What is Nitrate Reduction Test?
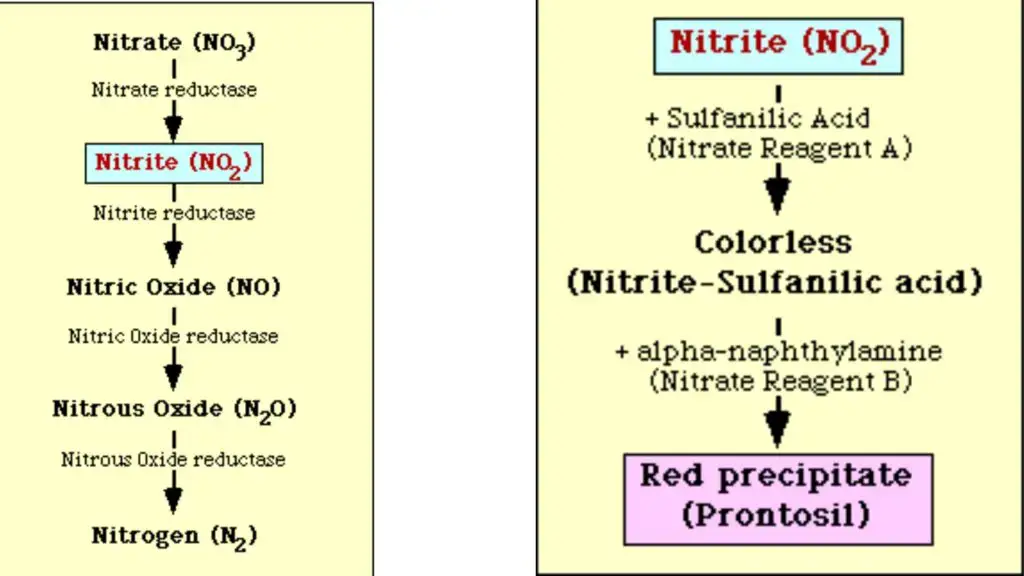
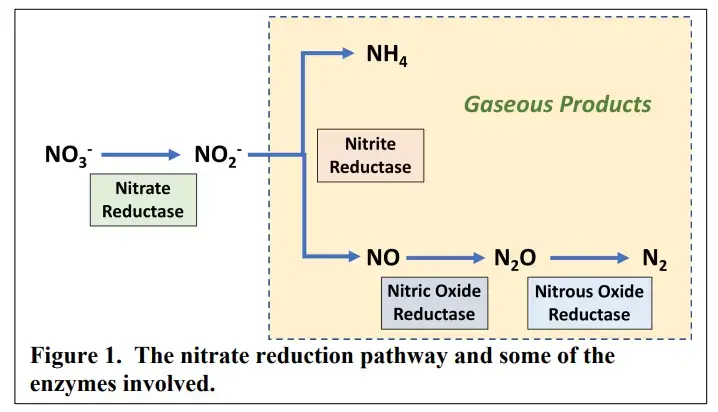
- Nitrate reduction test is used to differentiate Enterobacteriaceae based on their ability to produce nitrate reductase enzyme, which hydrolyzes nitrate (NO3–) to nitrite (NO2–), which may be further degraded to various nitrogen products such as nitrogen oxide, nitrous oxide, and ammonia (NH3), depending on the organism’s enzyme system and the environment in which it is growing.
- The nitrate/Nitrite reduction test is used to measure an organism’s ability to convert nitrate to nitrite and to identify the numerous ways in which an organism can decrease nitrate.
- Other than ambient oxygen, an electron acceptor is required for anaerobic metabolism. The final electron acceptor for numerous gram-negative bacteria is nitrate.
- The nitrate reduction test measures the production of the enzyme nitrate reductase, which is responsible for nitrate reduction.
- This test is effective for distinguishing organisms based on their capacity to convert nitrate to nitrite or nitrogen gas.
- Nitrate broth is used to test an organism’s ability to convert nitrate (NO3) to nitrite (NO2) via nitrate reductase. It also assesses an organism’s capacity to convert nitrate and nitrite into molecular nitrogen.
- Nitrate broth provides minerals as well as potassium nitrate, a nitrate source.
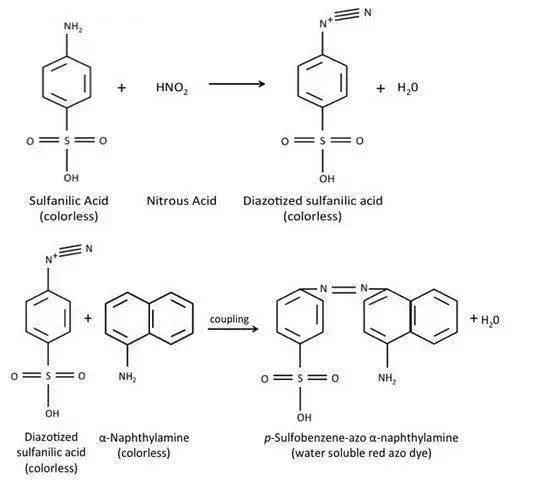
- After incubation the nitrate broth, one drop each of sulfanilic acid and -naphthylamine should be added. If the organism has converted nitrate to nitrite, nitrous acid will be produced from the nitrites in the medium.
- When sulfanilic acid is combined with nitrous acid, diazotized sulfanilic acid is produced. This interacts with the -naphthylamine to generate a product with a red hue. Therefore, if the medium appears red after the addition of nitrate reagents, the nitrate reduction result is regarded positive.
- If the medium does not become red following the addition of the reagents, the organism may not have been able to decrease the nitrate, or it may have been able to denitrify the nitrate or nitrite to form ammonia or molecular nitrogen. Therefore, the test requires an additional step.
- If the medium does not become red after adding the nitrate reagents, add a little quantity of zinc powder. Caution is required, as zinc powder is toxic! If the tube becomes red upon addition of zinc, this indicates the presence of unreduced nitrate.
- A red colour on the second step is therefore a negative result. The addition of zinc converted nitrate to nitrite, and the nitrite in the medium combined with sulfanilic acid to generate nitrous acid. The resulting diazotized sulfanilic acid interacted with the -naphthylamine to produce the red complex.
- Positive complete is the outcome if the medium does not become red after the addition of zinc powder. If there is no red colour, there is no nitrate to diminish. Since no nitrite nor nitrate was present in the media, denitrification occurred and ammonia or molecular nitrogen was produced.
Purpose of Nitrate Reduction Test
- This test is used to measure an organism’s capacity to convert nitrate to nitrite. Some members of the family Enterobacteriaceae convert nitrite to other molecules, while all members reduce nitrate.
Principle of Nitrate Reduction Test
- Incubate a high inoculum of test bacteria in nitrate broth. If bacteria capable of producing the nitrate reductase enzyme convert the nitrate in the broth to nitrite, the nitrite can be further reduced to nitric oxide (NO), nitrous oxide (N2O), or nitrogen dioxide (N2) (N2).
- This test is based on the detection of nitrite and its capacity to make a red compound when it combines with sulfanilic acid to form a complex, i.e. nitrite-sulfanilic acid, which then reacts with -naphthylamine to produce a red precipitate, i.e. prontosil, a water-soluble azo dye.
- The red colour will only be visible in the presence of nitrate. If there is no red hue in the medium after adding sulfanilic acid and -naphthylamine, then the media does not contain nitrite.
- When the nitrate has not been reduced, the organism under test is nitrate-negative. The test microorganism is nitrate-positive if the nitrate may have been reduced to nitrite, which was then entirely reduced to nitric oxide, nitrous oxide, or nitrogen, which does not react with the reagents that react with nitrite.
Reactions of Nitrate Reduction Test

Use of Zinc powder
- When nitrite is not found, it is required to determine whether the organism has reduced nitrate beyond nitrite.
- A pinch of zinc powder should be added to the culture. It catalyses the nitrate reduction to nitrite.
- The appearance of a red colour upon addition of zinc powder indicates that nitrate was not reduced by the test organism, indicating that the test organism is incapable of reducing nitrate.
- If there is no change in colour following the addition of zinc, the organism is a nitrate reducer because it has converted nitrate to one of the other nitrogen molecules.
Use of A Durham tube
- It is added to the nitrate broth to detect gas generation in the tube and to identify denitrification by organisms that produce gas through other pathways.
Requirements
- Nitrate broth
- Durham tube
- Reagent A
- Reagent B
- Zinc dust (Powder)
- Test organisms
- Bunsen burner
- Inoculating wire
- Incubator
- Control strains
Composition of Nitrate broth (medium)
| Ingredients | Gms / Litre |
| Peptic digest of animal tissue | 5.000 |
| Meat extract | 3.000 |
| Potassium nitrate | 1.000 |
| Sodium chloride | 30.000 |
| Final pH ( at 25°C) | 7.0±0.2 |
Preparation of Nitrate broth (medium)
- Suspend 39 grammes in 1000 ml distilled water.
- If necessary, apply heat to dissolve the medium thoroughly.
- Dispense into tubes and autoclave at 15 pounds of pressure (121 degrees Celsius) for 15 minutes.
Preparation of Reagents
1. Reagent A ( 0.8% Sulfanilic acid )
Composition
- Sulfanilic acid: 0.8 g
- Distilled water: 70 ml
- Glacial acetic acid: 30 ml
Preparation Steps
- Mix sulfanilic acid with water; heat to dissolve.
- Cool, and then add acetic acid.
- Store at 2 to 8°C.
- Shelf life is 3 months.
2. Reagent B (0.5% N,N-Dimethyl-alpha-naphthylamine)
Composition
- Glacial acetic acid :30 ml
- Distilled water:70 ml
- N,N-dimethyl-α-naphthylamine :0.5 g
Preparation Steps
- Combine acetic acid and water.
- Add α-naphthylamine.
- Store at 2 to 8°C.
- Shelf life is 3 months.
3. Zinc metal dust
Procedure
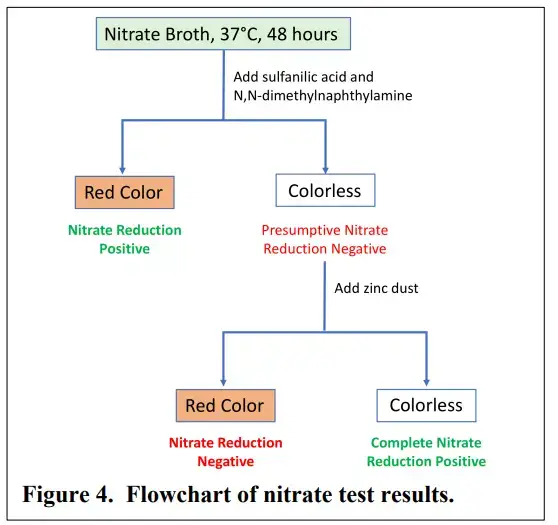
Occasionally, the nitrate reduction to nitrite test is completed in two phases.
First method
- Addition of Nitrate Reagents A and B determines the conversion of nitrate to nitrite.
Second method
- The decrease of nitrate beyond the detection of nitrite using zinc powder.
- Inoculate test organism in nitrate broth and incubate tubes at 30°C or 37°C for 24 hours at the appropriate temperature.
- After the completion of the incubation, look for the development of nitrogen gas before adding reagents.
- Add chemical A (6-8 drops).
- Likewise, add reagent B ( 6-8 drops).
- Observe for colour development in less than one minute.
- If no colour develops add zinc powder.
- After adding zinc, observe for at least three minutes for a red colour to appear.
Third Method
- Aseptically inoculate nitrate broth with a dense growth of the test organism.
- 24 to 48 hours of incubation at the proper temperature.
- In each broth, add one dropper of sulfanilic acid and one dropper of -naphthylamine.
- A change in hue to RED at this stage signifies a POSITIVE nitrate reduction test. If you observe a red colour at this moment, you must stop.
- The absence of a colour shift indicates the lack of nitrite. This may occur if nitrate was not reduced or if nitrate was reduced to nitrite, which was then further reduced to another molecule. If the colour is NOT red, you must advance to the next step.
- A toothpick’s worth of zinc should be added to each soup. Zinc catalyses the nitrate reduction to nitrite.
- At this point, a change in hue to RED signifies a NEGATIVE nitrate reduction test because it implies that nitrate was present and was reduced to nitrite.
- No colour shift indicates the absence of nitrate. Therefore, the lack of colour change at this time is a POSITIVE outcome.
Result and Interpretation of Nitrate Reduction Test
Nitrate/Nitrite reduction test: Positive
- Development of a cherry red hue with addition of reagents A and B Absence of red colour upon addition of zinc powder
Nitrate/Nitrite reduction test: Negative
- Red coloration resulting from the use of zinc powder
| Reaction | Gas | Color after addition of solution A and B | Color after addition of zinc | Interpretation |
| NO3 → NO2 | None | Red | – | NO3+, no gas |
| NO3 → NO2, gaspartialnongaseous endproducts | None | Red | – | NO3+, no gas |
| NO3 → NO2,gaseous endproducts | Yes | Red | – | NO3+, gas+ |
| NO3 → gaseousend product | Yes | None | None | NO3+, NO2+,gas+ |
| NO3 →nongaseous endproducts | None | None | None | NO3+, NO2+,no gas |
| NO3 → no reaction | None | None | Red | Negative |
| Organisms | Result |
| Acinetobacter calcoaceticus (19606) | Negative |
| Enterobacter aerogenes (13048) | Positive |
| Escherichia coli (25922) | Positive |
| Salmonella typhimurium (14028) | Positive |
| Bacillus cereus ATCC 10876 | Positive |


Limitations of Nitrate Reduction Test
- Inability to notice that the organism did not develop in the medium may lead to a false-negative test result (s).
- The interpretation of colour reactions should be determined immediately, as positive colour reactions might diminish swiftly.
- Following the addition of the nitrate reagents, a faint pink tint may be formed. This is not a favourable outcome.
- Due to the possibility of nitrite in the culture medium, Nitrate Agar or Nitrate Broth should be used for the nitrate reduction test.
- A negative zinc reduction (no colour change) test in conjunction with a negative nitrite reaction suggests that the nitrate has been reduced beyond the nitrite stage. Although nitrogen gas is a common byproduct of nitrate reduction, other byproducts may also be generated. Additional testing may be necessary to determine the final reaction products.
- To prevent false-negative nitrite reduction reactions, negative nitrite reactions must be confirmed using zinc dust.
- It has been reported that excessive zinc dust causes false-positive nitrite reduction reactions due to the complete reduction of unreduced nitrate to ammonia.
Quality Control
- Escherichia coli ATCC 25922—nitrate positive, gas negative
- Pseudomonas aeruginosa ATCC 27853—nitrate positive, gas positive
- Acinetobacter baumannii ATCC 19606—nitrate negative
Uses of Nitrate Reduction Test
- The nitrate reduction test is effective for distinguishing Enterobacteriaceae from other gram-negative bacteria since all members of this family reduce nitrate.
- It aids in the differentiation of Mycobacterium.
- In some species, Nitrate reduction may be connected to anaerobic respiration.
- Neisseria isolation from Moraxella and Kingella. This test is essential for distinguishing between N. gonorrhoeae and K. denitrificans, especially when Kingella denitrificans strains seem identical, i.e. gram-negative diplococci in gram-stained smears.
- It also facilitates Corynebacterium species identification.
References
- Tille P.M. 2014. Bailey and Scott’s diagnostic microbiology. Thirteen edition. Mosby, Inc., an affiliate of Elsevier Inc. 3251 Riverport Lane. St. Louis. Missouri 63043
- https://asm.org/ASM/media/Protocol-Images/Nitrate-and-Nitrite-Reduction-Test-Protocols.pdf?ext=.pdf
- https://microbeonline.com/nitrate-reduction-test/
- https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/product/documents/237/945/73426dat.pdf
- https://microbenotes.com/nitrate-reduction-test-objectives-principle-procedure-and-results/
- https://www.austincc.edu/microbugz/nitrate_reduction.php
- http://www.uwyo.edu/molb2021/additional_info/summ_biochem/nitrate_broth.html
- https://www.vumicro.com/vumie/help/VUMICRO/Nitrate_Reductase_Test.htm
- https://universe84a.com/nitrate-reduction-test/
- http://medbox.iiab.me/modules/en-cdc/www.cdc.gov/std/gonorrhea/lab/tests/nitrate.htm
- https://www.dalynn.com/dyn/ck_assets/files/tech/RN75.pdf
- https://himedialabs.com/td/m439s.pdf
- https://science.umd.edu/classroom/bsci424/LabMaterialsMethods/NitrateTest.htm
- http://crcooper01.people.ysu.edu/microlab/nitrate-test.pdf
- https://homepages.wmich.edu/~rossbach/bios312/LabProcedures/Nitrate%20reduction%20results.html
- http://faculty.collin.edu/dcain/CCCCD%20Micro/nitrate_reduction.htm
- https://microbiologyinfo.com/nitrate-reduction-test/