If the broth medium is supplemented with agar-agar it is referred to as agar medium like the nutrient Agar Medium (NAM) for the cultivation of bacteria potato dextrose (PDA) medium for the cultivation and storage of general fungi Czapek Dox agar (CDA) medium for fungi, starch-casein (SCA) media for the cultivation of actinomycetes, and so on.
Agar-agar may also be called *agar’. It is, however, the product from marine red algae, such as Gelidium or Gracilaria. Agar is a non-nitrogen-containing carbohydrate (i.e. complex polysaccharide) possessing 3,6-anhydro-L-galactose and D-galacto-pyranose units. Different types of plates may be made by using nutrients medium. For instance, a spread plate is prepared by spreading a tiny amount of liquid inoculum on the top of the solid material using a sterile glass rods or spreaders. Flood plates are made by inundating with a surface medium an inoculum of liquid and then removing the inoculum that is not used with an sterile Pasteur pipette. Microbial lawns are those with an emulsified growth that is deposited on top of the medium. A small bit of cotton known as the swab, which is attached by the thin woody or plastic stick. The swab can be used to inoculate all the surface of a plat.
Requirements
- Potato tubers -200 g
- Dextrose – 20 g
- Agar – 15 g
- Distilled water – 1lit
- NaOH 1N
- HLC – 1N
- Knife
- Muslin cloth
- Heater
- Beaker (1 litre capacity)
- Erlenmeyer flask (2) (500 ml capacity)
A. Preparation of PDA medium
- Cut potato tubers into pieces, peel off and weigh 200 grams.
- Cut the tubers into smaller pieces using the knife.
- Place the potatoes in an insulated beaker with around 100ml of water that has been distilled.
- Bring the contents to a boil using an electric heater for around 20 minutes.
- Decant supernatant, then filter it with four folds of fabric and place the filtrate in a beaker. The filtrate is referred to as potato extract.
- Add dextrose (20 grams) and Agar (15 grams) in the extract. Then gently stir to dissolve the ingredients.
- Transfer this medium into the measuring cylinder that has 1 litre capacity. Then, increase the volume up to 1 Liter by adding distillate water. Check your medium’s pH and then adjust it to 5.6 using IN HI and NaOH drops-wise.
- Pour the liquid inside two or three Erlenmeyer flasks. Place the cotton plugs in, cover the plug with aluminum foil or paper (Fig. 2.3) and then autoclave at 121degC over 20 minutes.
- Once the temperature has dropped, take the flasks out and use them if needed. keep them at the room temperature.
B. Preparation of agar deep tubes for cultivation of bacteria
If a culture tube that contains autoclaved agar media is placed in an upright position, it creates deep agar tubes upon it has solidified.
- Take the following steps (1) (1) – (3) in the instructions to prepare Agar Slants.
- Remove the tube of culture stand or iron basket with the medium.
- Set the tubes in an upright place. After 30 to 45 minutes, the medium begins to solidify and is referred to as deep tubes made of agar.
- Utilize them as soon as you need or store them for later use.
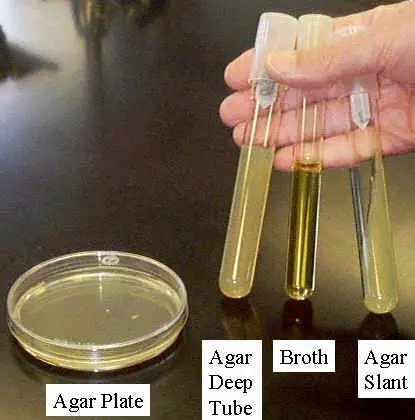
C. Preparation of agar slants in culture tubes
If the autoclaved solution is added to culture tubes and then the second is placed in a slanting position that gives the agar slants upon the solidification.
Requirements
- Any agar medium (e.g. PDA, CDA or NAM)
- Culture tubes (smooth mouth or screw-capped)
- Test tube stand
- Heater
- Aluminium foil or paper
Procedure
- Utilize already created and stored material (if the medium that was stored is going to be used, melt it using a heater that has gentle stirring).
- Distribute 8-10ml in each tube. Then place a cotton plug in the tube and secure it by the rubber band. There is no need for using aluminum foil or a cotton plugs if the tubes are screw-capped. The amount of medium could be reduced or increased in proportion to the number of tubes for culture.
- Place all the tubes in the test tube stand, or iron basket , and then autoclave at 121°C for 20 minutes.
- Remove the culture tubes after the temperature drops and put them on a slant with the support (Fig. 2.3B). You should wait for around 30 minutes.
- After that, the medium solidifies and agar slants are made.
- Use the slants for a culture transfer, if needed. You can also save them for later use.
D. Preparation of PDA plates
Requirements
- PDA medium
- Culture tubes (smooth mouth or screw-capped)
- Test tube stand
- Heater
- Aluminium foil or paper
Procedure
- Before beginning autoclaving, place a few Petri dishes in an oven and then sterilise them with 200 degC for around one hour.
- After cooling down when temperature is lower, place them in inside the chamber of laminar flow chamber or the inoculation chamber designed to inoculate. However, they need to be sterilized by ultraviolet light for 30 minutes prior to the commencement of work.
- Take the flask that contains PDA medium and pour approximately 15 to 20 ml of the medium in the bottom half of Petri dishes as long as the temperature remains at or around 40degC (care must be taken not to pour overly hot medium, as it could cause vapour to condense into water droplets, which will aid in the contamination).
- The plates should be placed in layers and then wait for around 20-30 minutes for the medium to set. medium.
- These plates that have solidified medium are referred to as PDA plates (agar plates) (Fig 2.3C).
- Utilize the plates right away to cultivate fungi or keep them in the freezer for later use.
- CDA plates NAM plates and other plates can also be made using the same method.
References
- https://www.sigmaaldrich.com/IN/en/applications/cell-culture-and-cell-culture-analysis/cell-culture-by-technique/cell-culture-media-preparation
- https://www.mt.com/at/de/home/applications/Laboratory_weighing/Culture-Media-Preparation.html
- https://microbiologyonline.org/teachers/preparation-of-media-and-cultures
- https://www.rpcau.ac.in/wp-content/uploads/2020/03/Media-and-their-composition.pdf
- https://www.technologynetworks.com/cell-science/articles/which-cell-culture-media-is-right-for-you-331552
- http://microbiologynetwork.com/quality_control_of_microbiological_culture_media.asp