
- The sludge collected after the main (mechanical) treatment of sewage is digested anaerobically (without oxygen) in a tank designed specifically for the purpose.
- Since anaerobic conditions predominate in this tank, the anaerobic microorganisms degrade organic matter into soluble chemicals and gaseous products (60–70% methane, 20–30% carbon dioxide, and lower amounts of H2 and N2).
- This gas mixture can be utilised to power the wastewater treatment facility or as a fuel.
- Recently, the Municipal Corporation of Delhi began providing this gas combination for cooking to around 100,000 individuals.
4. Advanced Treatment
- When it is necessary to remove chemicals in excess of those ordinarily removed by standard primary and secondary processes, advanced wastewater treatment is required.
- To remove nutrients, simple organic molecules, and complex synthetic organic chemicals, unit procedures have been created.
- There are biological processes, but physical-chemical methods prevail.
- Typical unit processes include of biological nitrification-denitrification, filtration, reverse osmosis, carbon adsorption, chemical addition, and ion exchange.
- The cost is the primary downside of sophisticated treatment processes.
5. Final Treatment or Tertiary treatment
- After other treatments are completed, the liquid effluent is typically disinfected and dumped into a body of water.
- When receiving waters are used for downstream water supply, recreation, agriculture, or shellfish harvesting, it is required to disinfect wastewaters to preserve public health. The vast majority of facilities employ chlorine for disinfection.
- Current study has demonstrated that chlorinated waters have a detrimental effect on the aquatic life of the receiving water. This has resulted in the development of alternate disinfection methods.
- Utilization of ozone and UV light is increasing. Many facilities that continue to use chlorine for disinfection now dechlorinate their effluent before releasing it into a body of water.
- Before final disposal, dissolved oxygen may also be added to treated wastewater.
- This procedure is referred to as previous aeration and is carried either by mechanical means or a slow cascading technique.
- Post-aeration reduces the drop in dissolved oxygen in the receiving water that ordinarily occurs after the discharge of treated wastewater effluent.
- Although surface water discharge is a common practise, land application has been and remains a viable alternative to surface water discharge.
- The Federal Water Pollution Control Amendment of 1972 gave land application of wastewaters major recognition in order to fulfil the “national aim of eliminating the discharge of pollutants into navigable waters by 1985.”
- It is true that municipal and industrial wastewaters have been employed for crop irrigation on a national scale.
- In the coming decade, we are expected to give land application of treated liquid waste from wastewater treatment plants greater consideration.
Tertiary treatment Summary
- Since the effluent after secondary treatment still contains non-biodegradable organic contaminants (if sewage contains industrial wastes) and mineral nutrients, especially nitrogen and phosphorus salts, it is subjected to tertiary (or final) treatment.
- If not, the nitrogen and phosphorus salts in sewage effluents can cause severe eutrophication in aquatic habitats. Activated carbon filters are typically used to remove non-biodegradable organic contaminants, whereas phosphorus and nitrogen salts are chemically treated.
- At a high pH, phosphorus salts are precipitated by liming, while ammonia-dominated nitrogen is removed by volatilization (vigorous aeration at elevated temperature). These treatments produce effluent of high quality that does not induce eutrophication.
- The first phase in tertiary treatment is disinfection, which is often achieved through chlorination with sodium or calcium hypochlorite (NaOCl or CaOCl2, respectively) or chlorine. Now, the effluent is considered microbiologically safe for human consumption and is therefore pure water.
Tertiary treatment process
- Tertiary treatment is the ultimate treatment for eliminating secondary effluents and removing fine suspended particles, organic traces, and microorganisms. In a flocculation tank, lime is added to the effluent from the secondary treatment plant to eliminate calcium phosphate.
- The solution is then injected into an NH3 stripping tower. At high pH, waste water nitrogen occurs as NH+4, which is transformed to gaseous ammonium ion (ll). Phosphorus is eliminated with the addition of ferric chloride or aluminium sulphate. Desalination, ion exchange, and chlorination are employed to remove the remaining organic materials and sterilise the water.
- Using adsorption (on activated charcoal), ion exchange, ultrafiltration, reverse osmosis, and electrodialysis, harmful, non-biodegradable compounds in industrial waste water can be eliminated.
6. Solids Processing
- Solids processing — the thickening, stabilisation, dewatering, and disposal of sludge — is a significant expense for modern wastewater treatment facilities.
- In the primary, secondary, and advanced stages of the treatment process, solids are eliminated.
- Typically, thickening is used to further concentrate the solids or sludge before stabilisation. Gravity thickening, analogous to sedimentation, or dissolved air flotation may be utilised to achieve thickening.
- Numerous stabilising techniques, including aerobic and anaerobic digestion, composting, chemical addition, and heat treatment, are utilised.
- The anaerobic digestion system is the most prevalent method employed by modern municipal treatment plants.
- Physical methods are utilised to achieve dewatering, which is frequently increased by the inclusion of polymer or other chemical coagulant aids.
- Vacuum filters, belt filter presses, plate and frame presses, and centrifuges are used for dewatering.
- Sand filter beds are still used to get rid of water in small treatment plants and older plants.
a. Anaerobic Sludge Digestion
- The solids that build during sedimentation are pumped into a separate tank specifically constructed for the controlled digestion of sludge.
- Solids recovered during aerobic treatment can also be reintroduced to the sludge digester.
- The microbial action on sludge components is known as sludge digestion.
- In these tanks, anaerobic conditions prevail, and both anaerobic and facultative microorganisms are active.
- These microbes reduce organic materials by breaking them down into soluble chemicals and gaseous products.
- During sludge digestion, large quantities of methane (60 to 70 percent), carbon dioxide (20 to 30 percent), and lower amounts of hydrogen and nitrogen are created.
- This gas mixture can be utilised as a fuel for both heating and power generation.
- Conditions impacting microbial growth and metabolism, such as inoculum, pH, and temperature, will be reflected in the degree of sludge digestion.
- Fresh sludge entering the tanks is inoculated with mature sludge, or seeded (sludge which has already undergone digestion).
- The mature sludge can be viewed as an actively expanding culture of microorganisms essential for quick sludge digestion.
- Important are the amount of inoculum employed and its complete mixing with the fresh sludge. The most fast digestion of sludge occurs at temperatures (50 to 60°C) that promote the growth of thermophilic bacteria.
- A neutral pH (7.0) is best for sludge digestion. These circumstances can only be regulated in tanks with a specific design.
- The temperature is kept around 30°C, which is below the above-mentioned ideal for the most rapid digestion, as thermophilic temperatures have not been shown to be practical.
- Complete digestion requires at least two to three weeks.
- In a contemporary wastewater treatment facility, the residual digester sludge is transferred to a mechanical drying machine.
- The sludge dries into “cakes” that are transported to incinerators using conveyor belts. The incinerator transforms the sludge cake into ash, which is then disposed of in a landfill.
- Alternately, stabilised dewatered sludge can be spread on land. Multiple municipalities use land-utilization approaches to dispose of processed sludge.
- However, such a practise necessitates affordable and accessible land. In addition, the volume of sludge produced by a large facility might be astonishing.
b. Composting
- Composting is the decomposition of dehydrated sludge, often within the thermophilic temperature range.
- Mixing dehydrated sludge with a bulking agent such as wood chips. The bulking material is added to help the stabilisation process by increasing air circulation throughout the sludge.
- The sludge and bulking material mixture is deposited in piles that are aerated. Oxygen is supplied by means of forcible aeration.
- The combination is left to cure or degrade biologically for a length of time.
- This time span is typically regarded to be 21 days for effective stabilisation.
- The bulking agent is extracted from the sludge after 21 days, and the sludge is allowed to continue cure for many weeks.
- Final curing transforms the sludge into a humus-like substance that is appropriate for use as a soil conditioner.
Chemical Treatment
- Although primary and secondary treatment procedures are effective in removing the majority of pollutants from wastewater, some pollutants require specialised forms of treatment.
- Phosphorous is one of these hazardous pollutants. Phosphorus included in the final effluent of a wastewater treatment facility could have a detrimental effect on receiving waters if left untreated.
- Phosphorous is one of the most important nutrients for aquatic plant growth. Human waste, detergents containing phosphate additions, corrosion control agents used in water supplies, and industrial emissions are all phosphorous sources.
- High quantities of phosphorus in receiving waterways promote excessive growth of algae and aquatic plants, which may disturb the receiving water’s natural biological balance.
- Rapid deterioration of water quality may hasten the eutrophication of the receiving water body. Phosphorus removal procedures can be classified as biological or chemical precipitation approaches.
- Utilizing a metal salt that reacts with soluble phosphorous to generate an insoluble precipitate is the current approach.
- During settling operations, this precipitate settles with the sludge and is therefore eliminated from the wastewater flow. The most often used metal salt is ferrous chloride, popularly known as “pickled liquor.”
- This metal salt solution is an easily accessible byproduct of steel production.
- Typically, iron solution application points are located immediately upstream of the major settling tanks, at the influent end of the aeration tanks, or at both locations simultaneously.
- Chemicals used for phosphorous precipitation must be well blended with wastewater to guarantee uniform dispersion and maximise removal efficiency.
- The final effluent is disinfected at wastewater treatment facilities, which is another crucial chemical treatment.
- Due to human emissions, disease-causing or pathogenic microorganisms may be present in all wastewaters. These microorganisms must be eliminated or killed prior to the discharge of treated wastewater into receiving waters.
- Chlorination for the aim of disinfection destroys virtually all dangerous microorganisms, hence preventing the spread of waterborne diseases.
- In order to further preserve receiving waterways, sodium bisulphate is added to dechlorinate wastewater effluent before to discharge.
Dewatering
- Dehydration is a solids processing activity. Biosolids stabilised after secondary anaerobic digestion are dehydrated to eliminate excess water.
- This technique decreases the particles’ volume and boosts their dryness prior to further processing.
- Through the use of mechanical belt filter presses, feed solids with typically 1.5 to 2% solids content are transformed into filter cake with typically 18 to 19% solids content.
- The feed solids are treated with a polymer-coagulating agent and compressed between filter belts made of woven mesh.
- The filtrate and wash water used to clean the woven filter belts are reintroduced into the wastewater flow for further treatment.
Discharge Criteria
Through the issuance of Certificates of Approval, the Ministry of the Environment determines and sets the permitted limits for effluent released from wastewater treatment facilities and rated hydraulic capacities within the province in order to protect the quality of our natural water resources. As expected, the size of the receiving water is a significant factor in determining acceptable discharge limits. Typically, discharge regulations are stricter the smaller the body of water that the receiving stream represents. The primary criteria for which discharge standards are established are:
- Rating for hydraulic capacity.
- TOD total oxygen demand.
- BOD biochemical oxygen demand.
- Total phosphorus (TP).
- Total suspended solids (TSS)
- CR chlorine residual.
- NH3 ammonia nitrogen.
- TKN total kjeldahl nitrogen.
- E. coli (EC).
Community growth-related issues are governed by hydraulic capacity ratings to ensure enough treatment capacity is available and maintained.
Excessive BOD in the final effluent may decrease the water body’s oxygen supply and lead to stagnant conditions if it exceeds the receiving water’s natural cleansing capacity.
High concentrations of TP or NH3 in effluent will promote the growth of algae and aquatic plants, which could disrupt the natural ecological cycle of the receiving water.
Large TSS discharges may also cause oxygen depletion due to the breakdown of organic matter in the receiving water.
Inadequate disinfection of a treatment facility’s final effluent may result in the discharge of harmful bacteria, as determined by FC bacteriological examination, to receiving waters, hence contributing to an outbreak of waterborne disease.
Measuring Treated Wastewater Quality
- The wastewater treatment process must be monitored to ensure that the discharged waters do not cause environmental or health hazards.
- Effectively treated water should contain a negligible amount of organic carbon. Carbon can be evaluated as total organic carbon (TOC), chemically oxidizable carbon by the chemical oxygen demand (COD) test, or biologically useable carbon by the biochemical oxygen demand (BOD) test during and after wastewater treatment.
- The TOC comprises all carbon, regardless of whether it is used by bacteria. It is measured by oxidising all organic materials in a sample to CO2 in an oxygen stream at high temperature.
- CO2 is measured using infrared or potentiometric techniques.
- The COD provides a comparable measurement, with the exception that lignin frequently does not react with the oxidising chemical employed in this method.
- The BOD test analyses only the portion of total carbon oxidised (consumed) by microorganisms over a five-day period at 20 degrees Celsius.
- It quantifies the quantity of dissolved O2 required for microbial oxidation of biodegradable organic matter.
- Ammonia produced during the oxidation of organic materials might also consume O2 during the BOD test.
- To prevent this “bottle effect,” 2-chloro-6-(trichloromethyl) pyridine is often used to suppress nitrification (nitrapyrin).
- Unless treated effluents are evaluated, nitrification is not a significant issue. TOC, COD, and BOD measurements are solely concerned with carbon removal.
- They do not specifically address the elimination of minerals like nitrate, phosphate, and sulphate. Contributing to eutrophication, these have an impact on the growth of cyanobacteria and algae in lakes, rivers, and seas.
Wastewater Treatment Methods Summary
Physical operations
- Screening: Screening is the mechanical separation of large suspended particles by means of parallel bars, rods, wires, grating, wire mesh, or perforated plates.
- Comminution: Before wastewater enters the primary settling tank, comminutors are employed to pulverise large floating material into fine particles.
- Flow equalization: Flow equalisation is the use of a basin or basins to regulate the rate of wastewater intake into secondary and tertiary treatment facilities.
- Sedimentation: This widely employed process, also known as clarifying, is based on the gravitational separation of big particles. Used in primary settling basins and activated sludge settling basins to separate biological floc. Also used to precipitate particles that have been chemically treated when chemical coagulation is applied.
- Flotation: The process of removing particles from water by bubbling air or other gas into it. The particles float to the surface and are collected by skimming; this method is more effective than sedimentation at removing fine particles. To boost particle removal, chemical additives can be used.
- Granular-medium filtration: Used to remove suspended solids and chemically precipitated phosphorus by passing wastewater through a filter bed comprised of granular material such as sand.
Chemical operations
- Chemical precipitation: Addition of coagulants such as alum, ferric chloride, ferric sulphate, or lime to wastewater prior to sedimentation; promotes flocculation of finely divided particles into flocs that settle.
- Adsorption: The collection of soluble material through the adsorption of activated carbon; typically performed after biological treatment to remove any leftover dissolved organic carbon.
- Disinfection: UV light, heat, gamma radiation, and chemical agents such as chlorine, bromine, ozone, phenolic compounds, and others are used to eradicate infections (and other microorganisms).
- Dechlorination: Dechlorination is the elimination of all chlorine residues prior to their release into receiving waters. These chemicals are potentially hazardous and reactive.
Biological operations
- Activated sludge process: After first settling, the activated sludge process is an aerobic, continuous flow system that uses microbes to decompose organic matter aerobically. To maintain aeration and prevent settling, the material is mechanically blended.
- Aerated lagoon: A basin with a depth between 1 and 4 metres in which wastewater is treated by a method comparable to activated sludge treatment. Aeration generates turbulence that keeps the lagoon’s contents mixing.
- Trickling filters: The most typical aerobic attached-growth biological therapy is trickling filters. A huge basin packed with rock or plastic packing material provides a substrate for the growth of microbial biofilm and increases the wastewater’s contact surface.
- Rotating biological contactors: Attached growth process with enormous cylinders partially immersed in wastewater. Biofilms develop on the continually rotating cylinders.
- Stabilization ponds: Stabilization ponds are shallow basins that are naturally or artificially mixed. Aerobic ponds handle soluble organic wastes and wastewater effluents, whereas anaerobic ponds stabilise organic wastes. The retention periods for wastewater range from one to three months.
- Anaerobic digestion: Anaerobic digestion is used to remediate sludge and wastewater containing a high concentration of organic material.
- Biological nutrient removal: Nitrogen removal by nitrification-denitrification and anammox reaction, as well as phosphorus removal by proprietary techniques (such as A/O, PhoStrip, and BR procedure).
References
- grégorio, Crini & Lichtfouse, Eric. (2018). Wastewater Treatment: An Overview. 10.1007/978-3-319-92111-2_1.
- https://web.iitd.ac.in/~arunku/files/CVL100_Y16/LecSep1220.pdf
- https://www3.epa.gov/npdes/pubs/bastre.pdf
- https://ec.europa.eu/environment/europeangreencapital/wp-content/uploads/2011/05/EGCNantesUKChap10-F.pdf
- https://phedharyana.gov.in/WriteReadData/Notice/2%20STP%20(1)%20%5BCompatibility%20Mode%5D.pdf
- https://guelph.ca/wp-content/uploads/IntroductionToWastewater.pdf
- http://web.deu.edu.tr/atiksu/ana58/c-6.pdf
- https://files.dep.state.pa.us/water/bsdw/operatorcertification/TrainingModules/ww01_intro_to_ww_treatment_wb.pdf