Plasma Membrane
- Plasma membrane refers to the envelope-like membrane or structure that surrounds the cell and its organelles.
- It is a double-membraned organelle that is present in both prokaryotic and eukaryotic cells and is also known as the phospholipid bilayer.
- In all live cells, the plasma membrane serves as a barrier and is selectively permeable, permitting the entry and exit of particular selected molecules.
- In addition to these functions, the plasma membrane also serves as a connection between the cell and its surroundings.
- The thickness of a plasma membrane is between 5 and 8 nm, and it is primarily composed of carbohydrates, phospholipids, proteins, and conjugated compounds.
- The plasma membrane is a bilayer of lipids that surrounds and contains the cell’s cytoplasm.
- Based on their molecular architecture and the presence of specific specialised components, this concept is also known as the fluid mosaic model.
- The fluid mosaic hypothesis was initially developed by American biologists Garth L. Nicolson and Seymour Jonathan Singer in 1972.
- The fluid mosaic model depicts in detail the structure of the plasma membrane in eukaryotic cells, as well as the arrangement of its constituents – phospholipids, proteins, carbohydrates, and cholesterol.
- These components give the plasma membrane a fluid look.
Functions of Plasma Membrane
- The plasma membrane serves as a physical barrier between the exterior environment and the intracellular organelles.
- The plasma membrane is a selectively permeable membrane that allows only specific molecules to enter and exit the cell.
- In both the endocytosis and exocytosis processes, plasma membranes play a crucial role.
- Additionally, the plasma membrane facilitates cell-to-cell communication and signalling.
- The plasma membrane performs a crucial role in attaching the cytoskeleton to give the cell its shape and preserve its potential.
Models of Plasma Membrane
Following are descriptions of the top four historical Plasma Membrane models. The examples are:
- Lipid and Lipid Bilayer Models
- Unit Membrane Model (Protein-Lipid Bilayer-Protein)
- Fluid Mosaic Model
- Dannelli Model.
1. Lipid and Lipid Bilayer Model
- Overton, Gorion, and Grendel proposed this model to describe the structure of the plasma membrane.
- Previously, only indirect evidence was available to describe plasma membrane structure.
- Overton discovered in 1902 that compounds soluble in lipid might pass selectively through membranes.
- On this premise, he stated that the plasma membrane consists of a thin lipid layer. Gorter and Grendel found in 1926 that the recovered from erythrocyte membranes was double the amount predicted if a single layer covered the entire surface area of these cells.
- On this premise, they concluded that the plasma membrane is composed of two layers of lipid molecules.
- These models of Gorter and Grendel were unable to explain the actual structure of the plasma membrane, but they laid the groundwork for future membrane structure models.
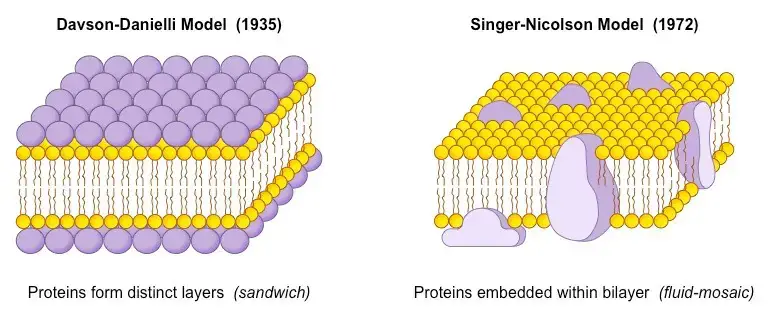
2. Unit Membrane Model (Protein-Lipid Bilayer-Protein)
- Also known as the unit membrane model Davson Daniell and Robertson proposed this concept.
- When membrane surface tension is measured, it suggests the presence of proteins.
- The first lipid bilayer model proposed by Gorter and Grendel was changed after the discovery of proteins. It has been proposed that the surface tension of cells is far lower than one would anticipate if only lipids were involved.
- When protein is added to a model lipid-water system, surface tension is shown to decrease. This indirectly suggested the presence of proteins.
- On the basis of this information, Davson and Danielli hypothesised that the plasma membrane had a lipid bilayer with protein on both surfaces.
- In the beginning, it was believed that proteins existed as covalently bonded globular structures attached to the polar ends of lipids. Consequently, they created a model in which the protein appears to be spread across the hydrophilic ends of the lipid bilayer.
- This model maintains its appeal throughout time. With the development of the electron microscope, it became possible to examine the intricate structure of the plasma membrane.
- On the surface of all cells, a plasma membrane of 6 nm to 10 nm (10nm = 100 ; 1 nm = 10_6mm) thickness was detected, and plasma membranes of two adjacent cells were found to be separated by a 1-15nm-wide interval.
- Additionally, it was noticed that the plasma membrane of the majority of cells looked to be tri-layered.
- Two outside dense layers measured approximately 2.0 nm in thickness, while the central layer was approximately 3.5 nm.
- Robertson initially codified the ideas of Gorter and Grendel, as well as those of Davson and Danielli, in 1959 with his unit membrane concept.
- This concept of a single membrane with three layers (two protein layers and one lipid bilayer) supports only the concept proposed by Davson and Danielli before.
- The less dense central layer of this unit membrane corresponds to lipid hydrocarbon chains.
- Plasma membrane unit membrane thickness (10nm) was discovered to be larger than intracellular membranes of endoplasmic reticulum or golgi complex.
3. Fluid Mosaic Model
- To describe the structure of the plasma membrane, many models have been proposed on occasion. None, however, were universally approved. In this regard, Gorter and Grendel, Davson and Danielli, etc. proposed models for plasma membranes, which were followed by the internationally acknowledged Fluid Mosaic Model for plasma membranes.
- Singer and Nicholson put forth the idea (1912). This hypothesis posits that lipids and integrated proteins are arranged in a mosaic-like pattern and that all biological membranes have a quasi-fluid structure in which both lipid and protein components are capable of performing transitional movement inside the lipid bilayer.
- In this concept, lipid molecules are capable of intramolecular movement, rotation along their axis, and flip-flop movement, which includes transfer from one side of the bilayer to the other.
- According to Gitler, this notion implies that the membrane’s major components, lipids, proteins, and oligosaccharides, are held together by non-covalent interactions (1972).
- Hartley (1936) created the word amphipathy to describe compounds with both hydrophilic and hydrophobic groups.
- Consequently, lipids and integrated proteins are amphipathic by nature.
- Our current understanding of the plasma membrane is based on the integration of data from chemical analysis and the investigation of biophysical aspects using numerous techniques.
- These are the primary components that are incorporated into the plasma membrane. The following four significant methodologies as discoveries have provided support for this relation: These are listed below:
- The technique of freeze fracture was employed to examine membrane. Freeze cracked electron microscopy indicated the presence of 7- 8 nm in diameter bumps and depressions. These remains are distributed at random. It was eventually determined that these are intramembrane protein particles that traverse the bilayer.
- Frye and Edidin (1970) selectively identified the species-specific proteins of human and mouse cells and then united the cells of the two species to create a heterokaryon. After 30 to 35 minutes of incubation at 37°C, human and mouse proteins in heterokaryons were observed to have intermixed (as shown by the use of particular antibodies), indicating that membrane proteins are mobile in the plane of the membrane.
- Patching and capping are processes that give evidence for the mobility of proteins within lipid bilayers. Cross-linking proteins tend to assemble into clusters when ligands such as antibodies have multiple binding sites for specific proteins on the cell surface. This suggests that proteins distribute laterally throughout the bilayer.
- Fluorescence recovery after photobleaching (FRAP) has also been applied to the measurement of protein lateral diffusion rates. An important cell surface protein is labelled with a fluorescent ligand (e.g., antibody). A laser beam is used to bleach the ligand in a tiny area, and the time required for fluorescent ligands to diffuse and mix is measured. The rate of protein diffusion is not constant.
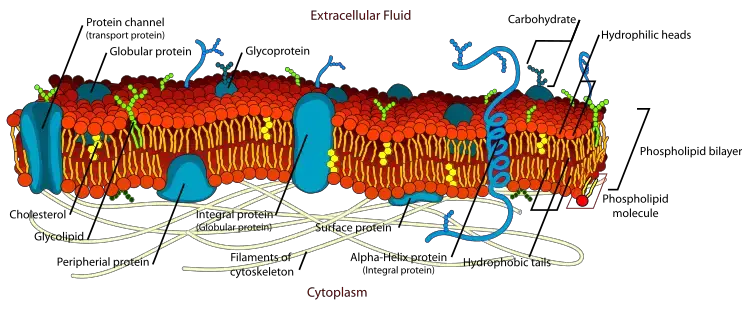
- The preceding evidence suggests that lipid bilayer has fluid qualities that allow membrane proteins to diffuse quickly. It is conceivable for proteins to undergo rotational diffusion.
- However, the flip-flop mechanism proposed for lipids has not been demonstrated for proteins.
- It was later proposed that not just proteins but also individual lipid molecules can flow freely within lipid bilayers. It was discovered to be true in both synthetic and isolated biological membranes produced from mycoplasmas, bacteria, and red blood cells.
- This was initially established in two forms of synthetic lipid bilayers, namely liposomes and black membranes, where liposomes are spherical vesicles.
- Black membranes ranging in diameter from 25nm to 1/nm (1000nm) span a hole in a partition between two aqueous compartments.
- Spin labelling permits the measurement of the motion of individual lipid molecules. (The spin of an unpaired electron generates a paramagnetic signal detectable using electron spin resonance (E.S.R) spectroscopy.
- From the ESR spectra, the mobility and orientation of a spin-labeled lipid can be determined.
- The lipid molecules can also rotate or rapidly interchange positions within the same monolayer (107 times per second) with a diffusion coefficient (D) of around 10-8 cm2/sec, so that a lipid molecule could diffuse the length of a large bacterial cell (–2m) in approximately one second.
- The hydrocarbon chains are flexible even while the lipid molecule is static. Except for the natural, separate biological membranes produced identical results.
- Singer and Nicolson offered a proposal to explain the structure of plasma membrane based on these observations.
- The term for this is fluid mosaic model. This model was essentially a modification of Robertson and Davson’s.
Modifications of Fluid Mosaic Model
- Cell membrane is a two-dimensionally oriented solution of integral proteins in a viscous phospholipids bilayer, according to the fluid mosaic model.
- Recent research has demonstrated that, despite the fact that lipids and a portion of the labelled protein population appear to diffuse freely, the mobility of other proteins is significantly more complicated than the fluid mosaic model originally predicted.
- A considerable proportion of proteins are, at least temporarily, limited to tiny domains in the cell membrane. A few membrane proteins undergo rapid, forward-directed transport toward the cell’s periphery, most likely with the assistance of cytoskeleton motors.
- The transitory confinement of integral proteins has been observed most clearly in cadherin, neural cell adhesion molecules, and receptors for nutrition and growth factor molecules, among others.
- Where proteins are confined, the domains have a diameter of 300-600 nm and the confinement lasts between 3 and 30 seconds. A “membrane skeleton fence” concept was recently developed to explain such protein confinement.
- On the basis of this membrane skeleton fence concept, it is possible to assert that a spectrin-like meshwork on the cytoplasmic side of the membrane transiently sterically limits some membrane-spanning proteins.
- The aforementioned characteristics of cell membrane, which necessitated a revision of our original concept of fluid mosaic model, were disclosed by at least three new techniques:
- Recovery of fluorescence following photo-bleaching (FRAP).
- Individual particle tracking (SPT).
- Optical laser trap (OLT).
- However, it is undeniable that the plasma membrane is a fascinating mixture of dynamic activities, in which its components may disperse randomly (as hypothesised in the fluid mosaic model), be temporarily confined to small domains, or undergo highly directed movement. These characteristics generate substantial lateral variability in the membrane.
1. Microvilli
- The plasma membrane of intestinal epithelial cells emits numerous microscopic, finger-like cytoplasmic structures known as microvilli.
- A single cell may have as much as 3,000 microvilli. The villi range in length from a few hundred to ten angstroms and in diameter from 800 to 1400 angstroms.
- The distance between microvilli is 100 degrees. They retain a unit membrane that is thicker (100-125 ) than the typical plasma membrane.
- In the case of nematodes, microvilli continue to be coated by fine filaments that run parallel to the villi.
- Beaded and adielectronic areas are separated by 130. Threadgold (1969) states that the cuticle of cestodes is emarginated into microtrichs, which appear to be a form of microvillus.
- Their distal extremities are adielectronic, their proximal portion is dielectronic, and their centres are filled with a variable amount of an amorphous granular substance.
- At specific locations, microvilli establish direct contact with the endoplasmic reticulum, creating a direct pathway into and out of the cytoplasm.
- The microvilli augment the surface area for absorption, operating similarly to villi.
- These microvilli are also present in hepatic cells, mesothelial cells of the convoluted tubules of the kidney, and gall bladder, uterine, and yolk sac epithelial cells.
- In some cells, the infoldings of the plasma membrane produce pocket-like or chamber-like structures with which mitochondria connect.
- These may release energy necessary for material movement across the plasma membrane.
2. Intercellular Spaces
- Intercellular voids or gaps separate the plasma membranes of neighbouring cells, which are not connected.
- There is a 20 -wide intercellular space between the plasma membranes of neighbouring cardiac cells.
- These gaps, which appear hexagonal in tangential section, are known as gap junctions.
3. Interdigitations
- In rare instances, the plasma membranes of two neighbouring cells exhibit finger-like protrusions. This is known as inter-digitation.
4. Desmosomes and Associated Zones
- These are the thicker portions of plasma membranes where many filaments shoot towards the cell’s interior surface.
- These filaments are referred to as tonofibrils (tonofilaments), whilst the thickened portions of plasma membranes are known as desmosomes or maculae adhaerens.
- Intercellular gap between desmosomes includes a covering substance that enables adhesion between neighbouring cells.
- Desmosomes seem to be present in vertebrate tissues. In general, the lateral plasma membrane has three components in the apical-basal direction: a tight region or zonula occludens, an intermediate junction or zonula adhaerens, and a desmosome or adhaerens macula.
(а) Zonula Occludens
- The region is 2000-5000 wide and begins at the junction of the apical and lateral cell boundaries.
- It appears to establish a belt-like connection around the cell. It is characterised by the fusing of adjacent plasma membranes, which eliminates intercellular space and acts as a barrier to the transport of big molecules.
- The zonula occludens is located between brain cells and epithelial cells. It allows electrical communication between cells and chemical communication of inorganic ions.
(b) Zonula Adhaerens
- As a continuation of the preceding zone, this zone stretches across 3000-5000A with a straight or undulating route.
- The membranes have an unique tripartite structure and are thicker, and the cytyoplasm shows a prominent densification of densely matted fibrils.
- A 200-angstrom-thick amorphous intercellular zone separates the two membranes.
(c) Macula Adhaerens
- It is approximately 200 away from the basal end of the zonula adhaerens. It is an oval or circular region between 2000 and 3000 in length that appears in pairs.
- Its exterior consists of tonofibrils (fine filaments) with a diameter of approximately 75.
- In Hydra and other invertebrates, thick transverse bars connect adjacent desmosomes to create a ladder-like structure.
- These desmosomes are known as septate. Lacking in septate desmosomes are tonofibrils.
4. Dannelli Model
According to this model:
- lipids and intrinsic proteins are arranged in a mosaic and
- Bilayers of biological membranes are semifluid, allowing lipids and intrinsic proteins to move inside the bilayer.
- This concept of fluidity implies that the locations of lipids, proteins, and oligosaccharides are maintained by non-covalent interactions. On this premise, it can be said that solvents or detergents can disperse components without disrupting their bonds. Intrinsic proteins are also intercalated in a continuous lipid bilayer to varying degrees. On both sides of the membrane, these proteins may be exposed to aqueous solvents.
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.