Reverse transfection is a technique used in molecular biology to introduce a sample of RNA, DNA or protein into cells in culture. Instead of adding the reagents to the cells and allowing them to take them up (forward transfection), reverse transfection involves incubating the cells on a surface that is coated with the reagents. The cells then attach to the surface and take up the reagents through endocytosis.
The history of reverse transfection is unclear, but it is likely to have been developed in the 1980s and 1990s, when the first transfection methods were being developed.
The importance of reverse transfection lies in its ability to introduce RNA, DNA or protein into cells in a manner that allows for better control over the amount and timing of uptake, as well as a lower risk of toxicity. This makes it a useful tool for studying gene expression, protein function and cellular biology. Additionally, reverse transfection can be used to deliver therapeutic genes or proteins to cells, which is an important aspect of regenerative medicine and gene therapy.
Reverse transfection Definition
In order to introduce new DNA into a cell, scientists employ a method called reverse transfection. For this method of transfection (the intentional introduction of nucleic acids into cells), DNA is printed on a glass slide and added to the culture before the adherent cells are added. In this sense, the word “reverse” is appropriate.
Process of Reverse transfection
Transfection-mix preparation for slide printing
- Slides can be printed with a DNA-gelatin mixture. It all starts with a 0.2% gelatin solution, which is made by dissolving gelatin powder in sterile Milli-Q water.
- The final concentration of gelatin is kept at or above 0.17%, and it is combined with purified DNA plasmid.
- Additionally to gelatin, atelocollagen and fibronectin have proven to be efficient transfection vectors for delivering foreign DNA into the nucleus of a cell.
Slide printing of DNA-gelatin mixture
- After the DNA-gelatin mixture has been prepared, it is pipetted onto a slide and then placed in a petri dish with the lid on. The solution is dried up by adding a desiccant to the plate.
- Last but not least, plated cells are added to the dish for plasmid uptake. However, with the development of various microarray printing techniques, hundreds of transfection mixes (each containing a unique DNA of interest) can be printed on a single slide to test the efficiency with which cells ingest plasmids.
- Microarray printing systems can be broken down into two broad categories: contact printing systems and non-contact printing systems.
- The Piezorray Flexible Non-contact Microarraying System is a non-contact printing system. Drops of about 333 pL in volume can be consistently squeezed out thanks to the pressure control and piezoelectric collar.
- There is less chance of cross-contamination and disruption of the target surface because the PiezoTip dispensers don’t touch the surface. A contact-spotting system such as the SpotArray 72 (Perkin Elmer Life Sciences) is an example of a contact printing system.
- It can print small arrays by raising and lowering groups of pins on its printhead, which can hold up to 48 in total. Carryover is avoided by washing the pins with a high-powered pressure-jet pin washer and then drying them in a vacuum after printing.
- The Qarray system is another kind of contact printing system (Genetix). The QArray Mini, QArray 2, and QArray Max are its three distinct printing systems. Once the ink has dried, the solution is allowed to dry, and the DNA-gelatin is fixed firmly in place on the array.
HybriWell in reverse transfection
- The HybriWell is then adhered to the slide over the region where the gelatin-DNA solution was printed. The second step is to pipette 200ul of transfection mix into one of the HybriWell ports; the solution will then flow through all of the wells in the array.
- After that, the array is incubated for a period of time and at a temperature that are specific to the cells they contain. The HybriWell is next detached from the transfection mixture using a thin-tipped forceps, and the third step is to pipette away the transfection mix.
- In step four, the transfection reagent-treated printed slide is positioned, printed side up, in a square dish.
- And then, in step five, the collected cells are carefully deposited onto slides (not on the printed areas). At the end, the dish is incubated at 37 degrees Celsius, 5% carbon dioxide humidity, for a full 24 hours.
Other reverse-transfection reagents
- The DNA condensation buffer (Buffer EC) and the enhancer work together with the Effectene Reagent to produce optimal transfection results.
- First, the DNA interacts with the enhancer in a defined-buffer system, which causes the DNA to condense. The condensed DNA is then mixed with Effectene Reagent to form Effectene-DNA complexes.
- When the Effectene-DNA complexes are introduced to the cells, they are diluted in the culture medium. The micelles formed by the Effectene Reagent are uniform in size and do not vary from batch to batch (as may be found with pre-formulated liposome reagents).
- This characteristic guarantees consistent complex formation during transfection experiments. Transferring DNA into eukaryotic cells is facilitated by highly compressing DNA molecules and covering them with Effectene Reagent.
Transfection Reagents That Can be Used for Reverse Transfection
All of the TransIT® transfection reagents can be utilised for reverse transfections with the following nucleic acid delivery considerations:
- Plasmid DNA, including plasmid DNA libraries encoding cDNA and shRNA: TransIT®-Express Transfection Reagent is a low-toxicity DNA transfection reagent created primarily for high-throughput applications and is effective with commonly utilised cell types. Modern transfection reagents with great efficacy, such as TransIT®-2020 Transfection Reagent, can also be utilised for the reverse transfection of DNA into cells that are difficult to transfect. In addition, all of the cell line-specific TransIT® transfection reagents can be utilised for reverse transfection of plasmid DNA into corresponding cell types. Here you can find a thorough technique with citations for reverse transfection of plasmid DNA using TransIT® reagents.
- siRNA/miRNA: Depending on the cell type, either TransIT-TKO® or TransIT-siQUEST® Transfection Reagent can be used for high-throughput transfection of siRNA/miRNA. Here you can find a full methodology with citations for the reverse transfection of siRNA using TransIT-TKO® reagent.
- Large mRNA: Using the TransIT®-mRNA Transfection Kit, reverse transfection of larger RNA species, such as mRNA, can be done.
- Both DNA and RNA Oligonucleotides: Using TransIT®-Oligo Transfection Reagent, a high throughput reverse transfection of various DNA and RNA oligonucleotides is possible.
Reverse transfection Protocol
Previous to the experiment: Prepare an Excel file containing all experiment-related calculations.
Materials
- a culture vessel holding the cells that is 80% confluent.
- Complete growth medium preheated to 37 degrees Celsius
- Prewarmed Phosphate-Buffered Saline (PBS) at 37°C.
- Prewarmed dissociation reagent, such as trypsin, at 37°C.
- Polyethyleneimine (PEI).
- Low serum medium, including Opti-MEM.
- plasmids of DNA at the desired concentration.
- The quantity of materials appropriate for a 10 cm2 plate.
Protocol
- Utilize a 70% ethanol spray to clean the hood and all the materials you will be utilising.
- Prepare the required amount of DNA in a 1.5 ml Eppendorf. **In the event of co-transfection, divide the whole amount of DNA between two plasmids.
- Using a vacuum pump, remove and discard the spent cell culture media from the culture vessel.
- Cells are washed with PBS (approximately 5 mL per 10 cm2 culture surface area). Add the wash solution to the vessel’s side with care and rock it back and forth several times.
- Remove the wash solution from the culture vessel and dispose of it.
- Add 2 mL of trypsin enzyme to the plate’s middle. To ensure that the cell layer is completely covered, gently shake the container.
- Incubate the culture vessel for approximately 2 minutes at 37 degrees Celsius.
- Observe the detachment of the cells under a microscope. Return the plate to the hood and add 8 mL of full growth medium when 90% of the cells have detached.
- Pipette the medium over the cell layer surface multiple times to disperse it. Avoid spores by transferring all 10 mL of cells to a 15 mL falcon and continuing to pipet.
- Mix 10 l of the cell suspension with 20 l of blue dye in an Eppendorf tube.
- Count the cells with a hemocytometer under a microscope.
- Adjust the concentration of the cell suspension stock with full growth medium.
- Mix PEI and OptiMEM according to the desired DNA:PEI ratio, then wait 5 minutes.
- Carefully pour the PEI-OptiMEM mixture to the DNA and thoroughly combine.
- Wait 15 minutes for the formation of DNA:PEI complexes.
- Add the required amount of DNA:PEI solution to each well of the transfection plate (6 wells plate, 96 wells plate etc.).
- Add the required quantity of cells to each well of the plate, and then rock the plate back and forth.
- At 37°C, incubate the plate.
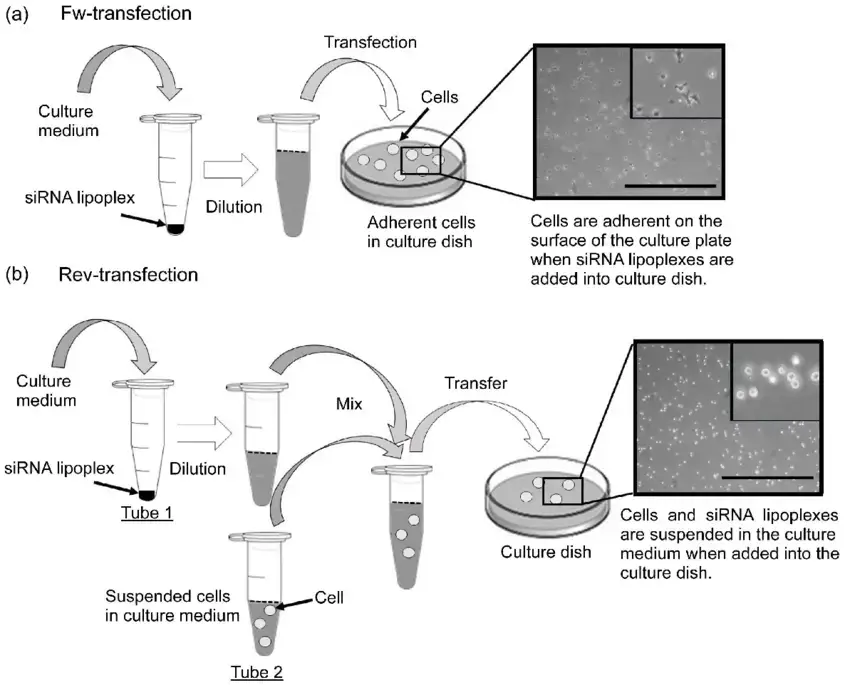
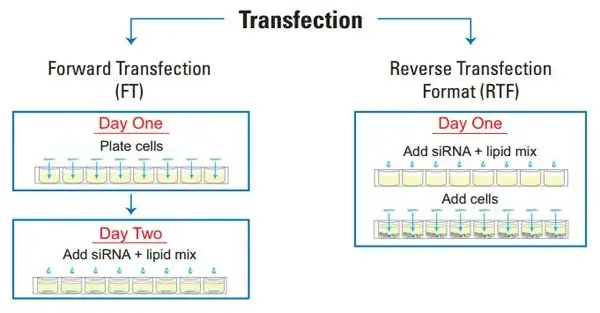
Forward Transfection or Reverse Transfection?
Both forward and reverse transfection procedures have major applications in scientific study. The primary difference between forward and reverse transfection protocols is whether the cells are seeded the same day as the transfection (as in forward transfection) or the day before (as in reverse transfection). Prior to the application of the nucleic acid + transfection reagent complex, forward transfection is typically utilised in instances where the cells must be attached and in a growth phase. In contrast, a reverse transfection involves assembling the nucleic acid + transfection reagent combination in the tissue culture plate and then seeding the cells in the wells. The reverse transfection approach has numerous advantages, including:
- Reverse transfection is compatible with mechanised robots, making it ideally suited for high-throughput screening.
- One day is saved by not needing to pre-plate cells.
- High reverse transfection efficiency reduces the amount of nucleic acid employed.
- In contrast to forward transfection, the transfection reagent can remain in contact with cells for 24-72 hours.
Advantages of Reverse transfection
Reverse transfection has several benefits over traditional transfection methods, including:
- Increased transfection effectiveness may result from the addition and attachment of target cells to the DNA-loaded surface, which increases the probability of cell-DNA contact.
- Tools that reduce manual labour costs (less DNA is required).
- High-throughput screening; a single microarray can be used to analyse the expression of hundreds of genes in cells.
- To improve the quality of screening results, parallel cell seeding in a single chamber for 384 experiments without any physical separation between experiments is recommended. When conducting research in multi-wall dishes, there can be differences from well to well.
- The same sample source plate can be dried and printed on many slides for at least 15 months without obvious loss of transfection effectiveness, allowing for the production of exact replication arrays.
Disadvantages of Reverse transfection
Reverse transfection’s drawbacks include the following:
- Due to the necessity for a precise and fast microarray printing technology to transfer the DNA-gelatin solution onto the slides, reverse transfection is more costly.
- Protocol modifications during the manufacturing of siRNA or plasmid arrays have (so far) been required for applications involving different cell lines, requiring extensive development and testing.
- As the number of spots increases, the chance of cross-contamination between them increases, making it crucial to optimise the array’s arrangement.
Applications of Reverse transfection
Reverse transfection is a technique used in molecular biology and biotechnology to introduce exogenous DNA or RNA into cells without the use of physical or chemical transfection methods. Some applications of reverse transfection include:
- Studying gene expression: reverse transfection can be used to introduce RNA into cells to study the expression of specific genes.
- Studying viral replication: reverse transfection can be used to introduce viral RNA into cells to study the replication of viruses.
- Gene editing: reverse transfection can be used to introduce RNA or DNA into cells to modify their genetic information, such as CRISPR/Cas9 system.
- Cell reprogramming: reverse transfection can be used to introduce RNA or DNA into cells to reprogram them into a different cell type.
- Studying protein-protein interactions: reverse transfection can be used to introduce RNA or DNA into cells to study the interactions between proteins.
What is Modified Reverse Transfection?
In “modified reverse” transfections, cells are passaged and plated prior to the addition of transfection complexes. In this circumstance, adherent cells are only lightly attached to the plate surface when they interact with transfection complexes.
What is Solid Phase Reverse Transfection?
If the nucleic acid to be transfected is already immobilised or spotted in a multi-well format, as in cDNA/shRNA/siRNA screens, the transfection process is called “solid phase reverse transfection.”
FAQ
What is reverse transfection?
Reverse transfection is a laboratory technique used to introduce nucleic acids into non-dividing cells. It allows researchers to manipulate the expression of genes in cells without relying on the cell’s natural ability to take up foreign material.
Why is reverse transfection important?
Reverse transfection is important because it allows researchers to study gene function in a controlled and specific manner. By manipulating gene expression, researchers can determine the role of specific genes in cellular processes and investigate the underlying mechanisms of diseases. Additionally, reverse transfection can be used to test the effectiveness of new drugs and therapies.
How is reverse transfection performed?
Reverse transfection is performed by adding plasmids, RNA, or siRNA directly to cells that have been cultured in a dish or well. The nucleic acids can be delivered using a variety of methods, including electroporation, liposomal transfection, or chemical transfection. The choice of delivery method depends on the type of cell being transfected and the goals of the experiment.
What are the limitations of reverse transfection?
The limitations of reverse transfection include low efficiency of transfection and potential toxicity of transfection reagents. Additionally, reverse transfection may not be applicable to all cell types, as some cells may not be amenable to the chosen transfection method. Furthermore, the expression levels of genes can vary depending on the method used and the specifics of the experiment.
What precautions should be taken when performing reverse transfection?
When performing reverse transfection, it is important to use appropriate safety precautions to minimize the risk of contamination and ensure the accuracy of results. This may include using sterile techniques when handling cells and reagents, wearing protective clothing, and regularly checking the quality of cells and reagents. Additionally, it is important to follow the manufacturer’s instructions for the transfection reagents and equipment being used, and to carefully monitor the cells for any signs of toxicity.