What is Mueller Hinton Agar (MHA)?
- Mueller Hinton Agar (MHA) is a widely used culture medium employed in antimicrobial susceptibility testing (AST) of bacteria. This standardized solid medium provides optimal conditions for the growth of clinically significant bacteria and is recommended for the Kirby-Bauer method of diffusion or dilution in agar for studying bacterial susceptibility to antimicrobial agents.
- Originally developed by Mueller and Hinton as a protein-free medium for the primary isolation of Neisseria species, MHA gained popularity due to its simplicity and transparency. Although other media were later developed for cultivating pathogenic Neisseria species, MHA remained extensively used for determining sulfonamide resistance in gonococci and other organisms. It was eventually recognized and adopted as the reference medium by various organizations such as CLSI (Clinical and Laboratory Standards Institute), CA-SFM (Comité de l’Antibiogramme de la Société Française de Microbiologie), and EUCAST (European Committee on Antimicrobial Susceptibility Testing).
- The selection of MHA as the reference medium was based on several factors.
- Firstly, MHA demonstrates good batch-to-batch reproducibility for susceptibility testing, ensuring consistent and reliable results.
- Secondly, it has a low content of sulfonamide, trimethoprim, and tetracycline inhibitors, which allows satisfactory growth of most non-demanding pathogens. This feature is crucial for accurate assessment of bacterial susceptibility to antimicrobial agents.
- Moreover, MHA supports the growth of a wide range of non-fastidious bacterial pathogens, making it suitable for testing susceptibility in various clinical isolates.
- Lastly, the extensive data and experience accumulated regarding its performance further support the use of MHA in antimicrobial susceptibility testing.
- The Kirby-Bauer method, utilizing antimicrobial-impregnated paper discs, is commonly employed with MHA for antibiotic susceptibility testing. The zone diameters observed around the discs correlate with minimum inhibitory concentration (MIC) values, providing information on the susceptibility of pathogenic microorganisms. The zone diameters have been established and standardized for different antimicrobial agents, allowing the determination of resistant, intermediate, and sensitive results.
- In specific cases, variations of MHA have been recommended. Mueller Hinton Agar with 5% sheep blood and Mueller Hinton Agar with Hemoglobin are suitable for antimicrobial susceptibility testing of Streptococcus pneumoniae and Haemophilus influenzae.
- The composition of MHA includes HM infusion B and acicase, which provide essential nutrients, nitrogenous compounds, carbon, sulfur, and other necessary elements for bacterial growth. Starch, acting as a protective colloid, helps protect against toxic substances present in the medium. Starch hydrolysis produces dextrose, serving as an energy source for bacteria. The ingredients in MHA are carefully chosen to have low thymine and thymidine content, as determined by MIC values for Enterococcus faecalis with sulfamethoxazole trimethoprim (SXT).
- Overall, Mueller Hinton Agar is a reliable and widely used culture medium in antimicrobial susceptibility testing, offering consistent results, broad bacterial growth support, and compatibility with the Kirby-Bauer method. Its standardized formulation and extensive usage experience make it a preferred choice in laboratories for assessing the susceptibility of bacteria to antimicrobial agents.
Why MHA is used for antibiotic susceptibility testing (AST)?
Mueller Hinton Agar (MHA) is commonly used for antibiotic susceptibility testing due to several key reasons:
- Non-selective and non-differential medium: MHA supports the growth of almost all organisms plated on it. This characteristic allows for testing a wide range of bacterial isolates and is not limited to specific species or strains.
- Starch content: MHA contains starch, which plays two important roles in antibiotic susceptibility testing. Firstly, starch has the ability to absorb toxins released by bacteria. This prevents the toxins from interfering with the action of antibiotics and ensures more accurate results. Secondly, starch mediates the rate of diffusion of antibiotics through the agar. This controlled diffusion enhances the formation of distinct zones of inhibition, providing a clearer indication of bacterial susceptibility.
- Loose agar consistency: MHA has a loose agar texture compared to other plates. This loose consistency facilitates better diffusion of antibiotics through the agar, leading to more accurate and reliable measurements of the zone of inhibition. Improved diffusion ensures that the antibiotics reach the bacteria effectively, reflecting the true susceptibility of the bacterial isolate.
- Batch-to-batch reproducibility: MHA demonstrates acceptable batch-to-batch reproducibility for susceptibility testing. This means that when multiple batches of MHA are used, consistent results are obtained. The standardized composition and formulation of MHA contribute to its reliable performance, allowing for consistent interpretation of susceptibility results over time.
- Low concentration of inhibitors: MHA has a low concentration of sulfonamide, trimethoprim, and tetracycline inhibitors. Specifically, the concentration of inhibitors such as thymidine and thymine is minimized in MHA. This reduction in inhibitors helps to minimize their interference with the antimicrobial activity being tested. It allows for a more accurate assessment of the susceptibility of bacterial isolates to sulfonamides and trimethoprim.
Mueller Hinton Agar (MHA) Principle
The principle of Mueller Hinton Agar (MHA) revolves around its composition and the controlled levels of various components to ensure optimal growth of bacteria and accurate antibiotic susceptibility testing.
The key components of MHA include Beef Extract, Acid Hydrolysate of Casein, Starch, and Agar. Beef Extract and Acid Hydrolysate of Casein provide essential nutrients such as nitrogen, carbon, sulfur, amino acids, vitamins, and other necessary elements for bacterial growth. These components support the metabolic requirements of bacteria and promote their proliferation on the agar medium.
Starch is added to MHA as a protective colloid. It serves to absorb any toxic metabolites released by bacteria during growth. By absorbing these toxins, starch prevents them from interfering with the action of antibiotics and helps maintain the accuracy of susceptibility testing.
Additionally, starch hydrolysis in MHA results in the production of dextrose, which serves as a source of energy for bacterial growth. This energy source supports the metabolic activities of bacteria, contributing to their viability and ensuring optimal growth on the agar medium.
Agar, a solidifying agent, is incorporated into MHA to provide a stable and solid matrix for bacterial growth. It allows the medium to maintain its physical structure while facilitating the diffusion of antimicrobial agents during susceptibility testing.
The levels of certain components, such as tetracycline and sulfonamide inhibitors, thymidine, thymine, magnesium, and calcium ions, are carefully controlled in MHA. The aim is to prevent interference with susceptibility testing and to promote robust bacterial growth. High levels of these inhibitors or nucleotides can affect the effectiveness of antibiotics and compromise the accuracy of susceptibility results. By minimizing their concentrations, MHA ensures reliable and reproducible testing outcomes.
Specifically, MHA is designed to minimize the presence of para-aminobenzoic acid (PABA) and its analogs, which can antagonize the activity of sulfonamides. Reduced levels of PABA in MHA prevent the inactivation of sulfonamides, allowing accurate assessment of bacterial susceptibility to these antibiotics.
Similarly, the thymidine and thymine content in MHA is reduced to a minimum. High levels of thymidine can lead to reduced activity of trimethoprim, resulting in smaller inhibition zones and the appearance of inner zonal colonies. By minimizing thymidine and thymine, MHA ensures that trimethoprim’s inhibitory effects are accurately reflected during susceptibility testing.
Composition of Mueller Hinton Agar (MHA)
| Ingredients | Gms/Litre |
| Beef extract | 2.0 |
| Acid hydrolysate of casein | 17.5 |
| Starch | 1.5 |
| Agar | 17.0 |
Final pH 7.3 +/- 0.1 at 25ºC.
Mueller Hinton Agar (MHA) Preparation
The preparation of Mueller Hinton Agar (MHA) involves the following steps:
- Weighing the appropriate amount: Weigh the required amount of dehydrated Mueller-Hinton agar powder as per the manufacturer’s instructions. Place the measured amount in a 2-liter flask.
- Mixing with water: Add 1 liter of distilled water to the flask containing the agar powder. Swirl the flask gently to disperse the powder evenly in the water.
- Heating and dissolving: Place the flask on a hot plate with a magnetic stirrer or other heating devices. Heat the mixture slowly until the powder is completely dissolved. It should come to a gentle boil. Avoid vigorous boiling, and ensure continuous stirring to prevent the medium from burning.
- Transfer and aliquoting: Once the agar powder is dissolved, carefully remove the flask from the heat source. Dispense the prepared MHA into the desired containers or aliquots. For example, you can dispense 250 ml volumes into 500 ml Erlenmeyer flasks.
- Loosely covering the containers: Cover the containers loosely to allow for proper air circulation. For example, you can insert stoppers into the mouth of the Erlenmeyer flasks.
- Autoclaving: Place the containers with the prepared MHA in an autoclave. Autoclave at 121°C for 15 minutes to sterilize the medium and eliminate any potential contaminants.
- Cooling: After autoclaving, remove the containers from the autoclave and allow them to cool. Place them in a 48°C water bath to facilitate the cooling process.
- Pouring into Petri plates: Arrange sterile Petri plates on a level surface. Measure accurate volumes of the molten MHA and pour it into the plates. The volumes will depend on the plate size: 60 to 70 ml for 150-mm plates and 25 to 30 ml for 100-mm plates.
- Removing bubbles: To eliminate bubbles that may be present on the surface of the molten agar, quickly and carefully pass the flame from a Bunsen burner over the agar. This helps ensure a smooth surface.
- Solidification: Allow the poured plates to solidify at room temperature. Keep the lids of the plates slightly ajar during the solidification process to allow for proper airflow.
- Checking pH: Once the plates have solidified, check the pH of the prepared MHA. The pH should be 7.3 ± 1 at 25°C. If the pH is below 7.2 or above 7.4, it may affect the performance and accuracy of susceptibility testing for certain drugs.
- Storage: Store the prepared MHA plates in tightly sealed packages at a temperature of 2 to 8°C. This helps maintain the quality and sterility of the agar medium.
Note: It is essential to ensure accurate volume measurement and proper plate depth for disk diffusion testing. An incorrect agar depth can lead to misleading results, such as false-susceptible or false-resistant outcomes.
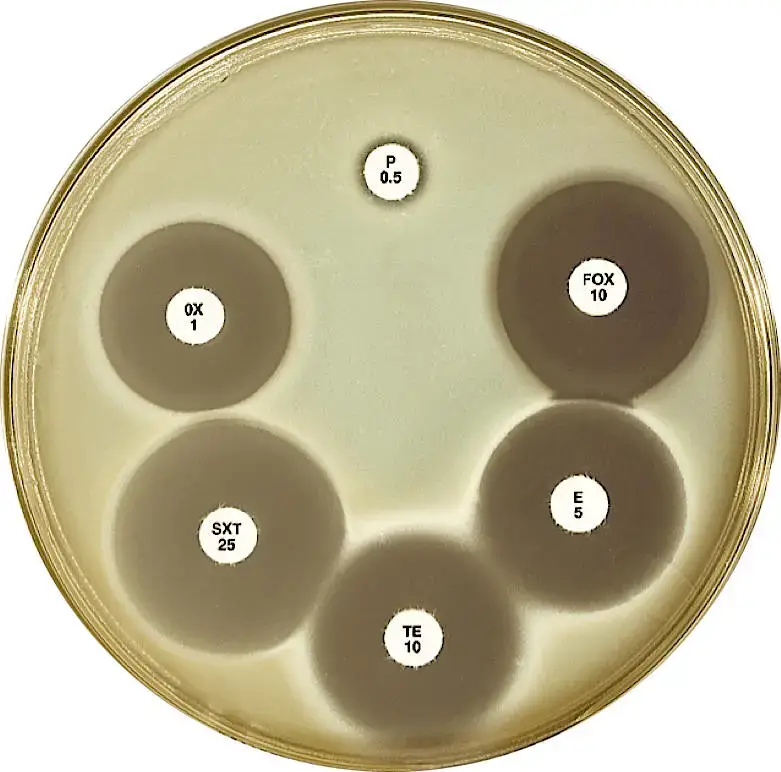
Sheep blood (5%)-supplemented Mueller-Hinton agar
Sheep blood (5%)-supplemented Mueller-Hinton agar is a variation of Mueller Hinton Agar (MHA) that incorporates 5% sterile defibrinated sheep blood into the medium. This addition provides additional nutrients and factors that enhance the growth of certain bacteria and supports the testing of specific organisms.
The preparation of sheep blood (5%)-supplemented Mueller-Hinton agar involves the following steps:
- Prepare 1 liter of MHA: Follow the steps described earlier to prepare 1 liter of Mueller Hinton Agar (MHA).
- Addition of sheep blood: Once the MHA has been prepared and cooled to approximately 48°C, add 50 ml of sterile defibrinated sheep blood to the molten agar. Defibrinated sheep blood is blood that has been treated to remove clotting factors, ensuring a liquid state.
- Mixing and pouring plates: Gently swirl the mixture to ensure proper mixing of the sheep blood with the agar. This step helps distribute the blood evenly throughout the medium. Pour the plates as described in the previous section.
- Storage: After the plates have solidified, store the prepared sheep blood (5%)-supplemented Mueller-Hinton agar plates in tightly sealed packages. Maintain them at a temperature of 2 to 8°C to preserve the sterility and quality of the medium.
The addition of 5% sheep blood to Mueller Hinton Agar provides a nutrient-rich environment that supports the growth of specific bacteria. This variation of MHA is commonly used in antimicrobial susceptibility testing for organisms such as Streptococcus pneumoniae and Haemophilus influenzae. The inclusion of sheep blood can enhance the growth and expression of characteristic properties of these organisms, aiding in their identification and susceptibility testing.
By supplementing MHA with sheep blood, the medium becomes tailored to the requirements of specific bacterial species and allows for more accurate susceptibility testing for these organisms.
Quality Control of Mueller Hinton Agar (MHA)
Quality control of Mueller Hinton Agar (MHA) is crucial to ensure the reliability and accuracy of antimicrobial susceptibility testing. The following measures can be taken to assess and maintain the quality of prepared MHA:
- Sterility checks: Before use, perform sterility checks on the prepared MHA. This involves incubating a portion of the medium under appropriate conditions to confirm that no microbial growth occurs. Sterility ensures that the medium is free from contaminants that could interfere with susceptibility testing.
- Measurement of pH: Measure the pH of the prepared MHA using a suitable pH indicator or meter. The pH should be within the range of 7.3 ± 1 at 25°C. Deviations from the specified pH range can affect the performance of antibiotics and lead to inaccurate susceptibility results.
- Measurements of fill: Measure the fill volume and depth of the poured MHA plates. Accurate volumes are critical for proper antibiotic diffusion and the formation of correct zone sizes. Inadequate fill volumes or uneven depths can lead to misleading results.
- Performance checks: Regularly perform performance checks on the MHA using specific strains of organisms recommended by the Clinical and Laboratory Standards Institute (CLSI). It is advisable to test the MHA at least weekly. The selected strains for performance testing typically include:
- Escherichia coli ATCC 25922
- Staphylococcus aureus ATCC 25923
- Pseudomonas aeruginosa ATCC 27853
- Enterococcus faecalis ATCC 29212
- Streptococcus pneumoniae ATCC 49619 (for MHA with 5% sheep blood)
These strains represent a range of bacteria commonly encountered in clinical settings. The MHA plates with the strains are incubated, and the resulting zone of inhibition around antibiotic disks is measured. The measured zone sizes are then compared to the CLSI-defined ranges for each antibiotic, ensuring that the susceptibility results fall within the expected range.
By regularly performing these quality control measures, laboratories can verify the performance of the prepared MHA and ensure that the media and disks are functioning as expected. It helps maintain the reliability and accuracy of antimicrobial susceptibility testing and provides confidence in the obtained susceptibility results.
Modifications of Muller Hinton agar
Modifications of Mueller Hinton Agar (MHA) are often employed to cater to the specific requirements of certain bacterial species or to enhance the accuracy of antimicrobial susceptibility testing. Here are two common modifications:
- Mueller Hinton agar supplemented with 5% sheep blood: This modification involves adding 5% sterile defibrinated sheep blood to the MHA medium. This modification is recommended for determining the antimicrobial susceptibility of certain bacteria, including Streptococcus pneumoniae, Neisseria meningitidis, and Haemophilus influenzae. The addition of sheep blood provides additional nutrients and factors that support the growth of these specific bacterial species and aid in accurate susceptibility testing.
- Haemophilus test medium (HTM): HTM is a preferred medium for the antimicrobial susceptibility testing of Haemophilus influenzae using the modified Kirby Bauer disk diffusion method. HTM is a specialized medium that consists of thymidine-free MHA supplemented with specific ingredients. These ingredients include 15 μg/ml NAD (nicotinamide adenine dinucleotide), 15 μg/ml bovine hemin, and 5 mg/ml yeast extract. This modified medium is designed to meet the specific growth requirements of H. influenzae and optimize the accuracy of susceptibility testing for this bacterial species.
These modifications address the unique nutritional and growth requirements of certain bacteria, allowing for more accurate and reliable antimicrobial susceptibility testing. By tailoring the medium to the specific needs of these bacteria, the modified agar formulations provide an optimal environment for their growth and ensure accurate assessment of their susceptibility to antimicrobial agents.
What is Mueller Hinton Broth?
Mueller Hinton Broth is a liquid medium used in microbiology, specifically for broth dilution minimum inhibitory concentration (MIC) studies. It has a similar nutrient composition to Mueller Hinton agar but lacks the solidifying agent, agar powder.
The key features of Mueller Hinton Broth are as follows:
- Nutrient formulation: Mueller Hinton Broth has an identical nutrient formulation to Mueller Hinton agar. The composition of the broth provides essential nutrients necessary for bacterial growth and allows for accurate MIC determination.
- Lack of agar: The main difference between Mueller Hinton Broth and Mueller Hinton agar is the absence of agar powder in the broth. Without agar, the medium remains in a liquid state, providing a suitable environment for bacterial growth and allowing for broth dilution methods.
- Supplementing with divalent cations: After sterilization, Mueller Hinton Broth needs to be supplemented with divalent cations. Specifically, it requires the addition of 10-12.5mg/liter of Mg2+ (magnesium) and 20-25mg/liter of Ca2+ (calcium), as recommended by the Clinical and Laboratory Standards Institute (CLSI). These divalent cations help support the growth and optimal performance of bacteria in the broth.
- Optional enhancements: Lysed horse blood or thymidine phosphorylase can be added to Mueller Hinton Broth as optional enhancements. These additives can improve the MIC endpoints for sulphonamides and trimethoprim, leading to more accurate susceptibility testing results for these antimicrobial agents.
The preparation of Mueller Hinton Broth involves the following steps:
- Add 21g of Mueller Hinton broth powder (CM0405B) to 1 liter of distilled water.
- Mix and dissolve the powder completely in the water.
- Pour the prepared broth into final containers, such as conical flasks.
- Sterilize the broth by autoclaving at 121°C for 15 minutes, ensuring the elimination of any contaminants.
Mueller Hinton Broth provides a liquid medium suitable for broth dilution MIC studies, where the antimicrobial agents are serially diluted in the broth to determine the minimum concentration that inhibits bacterial growth. It offers a convenient and reliable method for assessing bacterial susceptibility to antimicrobial agents in a liquid environment.
Differences between Mueller Hinton agar and Mueller Hinton broth
Mueller Hinton agar and Mueller Hinton broth are two variations of the same medium used in microbiology, particularly for antimicrobial susceptibility testing. Although they share the same composition, there are notable differences between the two:
- Agar Composition: Mueller Hinton agar contains 15 grams per liter of agar powder in addition to the other ingredients present in the medium. Agar is a solidifying agent that provides a solid surface for bacterial growth.
- Type of Medium: Mueller Hinton agar is a solid medium, while Mueller Hinton broth is a liquid medium. The addition of agar in MHA gives it a gel-like consistency, allowing the medium to solidify and provide a solid surface for bacteria to grow on. In contrast, MHB remains in liquid form, providing a nutrient-rich broth for bacterial growth.
- Containers Used: Mueller Hinton agar is typically poured into Petri dishes or plates. The medium solidifies in these containers, providing a surface on which bacterial colonies can grow and form visible zones of inhibition during susceptibility testing. On the other hand, Mueller Hinton broth is usually prepared in culture bottles or tubes, as the medium remains in a liquid state.
Both Mueller Hinton agar and Mueller Hinton broth serve specific purposes in microbiological testing. MHA is used for the diffusion-based Kirby-Bauer method, where antimicrobial discs are placed on the solid surface to assess susceptibility. MHB, being in liquid form, is commonly used for broth dilution or other liquid-based methods for determining antimicrobial susceptibility or for the growth of bacterial cultures.
Uses of Mueller Hinton Agar (MHA)
Mueller Hinton Agar (MHA) has several important uses in microbiology and antimicrobial susceptibility testing. Here are the key applications of MHA:
- Antimicrobial susceptibility testing: The primary use of MHA is in antimicrobial susceptibility testing, where it has become the standard medium for the Bauer Kirby method. This method involves placing antimicrobial discs on the surface of the agar to assess the susceptibility of bacteria to various antibiotics. The performance of MHA in this context is specified by organizations such as the NCCLS (National Committee for Clinical Laboratory Standards) or its successor, the Clinical and Laboratory Standards Institute (CLSI).
- Cultivation of Neisseria: MHA was originally formulated by Mueller and Hinton as a protein-free medium for the primary isolation of Neisseria species. Although other media have been developed for the cultivation of pathogenic Neisseria species, MHA is still suitable for their growth. It has been particularly used in determining sulfonamide resistance in gonococci and other organisms.
- Food testing: MHA is specified in the FDA Bacteriological Analytical Manual for food testing. It is commonly used for procedures performed on aerobic and facultative anaerobic bacteria in food samples. MHA provides a suitable medium for the growth and isolation of bacteria present in food samples, aiding in their identification and assessment of antimicrobial susceptibility if necessary.
Overall, Mueller Hinton Agar is primarily used for antimicrobial susceptibility testing, making it a widely recognized and standardized medium for this purpose. Additionally, it serves as a valuable medium for the cultivation of Neisseria species and is specified for various procedures in food testing, helping ensure the safety and quality of food products.
Limitations of Mueller Hinton Agar (MHA)
Mueller Hinton Agar (MHA) has certain limitations that should be considered when using it for microbiological testing. These limitations include:
- Factors affecting results: Several factors can influence the results obtained with MHA, including inoculum size, rate of growth, medium formulation, and pH. It is crucial to adhere strictly to the testing protocol to ensure reliable and accurate results.
- Inoculum density: The density of the bacterial inoculum can impact the zone size observed on MHA. A heavy inoculum may result in smaller zones of inhibition, while too little inoculum may lead to larger zones. It is important to standardize the inoculum density to maintain consistency in susceptibility testing.
- Fastidious organisms: MHA may not support the growth of fastidious organisms, which have specific nutritional requirements. In such cases, supplementation of the medium with blood or other growth factors may be necessary to promote the growth of these organisms.
- Fastidious anaerobes: MHA is not suitable for the growth of fastidious anaerobic organisms, which have specialized growth requirements. Alternative media or methods are required to support the growth and susceptibility testing of these organisms.
- Inapplicability to certain organisms: The disk diffusion method using MHA is not suitable for obligatory anaerobes, slow-growing organisms, and capnophiles. This method was standardized specifically for facultative organisms or rapid-growing aerobes. Different testing methods are necessary for these specific types of bacteria.
- Drug inactivation: Prolonged incubation times required for slow-growing organisms may result in drug inactivation. Some antimicrobial agents may lose their potency or efficacy over extended incubation periods, leading to unreliable susceptibility results.
- Variation in divalent cation concentration: Variations in the concentration of divalent cations, particularly calcium and magnesium, can affect the results of certain susceptibility tests. This can be observed with aminoglycosides, tetracycline, and colistin testing specifically with Pseudomonas aeruginosa isolates.
FAQ
What is Mueller Hinton Agar (MHA)?
Mueller Hinton Agar is a common culture medium used for antimicrobial susceptibility testing (AST) of bacteria. It provides optimal conditions for the growth of clinically significant bacteria.
What is the composition of Mueller Hinton Agar?
MHA contains Beef Extract, Acid Hydrolysate of Casein, Starch, and Agar. These ingredients provide essential nutrients for bacterial growth and support antimicrobial susceptibility testing.
What is the purpose of using MHA in antimicrobial susceptibility testing?
MHA is used as a standardized medium for the diffusion (Kirby-Bauer) or dilution methods of antimicrobial susceptibility testing. It allows the assessment of bacterial susceptibility to various antibiotics.
Can MHA be used for the cultivation of Neisseria species?
Yes, MHA was originally formulated for the primary isolation of Neisseria species. Although other media have been developed, MHA can still support the growth of Neisseria species.
Are there any limitations to using MHA?
Yes, there are limitations to consider. MHA may not support the growth of fastidious organisms, and variations in factors such as inoculum size, medium formulation, and pH can affect results. Additionally, MHA is not suitable for obligatory anaerobes or slow-growing organisms.
What is the difference between MHA and Mueller Hinton Broth?
The main difference is that MHA contains agar powder, making it a solid medium, while Mueller Hinton Broth lacks agar and remains in a liquid state. MHA is used for agar-based susceptibility testing, while the broth is used for broth dilution MIC studies.
How do you prepare MHA?
To prepare MHA, you weigh the appropriate amount of dehydrated MHA powder, add distilled water, heat until dissolved, dispense into containers, autoclave, and allow it to cool before using it for susceptibility testing.
Can MHA be supplemented with blood?
Yes, MHA can be supplemented with 5% sheep blood. This modification is recommended for determining the antimicrobial susceptibility of specific organisms like Streptococcus pneumoniae and Haemophilus influenzae.
Can MHA be used for testing sulfonamides and trimethoprim?
MHA can be used for testing the susceptibility of bacterial isolates to sulfonamides and trimethoprim. It has reduced levels of thymidine, thymine, and para-aminobenzoic acid (PABA) to minimize inactivation of these antimicrobials.
What quality control measures are important for MHA?
Sterility checks, pH measurement, fill volume measurement, and performance checks with specific strains should be performed to ensure the quality and reliability of MHA for antimicrobial susceptibility testing.
References
- https://www.himedialabs.com/media/TD/M173.pdf
- https://microbeonline.com/mueller-hinton-agar/#google_vignette
- https://microbiologie-clinique.com/mueller-hinton-agar.html
- https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/product/documents/368/488/70191dat.pdf
- https://biolabtests.com/the-origin-of-mueller-hinton-agar/
- https://hardydiagnostics.com/g45
- https://labmal.com/2019/11/20/mueller-hinton-agar-and-mueller-hinton-broth/