Nipah virus (NiV) is a zoonotic virus, which means it can be transmitted between animals and humans. Fruit bats, often known as flying foxes, serve as the natural reservoir for NiV. The Nipah virus is also known to infect humans and pigs. NiV infection is related with encephalitis (brain swelling) and can result in mild to severe sickness and death. Almost yearly outbreaks occur in regions of Asia, especially Bangladesh and India.
It is possible to prevent Nipah virus infection by avoiding ill pigs and bats in places where the virus is present and by avoiding drinking raw date palm sap, which may be contaminated by an infected bat. In hospital settings, regular infection control procedures can assist prevent transmission from patient to patient during an outbreak.
- The clinical manifestations of Nipah virus infection in humans range from asymptomatic illness (subclinical) to acute respiratory infection and deadly encephalitis.
- The estimated case fatality rate ranges from 40% to 75%. Depending on local capacities for epidemiological surveillance and clinical management, this rate can vary by outbreak.
- Nipah virus can be spread to humans by animals (such as bats or pigs) or contaminated food, as well as directly between humans.
- Nipah virus naturally infects fruit bats of the family Pteropodidae.
- There is no available cure or vaccine for humans or animals. Humans are primarily treated with supportive care.
- The 2018 annual evaluation of the WHO R&D Plan list of priority diseases indicates that Nipah virus research and development must be expedited immediately.
Structure of Nipah Virus (NiV)
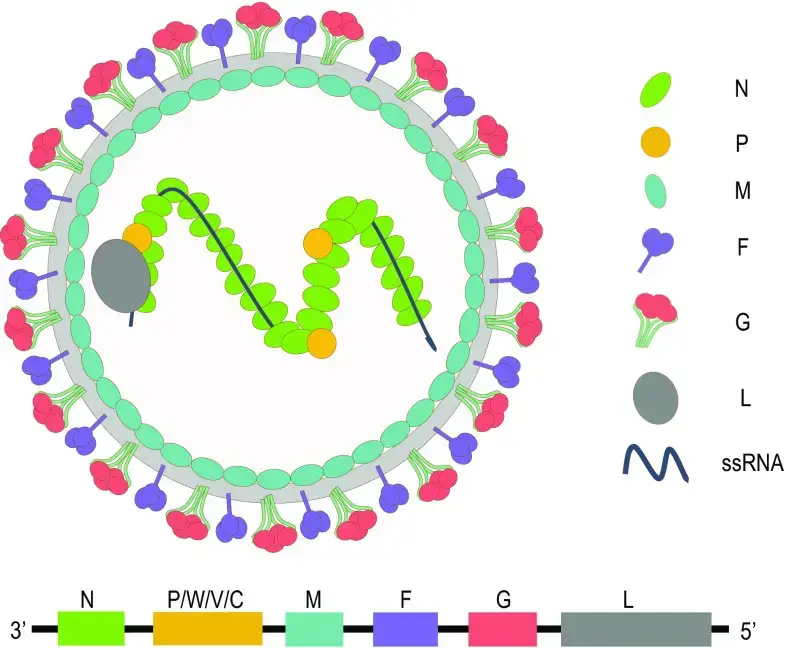
- Nipah virus (NiV) is an RNA virus that belongs to the family Paramyxoviridae (Order Mononegavirales), genus Henipavirus, and is closely linked to the horse-infecting Hendra virus.
- Nipah virus is an enveloped virus that ranges in size from 40 to 600 nm and is pleomorphic. The Nipah virus genome is a single-stranded, nonsegmented negative-sense RNA.
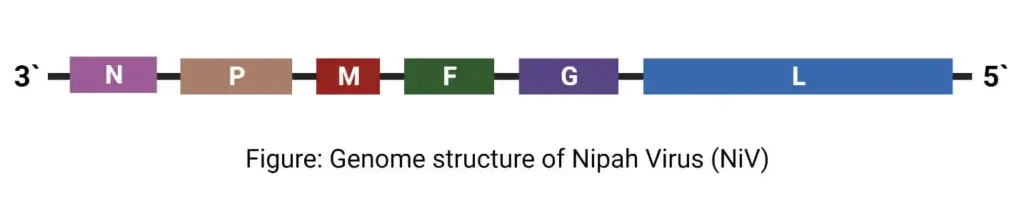
- The genome is 18,2 kilobytes long and contains six genes that correspond to structural proteins. It is composed of nucleocapsid (N), phosphoprotein (P), matrix protein (M), fusion protein (F), glycoprotein (G), and polymerase (L).
Genome structure of Nipah Virus (NiV)
- Single-stranded negative sense RNA of 18246 and 18252 base pairs (Malaysian isolate) (bngladesh isolate)
- There are six transcriptional units and six structural proteins in the genome. It is composed of nucleocapsid (N), phosphoprotein (P), matrix protein (M), fusion protein (F), glycoprotein (G), and polymerase (L)
- Genome-associated protein: large (L) protein and phosphoprotein (P)
- Fusion protein (F) and attachment glycoprotein protein are viral proteins (G)
- Phosphoprotein (P): its function as a polymerase cofactor, boosting polymerase processivity and permitting the encapsulation of newly synthesised viral genomes and antigenomes.
- Nipah virus phosphoprotein plays an additional role in immunosuppression by inhibiting interferon signalling by binding to host STAT-1.
The genetic makeup of the Nipah virus (NiV) is made up of a single-stranded RNA that is negative-sense, non-segmented, and has a genome size of approximately 18.2 kilobase pairs. This RNA codes for six structural proteins, namely nucleoprotein (N), phosphoprotein (P), matrix protein (M), fusion protein (F), attachment glycoprotein (G), and the large protein or RNA polymerase protein (L). In addition to these six proteins, the P gene is responsible for coding three nonstructural proteins through a process called RNA editing (V and W proteins) or an alternate open reading frame (C protein).
Epidemiology of Nipah Virus (NiV)
The initial occurrence of the Nipah virus outbreak happened in Malaysia-Singapore in the years 1998-1999. During the period of September 1998 to June 1999, there were 94 patients who had close contact with the swine population, and they were diagnosed with severe viral encephalitis. This showcased that the virus was directly transmitted from pigs to humans.
In Singapore and Malaysia, 246 patients were reported to have febrile encephalitis due to NiV between 1998 and 1999, and the mortality rate was around 40%. The second outbreak of the virus was reported in the non-contiguous area of Meherpur district in Bangladesh and Siliguri city in West Bengal, India, in 2001. Until the year 2010, there were nine outbreaks of the NiV reported in Bangladesh.
In the year 2011, another outbreak occurred in Bangladesh, which resulted in the deaths of 15 individuals due to the Nipah virus infection. Epidemiological investigations have confirmed that the NiV virus has spread throughout Asia, Africa, and the South Pacific Ocean, causing sporadic outbreaks through both human-to-human and zoonotic transmissions. It has caused hundreds of human deaths in the past two decades, posing a significant threat to both humans and domestic animals.
Transmission of Nipah Virus (NiV)
NiV is a zoonotic virus that can infect asymptomatic animals such as pigs and fruit bats (family Pteropodidae). It is now established that fruit bats belonging to the species Pteropus (also known as flying foxes) are the indigenous carriers of Nipah virus. Infected fruit bats release a virus in their saliva, urine, or other body secretions, which infects pigs and other domestic animals. Humans become infected by coming into close touch with these animals.
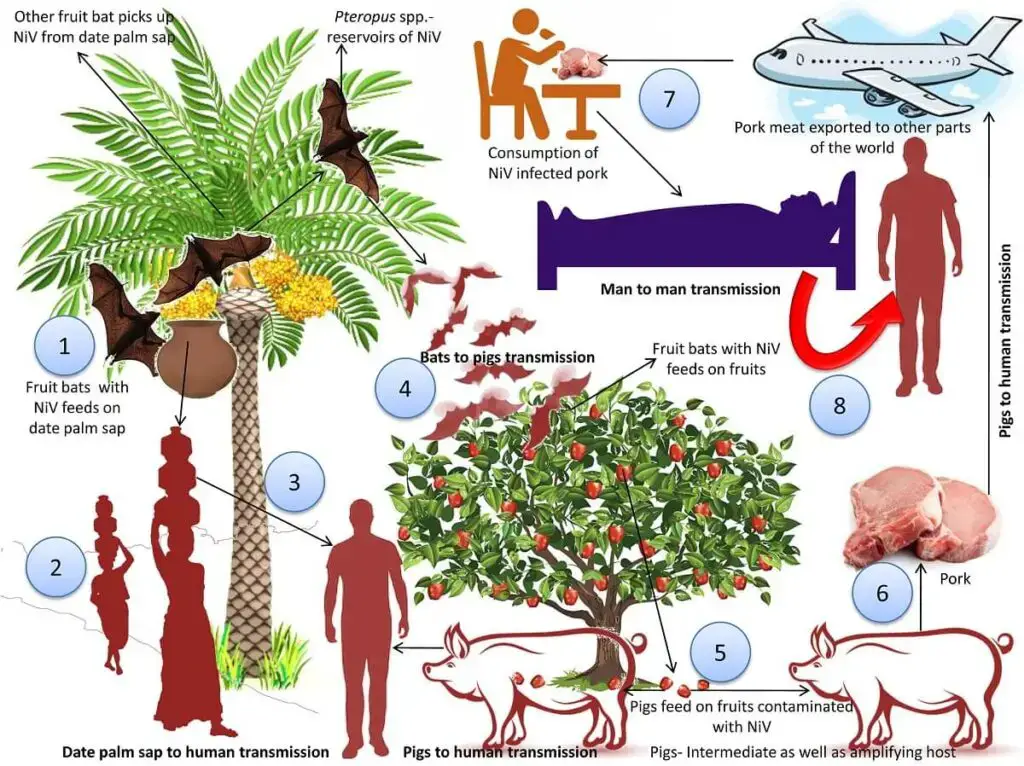
- Nipah virus is transmissible from animals to humans:
- By intimate contact with sick bats, pigs, and/or humans (NiV infected people).
- Infection by respiratory droplets, nasal or pharyngeal secretions of infected animals.
- Consuming contaminated fruits and juices containing animal secretions. Bat faeces frequently contaminate raw date palm sap (toddy), which is a substantial danger factor when consumed. It is known that bats drink toddy gathered in open containers and occasionally urinate in it, contaminating it with the virus.
- Person-to-Person transmission by direct contact with infected individuals, most usually among the patient’s family and carers.
Nipah virus’s natural hosts are fruit bats of the family Pteropodidae, notably species belonging to the genus Pteropus. Fruit bats do not appear to have any visible diseases. The geographic distribution of Henipaviruses is thought to overlap with that of the Pteropus group. Henipavirus infection in Pteropus bats from Australia, Bangladesh, Cambodia, China, India, Indonesia, Madagascar, Malaysia, Papua New Guinea, Thailand, and Timor-Leste provided support for this concept. Antibodies against Nipah and Hendra viruses were detected in African fruit bats of the species Eidolon, family Pteropodidae, indicating that these viruses may be present within the geographic distribution of Pteropodidae bats in Africa.
During the initial Malaysian outbreak in 1999, outbreaks of the Nipah virus in pigs and other domestic animals such as horses, goats, sheep, cats, and dogs were initially documented. In pigs, the virus is highly contagious. During the incubation period, which lasts four to fourteen days, pigs are contagious. Some infected pigs have acute feverish sickness, difficulty breathing, and neurological symptoms that include trembling, twitching, and muscle spasms. Mortality is generally low, with the exception of young piglets. Nipah virus should be detected when both pigs and humans have a peculiar barking cough or encephalitis.
Replication of Nipah Virus (NiV)
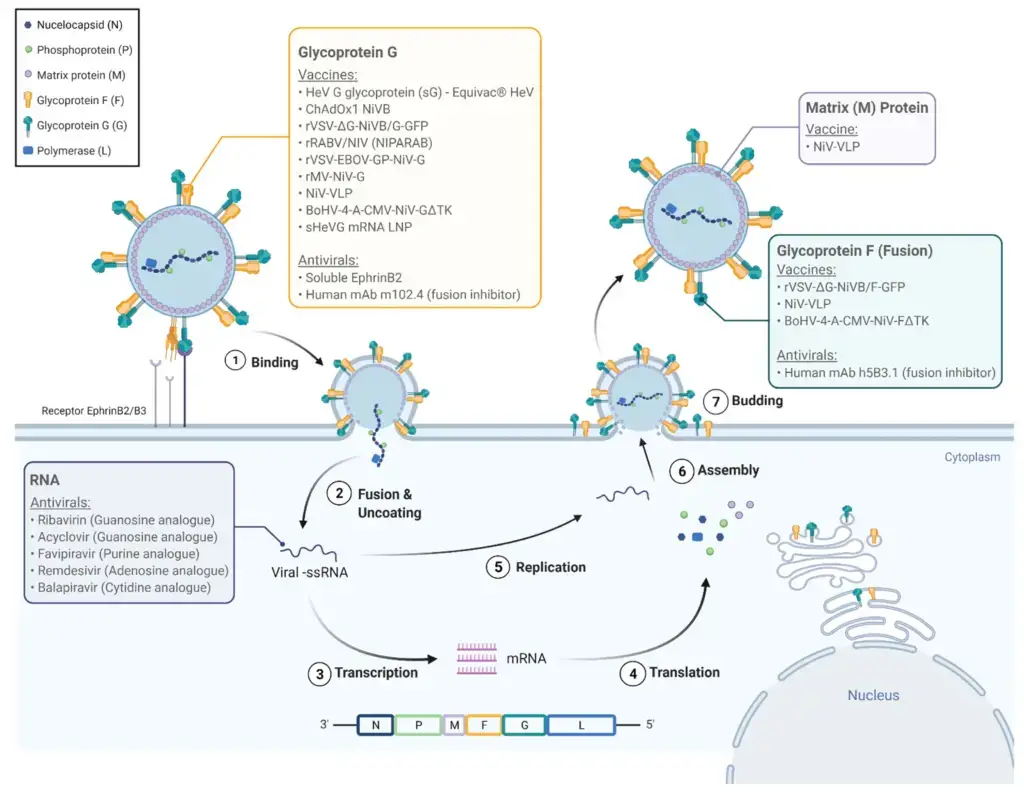
- The initial stage of viral infection is attachment/adsorption, wherein the virus affixes itself to the host cell through the viral glycoprotein G with the B2 receptor, and then fuses the viral membrane with the host membrane.
- Subsequently, the negative RNA genome serves as a template for the transcription of viral mRNA, which is then translated into viral proteins. The vRNA also functions as a template for the synthesis of cRNA(+), which further serves as the template for the synthesis of vRNA. Furthermore, the viral proteins perform a role in interferon signaling. The F proteins are also internalized and matured within the cells.
- The assembly process is primarily accomplished by the M protein, with N, P, C, M, F, and G incorporated in the virions.
- Finally, the virions bud through the host membrane that contains G proteins on its surface and are released from the cell.
Pathogenesis of Nipah Virus (NiV)
- NiV can be detected in the epithelial cells of the bronchiole in the initial stage of illness in humans.
- Viral antigens are present in bronchi and alveoli, mainly in the epithelium of bronchi and type II pneumocytes in experimental animal models.
- Inflammatory cytokines are induced due to infection of the respiratory tract epithelium, which recruits immune cells and leads to acute respiratory distress syndrome-like disease.
- Inflammatory mediators such as IL-1α, IL-6, IL-8, G-CSF, CXCL10, etc. are induced when smaller airway epithelium gets infected.
- The virus is disseminated to the endothelial cells of the lungs in the later stage of the disease, and it can enter the bloodstream and infect other organs, including the spleen, kidneys, and brain, leading to multiple organ failure.
- NiV can infect leukocytes and cause lethal infection in hamsters.
- Monocytes, natural killer cells, and CD6+ CD8+ T lymphocytes are infected in pigs.
- The virus can enter the CNS through the hematogenous route or anterogradely via olfactory nerves.
- Infection of the CNS by the virus disrupts the blood-brain barrier, induces IL-1β and TNF-α expression, and leads to the development of neurological signs.
- Infected CNS in humans may show inclusion bodies and plaques in both gray and white matter, along with necrosis.
- In several experimental animal models, the virus can directly enter the CNS via the olfactory nerve.
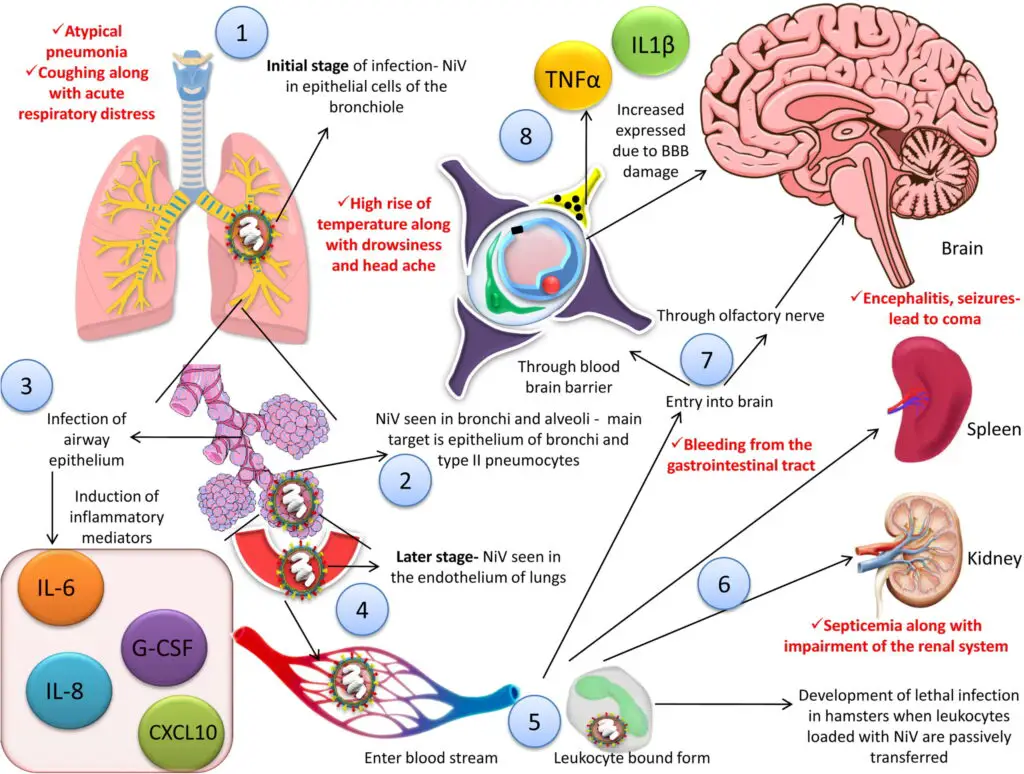
- NiV can infect the epithelial cells of the bronchiole in the initial stage of the disease.
- NiV antigen can be detected in bronchi and alveoli.
- Inflammatory mediators are activated due to infection of the airway epithelium, leading to the recruitment of immune cells and development of acute respiratory distress syndrome (ARDS)-like disease. Symptoms in humans include IL-1α, IL-6, IL-8, G-CSF, CXCL10, etc.
- In the later stage of the disease, the virus is disseminated to the endothelial cells of the lungs.
- The virus enters the bloodstream followed by dissemination, either freely or in host leukocyte bound form.
- NiV can reach the brain, spleen, and kidneys, leading to multiple organ failure in humans.
- There are two pathways for viral entry into the central nervous system (CNS): via hematogenous route or anterogradely via olfactory nerves.
- The blood-brain barrier (BBB) is disrupted, and IL-1β along with tumor necrosis factor (TNF)-α are expressed due to CNS infection by the virus, ultimately leading to neurological symptoms in humans.
Clinical Manifestations of Nipah Virus (NiV)
Infection with Nipah virus (NiV) can result in mild to severe illness, including brain swelling (encephalitis) and even death.
Typically, symptoms emerge 4-14 days after contact to the virus. Initial symptoms include fever and headache for three to fourteen days, and respiratory symptoms like as cough, sore throat, and difficulty breathing are frequently present. A phase of brain swelling (encephalitis) may ensue, with symptoms such as tiredness, disorientation, and mental confusion, which can proceed swiftly to coma within 24 to 48 hours.
Initial symptoms may include one or more of the following:
- Fever
- Headache
- Cough
- painful throat
- Trouble in respiration
- Vomiting
Serious symptoms may ensue, including:
- Disorientation, fatigue, and/or confusion
- Seizures
- Coma
- Brain swelling (encephalitis)
Mortality is possible in 40-75% of cases. There have been reports of survivors of Nipah virus infection experiencing recurrent convulsions and personality alterations.
Infections that cause symptoms and occasionally mortality months or even years after exposure (known as latent or dormant illnesses) have also been described.
Diagnosis of Nipah Virus (NiV)
- Laboratory tests: NiV can be detected by various laboratory tests such as enzyme-linked immunosorbent assay (ELISA), reverse transcription-polymerase chain reaction (RT-PCR), and virus isolation.
- Imaging studies: Imaging studies such as magnetic resonance imaging (MRI) and computed tomography (CT) scans can help detect neurological symptoms associated with NiV infection.
- Serological testing: Serological testing can be done to detect antibodies against NiV in the blood.
- Clinical presentation: The clinical presentation of NiV infection includes symptoms such as fever, headache, muscle pain, and respiratory distress, which can aid in the diagnosis of the disease.
- Contact tracing: Identifying and tracing contacts of infected individuals can aid in the diagnosis and prevention of further spread of the disease.
Treatment of NiV
- Supportive care: There is currently no specific antiviral treatment for NiV infection, so supportive care is the primary mode of treatment. This includes management of fever, respiratory distress, and neurological symptoms.
- Intensive care: Patients with severe respiratory distress or neurological symptoms may require intensive care, such as mechanical ventilation and hemodynamic support.
- Experimental treatments: Some experimental treatments, such as monoclonal antibodies and antiviral drugs, are being studied for their potential use in treating NiV infection. However, their effectiveness has not yet been established in clinical trials.
- Infection control measures: Preventing further spread of the disease through infection control measures, such as isolating infected individuals and using personal protective equipment, is crucial in the management of NiV outbreaks.
Prevention and Control of NiV
- Limiting exposure: The best way to prevent NiV infection is to limit exposure to infected animals, such as bats or pigs, and their contaminated products, such as raw date palm sap or meat.
- Personal protective measures: People who are in close contact with NiV-infected individuals or animals should wear personal protective equipment, such as gloves, gowns, and masks, to prevent transmission.
- Infection control measures: Isolation of infected individuals and proper disposal of contaminated materials, such as syringes or needles, is essential to prevent further spread of the virus.
- Public awareness: Raising public awareness about the risks and transmission routes of NiV infection can help prevent outbreaks and improve early detection and control.
- Vaccination: Currently, there is no licensed vaccine for NiV, but research is ongoing to develop effective vaccines for use in at-risk populations.
FAQ
What is Nipah Virus (NiV)?
Nipah Virus (NiV) is a deadly zoonotic virus that can cause severe respiratory illness and encephalitis in humans and animals.
How is NiV transmitted?
NiV can be transmitted through direct contact with infected animals, consumption of contaminated food or water, or person-to-person contact through respiratory secretions or bodily fluids.
What are the symptoms of NiV infection?
Symptoms of NiV infection can include fever, headache, dizziness, drowsiness, confusion, and seizures.
Is there a vaccine for NiV?
Symptoms of NiV infection can include fever, headache, dizziness, drowsiness, confusion, and seizures.
How is NiV diagnosed?
NiV can be diagnosed through laboratory tests that detect the virus in blood or other bodily fluids, or through imaging tests that show characteristic changes in the brain.
Is there a specific treatment for NiV infection?
There is no specific treatment for NiV infection, but supportive care can help manage symptoms and complications.
Can animals transmit NiV to humans?
Yes, animals such as pigs, bats, and horses can transmit NiV to humans through direct contact or consumption of contaminated products.
What measures can be taken to prevent NiV infection?
Measures to prevent NiV infection include avoiding contact with infected animals or their bodily fluids, practicing good hygiene such as washing hands frequently, and avoiding consumption of raw or undercooked fruits and vegetables.
Where is NiV commonly found?
NiV is commonly found in Southeast Asia, particularly in Bangladesh and India.
Are there any ongoing research efforts to better understand and treat NiV?
Yes, there are ongoing research efforts to better understand the transmission, pathogenesis, and treatment of NiV, including development of vaccines and antiviral therapies.
References
- Banerjee S, Gupta N, Kodan P, Mittal A, Ray Y, Nischal N, Soneja M, Biswas A, Wig N. Nipah virus disease: A rare and intractable disease. Intractable Rare Dis Res. 2019 Feb;8(1):1-8. doi: 10.5582/irdr.2018.01130. PMID: 30881850; PMCID: PMC6409114.
- Sun B, Jia L, Liang B, Chen Q, Liu D. Phylogeography, Transmission, and Viral Proteins of Nipah Virus. Virol Sin. 2018 Oct;33(5):385-393. doi: 10.1007/s12250-018-0050-1. Epub 2018 Oct 11. PMID: 30311101; PMCID: PMC6235768.
- Hauser N, Gushiken AC, Narayanan S, Kottilil S, Chua JV. Evolution of Nipah Virus Infection: Past, Present, and Future Considerations. Tropical Medicine and Infectious Disease. 2021; 6(1):24. https://doi.org/10.3390/tropicalmed6010024
- Saxena, S.K., Maurya, V.K., Kumar, S., Bhatt, M.L.B. (2020). Nipah Virus. In: Malik, Y.S., Singh, R.K., Dhama, K. (eds) Animal-Origin Viral Zoonoses. Livestock Diseases and Management. Springer, Singapore. https://doi.org/10.1007/978-981-15-2651-0_3
- Jensen, Kenneth & Adams, Ricky & Bennett, Richard & Bernbaum, John & Jahrling, Peter & Holbrook, Michael. (2018). Development of a novel real-time polymerase chain reaction assay for the quantitative detection of Nipah virus replicative viral RNA. PLOS ONE. 13. e0199534. 10.1371/journal.pone.0199534.
- Singh, Raj & Dhama, Kuldeep & Chakraborty, Sandip & Tiwari, Ruchi & Natesan, Senthilkumar & Khandia, Rekha & Munjal, Ashok & K, Vora & Latheef, Shyma & Karthik, Kumaragurubaran & Malik, Yashpal & Singh, Ravpreet & Chaicumpa, Wanpen & Mourya, Devendra. (2019). Nipah virus: epidemiology, pathology, immunobiology and advances in diagnosis, vaccine designing and control strategies – a comprehensive review. The Veterinary quarterly. 39. 26-55. 10.1080/01652176.2019.1580827.
- Reddy, KR. (2018). Nipah Virus (NiV) Infection: An Emerging Zoonosis of Public Health Concern. Journal of Gandaki Medical College-Nepal. 11. 10.3126/jgmcn.v11i02.22897.
- Hayman, D. T. S., & Johnson, N. (2014). Nipah Virus. The Role of Animals in Emerging Viral Diseases, 293–315. doi:10.1016/b978-0-12-405191-1.00011-9
- Rollin, P. E. (2014). Nipah Virus Disease. Emerging Infectious Diseases, 175–184. doi:10.1016/b978-0-12-416975-3.00013-3
- https://www.onlinebiologynotes.com/nipah-virus-structure-and-genome-mode-of-transmission-pathogenesis-symptoms-prevention-and-treatment/
- https://ncdc.gov.in/showlink.php?id=302
- https://microbewiki.kenyon.edu/index.php/Nipah_Virus
- https://www.business.qld.gov.au/industries/farms-fishing-forestry/agriculture/biosecurity/animals/diseases/guide/nipah-virus
- https://www.woah.org/en/disease/nipah-virus/
- https://www.cdc.gov/vhf/nipah/index.html#:~:text=Nipah%20virus%20(NiV)%20is%20a,illness%20in%20pigs%20and%20people.
- https://www.who.int/news-room/fact-sheets/detail/nipah-virus