What is the ornithine decarboxylase test?
- The Ornithine Decarboxylase Test is a critical biochemical assay used in microbiology, particularly for differentiating members of the Enterobacteriaceae family. This test is centered on the enzymatic activity of decarboxylases, which are enzymes that catalyze the decarboxylation of amino acids. Specifically, it focuses on the ability of bacteria to produce ornithine decarboxylase, an enzyme that acts on the amino acid ornithine.
- In the metabolic context, amino acids can be metabolized through various pathways such as decarboxylation, hydrolysis, or deamination. Decarboxylation, the focus of this test, involves the removal of a carboxyl group from an amino acid. This process is facilitated by specific decarboxylases, and in the case of the ornithine decarboxylase test, the enzyme targets ornithine.
- The test is designed to identify and differentiate bacteria based on their enzymatic activities. For instance, it is particularly significant in distinguishing members of the Klebsiella-Enterobacter-Serratia group and identifying species of Proteus within the Enterobacteriaceae family. The production of enzymes like ornithine decarboxylase is a key parameter in bacterial differentiation.
- The methodology of the test involves using decarboxylase media, initially introduced by Moeller. This media comprises various components such as peptone, beef extract, indicators like bromocresol purple and cresol red, pyridoxal, and glucose. To test for decarboxylase activity, an amino acid (in this case, ornithine) is added. The medium is then autoclaved and covered with a layer of sterile paraffin oil. The presence of decarboxylase activity is indicated by a pH rise in the amino acid reagent, observable through color changes in the medium.
- Modifications of the original method have been made to enhance the test’s efficiency and reduce incubation times. For example, Fay & Barry modified Moeller’s method by omitting glucose, reducing the pH, and decreasing the test volume, which allowed for the detection of ornithine decarboxylase activity within 2-4 hours. This rapid test proved particularly useful in differentiating Klebsiella from the Enterobacter-Serratia group and in separating species of Proteus based on their ornithine decarboxylase activity.
- The ornithine decarboxylase test, therefore, serves as a fundamental tool in microbial identification and classification. Its ability to quickly and accurately distinguish between different bacteria based on specific enzymatic activities makes it invaluable in clinical diagnostics and microbiological research.
Objectives of Decarboxylase Test
- Assessing Decarboxylase Enzyme Production: The primary objective of the decarboxylase test is to evaluate the ability of an organism to produce decarboxylase enzymes. These enzymes are responsible for catalyzing the decarboxylation process, which involves the removal of a carboxyl group from an amino acid. This test specifically focuses on the production of enzymes like ornithine decarboxylase, arginine decarboxylase, and lysine decarboxylase. The presence or absence of these enzymes in a bacterial culture is indicative of the metabolic pathways the organism utilizes.
- Differentiation within Enterobacteriaceae Family: Another crucial objective of the decarboxylase test is to differentiate members of the Enterobacteriaceae family. This family of bacteria encompasses a wide range of species, many of which are significant in clinical contexts. The ability of these bacteria to produce decarboxylase enzymes varies, and this variation is utilized as a key parameter for differentiation. The test aids in separating bacteria within the Enterobacteriaceae family, especially in distinguishing members of the Klebsiella-Enterobacter-Serratia group and identifying specific species of Proteus. The differential production of decarboxylase enzymes, therefore, provides essential information for the accurate identification and classification of these bacteria.
Principle of Ornithine decarboxylase test/Principle of Decarboxylase Test
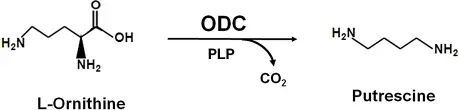
- Decarboxylation Reaction Basis: The principle of the Ornithine Decarboxylase Test, as well as other decarboxylase tests, is founded on the ability of certain microorganisms to decarboxylate or hydrolyze amino acids, forming an amine. This reaction leads to the production of an alkaline pH environment. Amino acids such as arginine, lysine, and ornithine are the primary substrates used to assess the production of specific enzymes that catalyze these reactions.
- Composition of the Basal Medium: The basal medium, often following Moeller’s formula, comprises meat peptones and beef extract. These components provide essential nitrogenous nutrients to support bacterial growth. The medium also contains glucose, a fermentable carbohydrate, and pyridoxal, an enzyme cofactor enhancing decarboxylase activity. The pH indicators in the medium, bromocresol purple and cresol red, facilitate the observation of pH changes.
- Initial Acid Production from Glucose Fermentation: Upon the fermentation of glucose in the medium, acids are produced, leading to a lowering of pH. This acidification of the medium is visually indicated by a color change from purple to yellow.
- Role of Decarboxylase in Alkaline Shift: In the presence of a decarboxylase-producing organism, decarboxylation or hydrolysis of the amino acid occurs in response to the acidic environment. This results in the formation of alkaline end products (amines), which cause the medium to revert to its original purple color.
- Control Mechanism for Non-Fermenters: For organisms that do not ferment glucose, the medium will not exhibit a yellow color change. However, the decarboxylase test can still be conducted by including a control medium without amino acids for comparison purposes.
- Specific Amines Produced: The decarboxylation of lysine yields cadaverine, while the decarboxylation of ornithine produces putrescine. Arginine, upon decarboxylation, results in agmatine, which is further hydrolyzed by a dihydrolase to form putrescine. Additionally, arginine dihydrolase catalyzes the conversion of arginine to citrulline, which is further converted to ornithine, ultimately producing putrescine.
- Anaerobic Conditions for Decarboxylation: It is crucial to maintain an anaerobic environment for the decarboxylation reaction. Therefore, the contents of each test tube are sealed with oil or paraffin to prevent oxygen exposure.
- Microorganisms Tested: The test is extensively used for identifying various microorganisms to the species level. These include Enteric Gram-negative rods, Vibrio, Plesiomonas, and Aeromonas. It is also applicable for organisms like Stenotrophomonas, Burkholderia, Fluorescent Pseudomonas, Coagulase-negative staphylococci, Viridans group streptococci, miscellaneous non-glucose-fermenting Gram-negative rods, and spreading indole-negative Proteus.
Requirement
- Media Overview: The Decarboxylase Test primarily utilizes the Decarboxylase Test Medium Base, often following Moeller’s formula, which is specifically designed for testing amino acid decarboxylase activity. Alternative media options include Motility-indole-ornithine medium (MIO) and Lysine iron agar, which serve similar purposes in identifying enzymatic activities related to amino acid decarboxylation.
- Composition of the Decarboxylase Medium Base: The specific constituents of the decarboxylase medium base are critical for its functionality. This includes:
- Peptic digest of animal tissue: 5.0 grams per liter, which serves as a source of nitrogen and other essential growth factors.
- Yeast extract: 0.3 grams per liter, providing additional nutrients and vitamins.
- Dextrose: 1.0 gram per liter, acting as a fermentable carbohydrate source.
- Bromo cresol purple: 0.1 gram per liter, a pH indicator that changes color in response to pH alterations.
- Cresol red: 0.005 grams per liter, another pH indicator complementing the bromo cresol purple.
- Pyridoxal: 0.005 grams per liter, which functions as an enzyme cofactor enhancing decarboxylase activity.
- Reagents Used:
- Mineral oil is employed to create an anaerobic environment within the test tubes, essential for the decarboxylation process.
- Vaspar, liquid paraffin, or petroleum jelly, maintained at 56°C in liquid form, are also used for sealing the test tubes to prevent oxygen ingress.
- Supplies Used:
- Sterile sticks or inoculating loops are essential for introducing the bacterial sample into the test medium.
- An incubator, maintained at 35°C, is required to provide optimal growth conditions for the bacteria during the test period.
The combination of these specific media, reagents, and supplies facilitates the accurate execution of the Decarboxylase Test. Each component plays a distinct role, from providing essential nutrients and pH indicators in the medium to ensuring the proper anaerobic conditions for the decarboxylation reaction to occur. This systematic arrangement of materials is crucial for the successful identification and differentiation of bacterial species based on their decarboxylase enzyme production.
Composition of Ornithine decarboxylase broth
| Peptone | 5.0g |
| Meat Extract | 5.0g |
| Pyridoxal | 0.005g |
| Dextrose | 0.5g |
| L-Ornithine | 10.0 |
| Bromocresol Purple | 0.010 |
| Cresol Red | 0.005 |
Final pH 6.0 ± 0.2
Preparation of the media
- Initial Media Preparation: The process begins by accurately measuring 9.02 grams of dehydrated decarboxylase test medium powder. This powder can either be a commercially prepared mix or a laboratory-prepared media blend. The measured quantity is then added to 1000 milliliters of distilled or deionized water. This type of water is used to ensure purity and absence of contaminants that could affect the test results.
- Dissolving the Medium: The solution containing the medium and water is then heated. The purpose of this heating is to bring the solution to a boil, which is necessary for completely dissolving the medium. This step is crucial for ensuring uniformity in the solution, thereby allowing for consistent test results.
- Division and Labeling of Media: After the medium is fully dissolved, it is divided into four equal parts. One of these parts is tubed without the addition of any amino acid. This portion serves as the ‘Control’, which is essential for comparison with the test samples. The control tube is appropriately labeled to avoid any confusion during the testing process.
- Addition of Amino Acids: The remaining three parts of the medium are dispensed into three separate tubes. To each of these tubes, a different amino acid is added: L-lysine hydrochloride, L-arginine hydrochloride, and L-ornithine hydrochloride, respectively. These amino acids are added to reach a final concentration of 0.5%. The addition of these specific amino acids allows for the testing of the organism’s ability to decarboxylate or hydrolyze each particular amino acid.
- Dispensing and Sterilization: Approximately 3-4 ml of the prepared media are dispensed into screw-capped tubes. These tubes are then sterilized to eliminate any potential contaminants. Sterilization is achieved through autoclaving at 10 lbs pressure (115°C) for 20 minutes. This step is critical for maintaining the sterility and integrity of the media.
- Prevention of False Alkalinization: Prior to sterilization, it is recommended to add a layer of liquid paraffin to a height of about 5 mm in each tube. This layer of paraffin is intended to prevent false alkalinization at the surface of the medium, which could otherwise lead to inaccurate test results.
Procedure of Ornithine decarboxylase test
- Procedure for Glucose-Fermenting Organisms:
a. Inoculation: A drop of an 18-24 hour culture, grown in brain heart infusion broth, is added to each of the three decarboxylase broths. These broths are specifically prepared with arginine, lysine, and ornithine to test for the organism’s ability to decarboxylate each of these amino acids.
b. Control Tube: For glucose-fermenting organisms, a control tube is not required. This is because these organisms are expected to alter the pH of the medium through glucose fermentation.
c. Adding Sterile Mineral Oil: A 4 mm layer of sterile mineral oil is added atop each of the tubes. This layer is critical as it creates an anaerobic environment within the tube, essential for the decarboxylation process.
d. Incubation: The inoculated tubes are then incubated for 4 days at a temperature range of 35-37°C, in ambient air conditions.
e. Observation of Color Change: During the incubation period, the tubes are observed for any color change at 24, 48, 72, and 96 hours. A color change indicates a pH shift due to decarboxylation. - Procedure for Glucose-Nonfermenting Organisms:
a. Preparation of Suspension: A bacterial suspension, denser than McFarland No. 5, is prepared in brain-heart infusion broth. This suspension is derived from an overnight culture (18-24 hours old) growing on 5% sheep blood agar.
b. Inoculation with Suspension: Each of the four decarboxylase broths, including a control, is inoculated with four drops of the prepared bacterial suspension. The control tube, without amino acids, is essential in this case for comparison purposes.
c. Adding Sterile Mineral Oil: Similar to the procedure for glucose-fermenting organisms, a 4 mm layer of sterile mineral oil is added to each tube to ensure anaerobic conditions.
d. Incubation: The tubes are then incubated at 35-37°C in ambient air for 4 days. This period allows sufficient time for the bacteria to grow and exhibit decarboxylase activity if present.
e. Observation of Color Change: Observations for color change are made at 24, 48, 72, and 96 hours during the incubation. The presence or absence of color change in comparison to the control tube provides information about the decarboxylase activity of the organism.
Ornithine decarboxylase test results
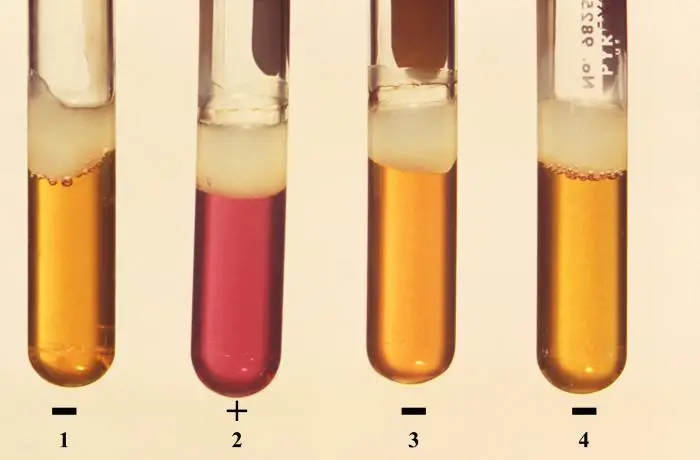
- Positive Test Indicators: A positive result in the decarboxylase test is indicated by a turbid purple or a faded-out yellow-purple color. This color change to alkaline signifies that the organism has decarboxylated the amino acid, producing an alkaline end product. Such a reaction confirms the presence of decarboxylase activity for the specific amino acid tested.
- Negative Test Indicators: A negative test is characterized by a bright clear yellow color, indicative of an acidic environment. This occurs when the organism is unable to decarboxylate the amino acid, resulting in the accumulation of acid from glucose fermentation. Additionally, a lack of color change, particularly in nonfermenting rods, also signifies a negative result.
- Control Tube Importance: The control tube plays a crucial role in validating the test. It should either retain its original color or turn yellow. If the control tube exhibits turbidity and an alkaline or purple color, it invalidates the test. Therefore, questionable results are always compared against the control tube to ascertain their validity.
- Interpretation of Specific Organism Results:
- Enterobacter aerogenes: Shows a positive reaction (color change to purple) for lysine and ornithine decarboxylation, but a negative reaction (yellow-colored broth) for arginine decarboxylation.
- Escherichia coli: The results are variable across lysine, arginine, and ornithine decarboxylation.
- Klebsiella pneumoniae: Exhibits a positive reaction for lysine decarboxylation but negative reactions for arginine and ornithine decarboxylation.
- Proteus vulgaris: All tests (lysine, arginine, and ornithine) yield negative reactions.
- Pseudomonas aeruginosa: Positive for arginine decarboxylation, but negative for lysine and ornithine decarboxylation.
- Salmonella Typhi: Positive for lysine decarboxylation, variable for arginine, and negative for ornithine decarboxylation.
- Serratia marcescens: Positive reactions for lysine and ornithine decarboxylation, but negative for arginine.
- Shigella flexneri: Negative reactions for all three amino acids, with variability in arginine decarboxylation.
- Vibrio cholerae: Shows positive reactions for arginine and ornithine decarboxylation but a negative reaction for lysine.
Decarboxylase Test Interpretations
- Monitoring Medium Color Change: The primary aspect of interpreting results in the Decarboxylase Test involves checking the color of the medium daily for up to 10 days. This monitoring is essential to detect signs of fermentation and decarboxylation. The color changes, or lack thereof, provide crucial insights into the metabolic activities of the test organism.
- Baseline Color of the Media: Uninoculated Moeller’s base media, as well as media with an amino acid, typically exhibit a light brown color. This baseline color serves as a reference point for detecting any subsequent changes that occur due to the activities of the inoculated organism.
- Interpreting No Color Change: If there is no change in the color of the medium, it indicates that the organism does not ferment glucose and is decarboxylase negative (-) for that specific amino acid. This result suggests the absence of enzyme activity necessary for decarboxylating the amino acid.
- Yellow Color Indicating Glucose Fermentation: A medium that changes to a yellow color, but remains yellow without transitioning to purple, indicates that the organism ferments glucose, and acidic by-products are formed. However, in this scenario, the organism is still decarboxylase negative (-) for that amino acid. The appearance of yellow broth is an indication of fermentation of glucose but not an indication of decarboxylation.
- Purple Color Indicating Decarboxylation: If the color of the media changes to purple, it signifies the decarboxylation of the amino acid and the formation of amine (alkaline by-products) by the test organism. This result means that the organism is decarboxylase positive (+) for that amino acid. Conversely, the lack of a purple color indicates that the amino acid has not been decarboxylated, and the organism did not produce the decarboxylase enzyme.
- Summary of Result Interpretations:
- Media color: No Change (Media remains light brown)
- Bacterial reaction: Decarboxylase negative (-)
- Media color: Changes to yellow but does not change to purple
- Bacterial reaction: Decarboxylase negative (-)
- Media color: Changes to purple
- Bacterial reaction: Decarboxylase positive (+)
- Media color: No Change (Media remains light brown)


| Media Color | Bacterial Reaction | Interpretation |
|---|---|---|
| No Change | Decarboxylase Negative (-) | Organism does not ferment glucose and is negative for decarboxylase for the tested amino acid. |
| Yellow | Decarboxylase Negative (-) | Organism ferments glucose, but is negative for decarboxylase for the tested amino acid. No decarboxylation occurred. |
| Purple | Decarboxylase Positive (+) | Organism is positive for decarboxylase for the tested amino acid, indicating decarboxylation and formation of alkaline by-products. |


Ornithine decarboxylase test results of some organisms
| Test Organism | Lysine Decarboxylation | Arginine Decarboxylation | Ornithine Decarboxylation |
|---|---|---|---|
| Enterobacter aerogenes | Positive (Purple) | Negative (Yellow) | Positive (Purple) |
| Escherichia coli | Variable | Variable | Variable |
| Klebsiella pneumoniae | Positive (Purple) | Negative (Yellow) | Negative (Yellow) |
| Proteus vulgaris | Negative (Yellow) | Negative (Yellow) | Negative (Yellow) |
| Pseudomonas aeruginosa | Negative (Yellow) | Positive (Purple) | Negative (Yellow) |
| Salmonella Typhi | Positive (Purple) | Variable | Negative (Yellow) |
| Serratia marcescens | Positive (Purple) | Negative (Yellow) | Positive (Purple) |
| Shigella flexneri | Negative (Yellow) | Variable | Negative (Yellow) |
| Vibrio cholerae | Negative (Yellow) | Positive (Purple) | Positive (Purple) |
Quality Control
Control organisms play a pivotal role in the decarboxylase test. They are essential for validating the accuracy and reliability of the test results. Control organisms are divided into two categories: positive controls, which are expected to show a specific reaction, and negative controls, which are expected to show no reaction.
- Positive Control Organisms:
- a. Proteus mirabilis: This organism serves as a positive control and is usually incubated for a period ranging from 4 to 18 hours. It is expected to show positive decarboxylase activity, confirming the test’s ability to detect such activity.
- b. Staphylococcus lugdunensis: Used as another positive control, this bacterium should be incubated for 30 minutes to 2 hours. It provides a rapid confirmation of the test’s effectiveness in detecting decarboxylase activity.
- Negative Control Organisms:
- a. Proteus vulgaris: As a negative control, this organism is held for 18 hours. It is not expected to exhibit decarboxylase activity, thereby confirming the test’s specificity.
- b. Staphylococcus epidermidis: This bacterium, incubated for 2 hours, also serves as a negative control. Its lack of decarboxylase activity is essential for ensuring the test’s accuracy.
- Control Organisms and Test Results: The control organisms are used in conjunction with specific test results for different amino acids. For example:
- a. Arginine: The color of the control tube and the results observed with different organisms (such as Klebsiella pneumoniae and Enterobacter cloacae) provide crucial information about the organism’s ability to decarboxylate arginine.
- b. Lysine: Similarly, the response of the control organisms to lysine decarboxylation aids in the interpretation of the test results.
- c. Ornithine: The ornithine control tube’s color change, or lack thereof, in conjunction with the control organisms’ behavior, offers valuable insights into their metabolic capabilities regarding ornithine decarboxylation.
- Significance of Control Organisms in Decarboxylase Test: Therefore, control organisms are indispensable in the decarboxylase test. They provide a benchmark against which the test results can be compared, ensuring the validity and reliability of the test. The use of both positive and negative controls, in conjunction with specific color changes for different amino acids, allows for a comprehensive assessment of an organism’s decarboxylase activity. This systematic approach underscores the precision and accuracy inherent in microbiological testing methodologies.
Uses of Decarboxylase Test
- Differentiation within Enterobacteriaceae Family: The Decarboxylase Test plays a crucial role in differentiating members of the Enterobacteriaceae family. This family includes a wide variety of species with closely related physiological characteristics. The test’s ability to identify specific enzymatic activities helps in distinguishing between these similar species. By assessing the presence or absence of decarboxylase enzymes, microbiologists can accurately classify different members of this family, which is essential in both clinical diagnostics and research.
- Identification of Enterococcus Species: Specifically, the arginine decarboxylase aspect of the test is instrumental in identifying Enterococcus species to the species level. For instance, Enterococcus faecalis and Enterococcus faecium are arginine positive, meaning they show a positive reaction in the arginine decarboxylase test. Conversely, Enterococcus avium is arginine negative. This distinction is vital in clinical settings, as different Enterococcus species can have varied implications for patient treatment and disease management.
- Distinguishing Between Salmonella and Shigella: The lysine decarboxylase component of the test is used to differentiate between Salmonella and Shigella species. Salmonella species typically show a positive reaction (lysine decarboxylase positive), while Shigella species are usually negative (lysine decarboxylase negative). This differentiation is particularly significant in the field of medical microbiology, as Salmonella and Shigella are both important enteric pathogens but require different approaches for treatment and control.
- Microbial Taxonomy and Classification: The Decarboxylase Test is integral to microbial taxonomy and classification. By identifying the specific enzymatic profiles of bacteria, microbiologists can categorize and classify organisms into their respective genera and species. This classification is fundamental for understanding microbial diversity and relationships among different bacterial groups.
- Antimicrobial Susceptibility Testing: In some cases, the Decarboxylase Test can aid in antimicrobial susceptibility testing. Certain bacterial responses to specific amino acids can indicate their susceptibility or resistance to particular antibiotics. Therefore, this test can be an auxiliary tool in determining the most effective antimicrobial treatment for bacterial infections.
- Epidemiological Studies: The Decarboxylase Test is useful in epidemiological studies to track and identify the sources of bacterial infections, especially in cases of foodborne illnesses. By differentiating between various strains of pathogens like Salmonella and Shigella, public health officials can trace outbreaks to their sources and implement control measures.
- Quality Control in Food Industry: In the food industry, the Decarboxylase Test is used for quality control purposes. The presence or absence of certain decarboxylase-positive bacteria can indicate spoilage or contamination in food products, thereby assisting in maintaining food safety standards.
- Research and Development: The test is also valuable in research settings for studying bacterial metabolism and genetic expression. Understanding how different bacteria metabolize amino acids can provide insights into their metabolic pathways and genetic regulation, which is important for advances in microbiology and biotechnology.
- Clinical Diagnostics: Clinically, the Decarboxylase Test assists in diagnosing infections caused by enteric bacteria. By identifying the bacterial species responsible for an infection, clinicians can make more informed decisions regarding patient treatment and management.
- Environmental Monitoring: This test is also employed in environmental monitoring to detect and identify bacteria in various ecosystems. Understanding the bacterial composition of different environments can inform studies related to ecology, pollution, and the impact of human activities on natural habitats.
Limitations of Decarboxylase Test
- Necessity of an Oil Barrier: The Decarboxylase Test requires the application of mineral oil or a similar barrier on the surface of each inoculated broth medium. This layer is crucial as it reduces the possibility of an alkaline shift occurring due to oxidation. Without this barrier, the test results could be compromised, leading to inaccurate interpretations.
- Timing of Result Interpretation: Results of the Decarboxylase Test should not be interpreted prior to 18 to 24 hours of incubation. Interpreting results too early, particularly within the first 10 to 12 hours of incubation, may lead to erroneous conclusions. This is because glucose fermentation, which creates the acidic environment necessary for decarboxylase production, occurs within these initial hours.
- Challenges with Glucose-Nonfermenting Microorganisms: Glucose-Nonfermenting microorganisms may display weak decarboxylase activity, resulting in insufficient production of amines necessary to convert the pH indicator system. This can lead to a less pronounced color change, making it difficult to interpret the results accurately. Conversely, some non-fermenters can produce sufficient amines to result in a deeper purple color compared to the uninoculated tube, which can also complicate result interpretation.
- Indicator Reduction vs. Alkaline End Products: A grey color in the tube may not necessarily indicate the production of alkaline end products, but rather a reduction of the indicator. This can lead to confusion in interpreting the results. To aid in accurate reading, additional bromcresol purple may be added to the medium.
- Handling Tubes with Dual Color Layers: If a tube shows two layers of different colors, it should be gently shaken before interpreting the reaction. This step is important to ensure that the color change throughout the medium is uniform and representative of the overall reaction.
- Considerations for Arginine Positive Bacteria: In the case of glucose-nonfermenting bacteria that are arginine positive, it is essential that they also test negative for lysine and ornithine decarboxylation. This specificity is important for accurate differentiation and identification of certain bacterial species.
Tips for Decarboxylase Test
- Inoculation of Control Tube: It is essential to inoculate each isolate into a basal medium tube without the amino acid to serve as a growth control and negative reference. This control is critical for comparing the organism’s growth in the absence of the specific amino acid and ensuring the reliability of the test results.
- Protection from Air Exposure: Inoculated broth must be protected from air by adding a layer of sterile mineral oil before incubation. Exposure to air can lead to the alkalization of the surface of the medium, potentially resulting in false positive results. The oil layer creates an anaerobic environment, which is crucial for the accurate determination of decarboxylase activity.
- Accurate Labeling of Broths: Since the broths containing different amino acids appear similar, careful labeling is imperative to distinguish which amino acid is present in each tube. Accurate labeling ensures that the correct interpretation is made for each specific decarboxylase test.
- Timing of Test Interpretation: Interpretation of the test should only be made after at least 24 hours of incubation. Conducting the interpretation before this period can lead to premature and possibly incorrect results, as the full extent of the organism’s decarboxylase activity may not have been expressed.
- Extended Incubation for Certain Microorganisms: For some microorganisms, an increased incubation period of up to 10 days may be necessary. This extended period allows for the full expression of decarboxylase activity in organisms that may have slower metabolic rates or delayed enzyme production.
- Sterilization of Mineral Oil: Autoclaving mineral oil is not recommended due to the difficulty in achieving complete sterility and the risk of introducing water or condensation. Dry oven sterilization of small aliquots or filtration of warmed mineral oil is preferable. Moreover, autoclaving can be hazardous due to the potential for steam/oil mixtures to explode.
- Use of Specific Media for Differentiation: Different media are used for distinguishing certain members of the Enterobacteriaceae family. Lysine Iron Agar (LIA) is recommended for differentiating Salmonella species by detecting hydrogen sulfide production and the decarboxylation or deamination of lysine. Motility, Indole, Ornithine (MIO) medium is advised for testing the motility, indole production, and ornithine-decarboxylase activity of enteric bacilli.
FAQ
how is ornithine decarboxylase test used for identification of enterobacteriaceae?
An inoculum from a pure culture is transferred aseptically to a sterile tube of ornithine decarboxylase broth. The inoculated tube is incubated at 35-37 C for 24 hours and the preliminary results are determined. The microbe must first use the glucose present to cause the pH to drop. Increases the acidity of the solution, thereby changing colour of indicator from yellow to purple.
If the organism fails to produce ornithine-decarboxylase, the medium is acid (yellow).
What is the basis for the key test for ornithine or lysine decarboxylase?
production of decarboxylase enzyme by the sleeted organisms.
References
- Fay GD, Barry AL. Rapid ornithine decarboxylase test for the identificatioan of enterobacteriaceae. Appl Microbiol. 1972 Apr;23(4):710-3. doi: 10.1128/am.23.4.710-713.1972. PMID: 4553140; PMCID: PMC380423.
- Kumar, Shiv & endra, Jit & Das, Anup & Mane, Pratibha & Sangwan, Jyoti & Kumari, Saroj. (2018). Isolation, Identification and Antibiogram of Coagulase Negative Staphylococcus (CoNS) Isolated from Various Clinical Samples at a Tertiary Care Teaching Hospital, Jaipur, India. International Journal of Current Microbiology and Applied Sciences. 7. 3048-3059. 10.20546/ijcmas.2018.701.362.
- http://www.liofilchem.net/login/pd/ts/610305_TS.pdf
- https://medical-dictionary.thefreedictionary.com/ornithine+decarboxylase+test
- https://www.keyscientific.com/prodct.php?productid=144
- https://himedialabs.com/TD/M1223.pdf
- https://www.vumicro.com/vumie/help/VUMICRO/Ornithine_decarboxylase_Test.htm
- https://vumicro.com/docs/ornithine-decarboxylase-test/
- https://www.austincc.edu/microbugz/decarboxylation_test.php
- https://phil.cdc.gov/Details.aspx?pid=15379
- https://socratic.org/questions/why-is-the-ornithine-decarboxylation-reaction-indicated-by-a-purple-color-and-a-