Nelson–Somogyi method is a classical colorimetric method used for the quantitative determination of reducing sugars such as glucose and fructose. It is a highly sensitive method and is commonly used in biochemical and carbohydrate analysis. It is considered as a standard method because the estimation is based on stoichiometric reduction reaction and gives accurate measurement of reducing groups present in the sample.
This method is mainly preferred over some other reducing sugar assays due to formation of a stable color complex and higher sensitivity. It can detect very small amount of reducing sugars and the colour produced remains stable for longer time.
It is based on reduction–oxidation reaction. Reducing sugars present in the sample reduces cupric ions (Cu²⁺) to cuprous ions (Cu⁺) under alkaline conditions. This reaction is carried out using Somogyi’s alkaline copper reagent and heating in boiling water bath.
The cuprous ions formed then reacts with arsenomolybdate reagent (Nelson’s reagent). The cuprous ions reduce the arsenomolybdate complex to form a deep blue coloured compound known as molybdenum blue. The intensity of blue colour formed is directly proportional to the concentration of reducing sugars present in the given sample.
This method is highly sensitive and can detect as low as 10 µg of glucose. The colour formed is stable and suitable for accurate spectrophotometric analysis. However one limitation of this method is the use of arsenic containing reagent which is toxic and requires careful handling.
Principle of Nelson-Somogyi method
It is a colorimetric method based on the reducing property of sugars. Reducing sugars such as glucose possess free aldehyde or ketone group which has the capacity to reduce metal ions under suitable conditions. This principle is utilised in Somogyi method for estimation of reducing sugars.
In this method the sample containing reducing sugars is heated with alkaline copper tartrate reagent. Under alkaline condition the reducing sugars reduce cupric ions (Cu²⁺) to cuprous ions (Cu⁺). As a result cuprous oxide (Cu₂O) is formed which appears as a precipitate. This step is essential and occurs only when free reducing groups are present in the sugar molecules.
The cuprous ions produced in the first step further reacts with arsenomolybdate reagent known as Nelson’s reagent. The cuprous ions reduce molybdic acid present in the reagent to form a stable blue coloured complex called molybdenum blue. The intensity of blue colour formed depends upon the amount of reducing sugar present in the sample.
The developed blue colour is measured using a spectrophotometer at a wavelength range of 500–620 nm. The absorbance obtained is directly proportional to the concentration of reducing sugars. Sodium sulfate is present in the copper reagent which helps in reducing the solubility of oxygen and prevents re-oxidation of cuprous ions by atmospheric oxygen, thus ensuring accuracy of the method.
Objectives of Nelson-Somogyi method
- To determine quantitatively the concentration of reducing sugars such as glucose lactose maltose and galactose present in given sample.
- To utilise the reducing property of carbohydrates which possess free aldehyde or ketone group for reduction of cupric ions to cuprous ions in alkaline medium.
- To produce a stable blue coloured complex (molybdenum blue) by reaction of cuprous ions with arsenomolybdate reagent for colorimetric estimation.
- To carry out spectrophotometric measurement of reducing sugars based on intensity of colour formed which is proportional to sugar concentration.
- To prevent self reduction of copper reagent and improve reagent stability by suitable composition of carbonate and tartrate.
- To prevent re-oxidation of cuprous oxide by atmospheric oxygen during heating and cooling by addition of sodium sulphate.
- To remove interfering non sugar reducing substances from biological samples by deproteinization so that true sugar is estimated.
- To prepare a standard calibration curve using known glucose concentration for calculation of unknown samples.
Requirements for Nelson-Somogyi method
Apparatus
- Spectrophotometer or colorimeter for measuring absorbance in the range of 500–620 nm.
- Boiling water bath maintained at 100°C for heating the reaction mixture.
- Clean dry test tubes of suitable size for carrying out reaction.
- Glass marbles aluminium foil or tube covers to prevent evaporation during heating.
- Pipettes for accurate measurement of sample and reagents.
- Centrifuge for removal of precipitate when deproteinization is carried out.
- Cuvettes for spectrophotometric reading.
Reagents
- Somogyi’s alkaline copper reagent containing copper sulphate sodium carbonate sodium bicarbonate potassium sodium tartrate and sodium sulphate.
- Nelson’s arsenomolybdate reagent consisting of ammonium molybdate sulphuric acid and sodium arsenate (toxic in nature).
- Standard glucose solution of known concentration for preparation of standard curve.
- Test sample containing reducing sugars to be estimated.
Additional Requirements (if needed)
- Deproteinizing agents such as barium hydroxide and zinc sulphate for removal of proteins from biological samples.
- Ethanol for extraction of sugars from plant materials.
Procedure of Somogyi Method for Determination of Reducing Sugars
- Prepare Somogyi alkaline copper reagent by mixing reagent A and reagent B in required ratio just before use. Nelson’s arsenomolybdate reagent is prepared earlier and allowed to mature properly.
- If the sample is biological in nature deproteinization is carried out using barium hydroxide and zinc sulphate to obtain clear filtrate. In plant samples sugars are extracted using hot ethanol.
- Prepare a series of glucose standard solutions of known concentration. Take fixed volume of standards test sample and distilled water blank in separate clean test tubes.
- Add equal volume of alkaline copper reagent to each tube. Mix well and cover the tubes with glass marble or foil to prevent evaporation.
- Keep the tubes in boiling water bath for about 10–20 minutes. During this step reduction of cupric ions to cuprous ions takes place.
- Remove the tubes from water bath and allow them to cool to room temperature. Shaking is avoided during cooling to prevent re oxidation of cuprous oxide.
- Add measured volume of Nelson’s arsenomolybdate reagent to each tube. Mix until the precipitate dissolves completely and blue colour develops.
- Dilute the contents with distilled water to required volume and allow the colour to stabilise for few minutes.
- Measure the absorbance using spectrophotometer or colorimeter at suitable wavelength between 500–620 nm.
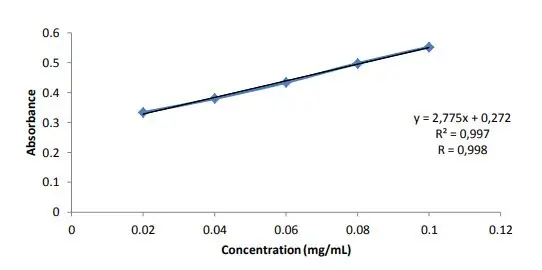
- Prepare standard curve by plotting absorbance against concentration of glucose. The amount of reducing sugar in test sample is calculated using this standard curve.
Calculation
Prepare a series of standard glucose solutions of known concentration and treat them in the same manner as the test sample using alkaline copper reagent and arsenomolybdate reagent.
Measure the absorbance of standards and test sample using spectrophotometer at suitable wavelength between 500–620 nm.
Plot a standard calibration curve by taking concentration of glucose on X-axis and corresponding absorbance on Y-axis. A straight line is obtained which follows Beer’s law.
Note the absorbance value of the unknown sample and determine the corresponding concentration of reducing sugar from the standard curve.
If linear equation is used the concentration is calculated using the equation Y = mx + c where Y is absorbance and x is concentration of reducing sugar.
Multiply the obtained value with dilution factor if the sample was diluted during extraction or deproteinization.
Express the final amount of reducing sugar in required units such as mg per 100 ml mg per g of sample or percentage concentration.
Result of Somogyi Method
- On heating the sample with alkaline copper reagent reducing sugars reduce cupric ions to cuprous ions leading to formation of cuprous oxide precipitate which appears yellow to brick red in colour.
- After addition of Nelson’s arsenomolybdate reagent the cuprous oxide reduces molybdic acid resulting in formation of stable blue coloured complex known as molybdenum blue.
- The intensity of blue colour developed is measured using spectrophotometer or colorimeter at wavelength between 500–620 nm.
- The absorbance obtained is directly proportional to the concentration of reducing sugars present in the sample and follows Beer’s law.
- The exact amount of reducing sugar present in the given sample is determined by comparing the absorbance value with the standard calibration curve prepared using glucose standards.
Uses of Somogyi Method
- It is used for quantitative estimation of reducing sugars such as glucose fructose and maltose in laboratory samples.
- It is used in clinical laboratories for determination of blood glucose level for diagnosis and monitoring of diabetes.
- It is used in food industry for analysis of reducing sugars present in fruits fruit juices honey syrups and processed food products.
- It is used for estimation of sugar content in beverages such as soft drinks wines and fermented products.
- It is used in enzyme assays for determination of activity of carbohydrase enzymes like amylase cellulase xylanase and other polysaccharide degrading enzymes.
- It is used in plant physiology studies to estimate reducing sugars in plant tissues during growth development and stress conditions.
- It is used in biomass and biofuel research to measure reducing sugars released after hydrolysis of plant and algal biomass.
- It is used for determination of total sugars after hydrolysis of non reducing sugars into reducing monosaccharides.
Advantages of Somogyi Method
- It gives accurate quantitative estimation of reducing sugars due to stoichiometric nature of copper reduction reaction.
- It is highly sensitive method and can detect very small amount of reducing sugars as low as microgram level.
- The blue coloured complex (molybdenum blue) formed is stable for long time which allows accurate spectrophotometric reading.
- The intensity of colour formed is directly proportional to concentration of reducing sugar and follows Beer’s law over a wide range.
- The method shows good reproducibility and precision when performed under controlled conditions.
- Self reduction of copper reagent is minimised due to improved composition of Somogyi reagent which increases reliability of results.
- It can be used for different types of samples such as blood plant extracts food products and beverages without interference from sample colour.
Limitations of Somogyi Method
- The method uses arsenomolybdate reagent which contains arsenic and is highly toxic and hazardous to handle.
- It is not specific for a particular sugar and estimates total reducing substances present in the sample.
- Non sugar reducing compounds such as proteins uric acid and other metabolites can interfere and give higher values.
- Deproteinization step is required for biological samples which makes the procedure lengthy and sometimes incomplete.
- Cuprous oxide formed during reaction can undergo re oxidation by atmospheric oxygen especially during cooling step.
- Shaking of tubes during heating or cooling may lead to error in results due to re oxidation of copper.
- Certain buffer solutions like citrate and Tris interfere with the reaction and affect accuracy of estimation.
- Harsh alkaline and boiling conditions may destroy some labile carbohydrates and polysaccharides.
- The procedure is time consuming and requires careful control of heating and cooling conditions.
- Different reducing sugars may not follow same stoichiometry hence separate standard curve is required for each sugar.
- Agency for Toxic Substances and Disease Registry. (n.d.). Medical management guidelines for arsenic (As) and inorganic arsenic compounds.
- Asquieri, E. R., et al. (2019). Comparison of titulometric and spectrophotometric approaches towards the determination of total soluble and insoluble carbohydrates in foodstuff.
- BenchChem. (n.d.). Molybdenum blue.
- Biocyclopedia. (n.d.). Determination of reducing sugars by Nelson-Somogyi method.
- Boston University. (n.d.). Chapter 7: Quantitative determination of sugar in blood.
- Comprehensive analytical evaluation of the Nelson-Somogyi methodology for reducing sugar quantification. (n.d.).
- El-Shishtawy, R. M., et al. (2023). Novel and facile colorimetric detection of reducing sugars in foods via in situ formed gelatin-capped silver nanoparticles.
- Freeman, S. (2015). How do you choose an optimal wavelength for absorbance readings?
- Green, F., III, et al. (1989). Adaptation of the Nelson-Somogyi reducing-sugar assay to a microassay using microtiter plates.
- Guo, R., et al. (2025). Limitations of the molybdenum blue method for phosphate quantification in the presence of organophosphonates.
- Gusakov, A. V., et al. (2011). Comparison of two methods for assaying reducing sugars in the determination of carbohydrase activities.
- Hatanaka, C., & Kobara, Y. (1980). Determination of glucose by a modification of Somogyi-Nelson method.
- Kaczmarek, M. (n.d.). Estimation of reducing sugar by Nelson-Somogyi method.
- King Saud University. (n.d.). Determination of reducing sugars by Somogyi-Nelson method.
- Kozitsina, A. N., et al. (2014). Determination of urea and creatinine by chronoamperometry.
- McCleary, B. V., & McGeough, P. (2015). A comparison of polysaccharide substrates and reducing sugar methods for the measurement of endo-1,4-β-xylanase.
- MilliporeSigma. (2025). Safety data sheet: Arsenic.
- New Jersey Department of Health. (2008). Hazardous substance fact sheet: Arsenic.
- Oborn, R. E., et al. (1971). Automated determination of reducing sugar and sucrose in food products.
- Occupational Safety and Health Administration. (n.d.). 1910.1018 – Inorganic arsenic.
- Occupational Safety and Health Administration. (n.d.). Semiconductors – Arsenic.
- Petrillo, T. (2015). Do you have any good protocol for a modified Nelson-Somogyi?
- Romadhoni, R. P., et al. (2017). Determination of reduction sugar from banana (Musa acuminata balbisiana Colla) with different cooking process by UV-visible spectrophotometer.
- Scientific Research Publishing. (n.d.). Nelson, N. (1944) A photometric adaptation of the Somogyi method… – References.
- Scribd. (n.d.). Determination of reducing sugars by the method of Somogyi-Nelson.
- Scribd. (n.d.). EXPT NO : 1 Estimation of glucose by ‘Somogyi-Nelson method’.
- Scribd. (n.d.). Glucose analysis via Nelson-Somogyi method.
- Scribd. (n.d.). Nelson-Somogyi method and result.
- Scribd. (n.d.). Reducing sugars in carbonated drinks.
- Sharma, N. C., et al. (1972). A simplified technique for estimation of blood glucose.
- Somogyi, M. (1952). Notes on sugar determination.
- Study.com. (n.d.). What is the theory behind Nelson-Somogyi’s method?
- Syal, K., et al. (2013). Creatinine estimation and interference.
- The BU Biochemistry Laboratory Manual. (n.d.). Nelson-Somogyi method.
- Weber, J. A., & van Zanten, A. P. (1991). Interferences in current methods for measurements of creatinine.
- Wikipedia. (n.d.). Molybdenum blue.
Nelson-Somogyi method can be used to quantify the reduction of sugar using arsenolmolibdat and copper reagents. The principle behind the Nelson Somogyi method is the quantity of deposro oxide deposits which react with arsenomolibdate, which reduces to molybdine blue. The blue hue is determined by as absorbance.
The keto and aldehyde free groups are regularly studied when reducing sugars. Lactose, galactose and maltose are all examples of sugars that are reduced. The cyclic form has to first break the ring to create reactive aldehyde prior to the process of oxidation can begin.