What is Microtome?
A microtome is a laboratory device that is used for cutting very thin sections of different materials for microscopic examination. It is the instrument by which uniform slices are produced, and it is the process that helps the light or the electron to pass through the specimen so that the internal structures become visible under microscope.
The specimen is usually embedded in a supporting medium like paraffin or resin, which provides stability during cutting. In this method steel, glass or diamond blades is used depending on the hardness of the material. It is possible to obtain sections ranging from 1 μm to even 100 nm in advanced microtomes, and these thin sections is important for studying tissues, cells and other structural details. It is the use of the microtome that makes histology possible and it also helps in diagnosis of diseases because biopsy sections are prepared by this technique.
Microtomy is also applied in research related to developmental biology, pathology, botany and materials science, as uniform and comparable slices are obtained in this way. Before the introduction of mechanical microtomes the sections were cut free-hand with razor blades, which often produced irregular slices.
The development of microtome started in the 18th and 19th centuries when several devices were made to improve section quality. In 1770 George Adams Jr. prepared a device for cutting thin slices of wood and plant tissues, and later Alexander Cummings improved it. In 1835 Andrew Prichard made a table-based model that reduced vibration during cutting. Wilhelm His Sr. is often considered as the inventor of the microtome in 1865 when he described Beschreibung eines Mikrotoms.
There were also early sliding microtome designs by Jan Evangelista Purkyně and his assistants. During the 19th and 20th centuries many modifications appeared like rocking microtomes, rotary microtomes and cryostats. In the 1950s Pearse and Slee developed cryostat microtomes for frozen sections. Today ultramicrotomes, advanced rotary microtomes and laser microtomes is used to obtain ultra-thin slices especially for electron microscopy.
Principle of Microtome
The principle of microtome is based on mechanical cutting of the specimen into very thin and uniform sections that can be used for microscopic study. It is the method where the specimen block is slowly advanced towards a very sharp knife while the knife is kept fixed in many designs. The controlled advancement of the block produces sections at regular intervals, and the feed mechanism determines the thickness which is usually between 1–10 µm in routine work. The specimen is commonly embedded in paraffin (wax) or resin to make it hardened before cutting so that tearing and distortion is minimized during sectioning.
In this method the cutting action is performed by moving the specimen by a screw or rotary feed, or in some types the knife is moved instead, but the control of movement is always precise. The angle between the specimen and knife edge is adjusted because correct angle, steady pressure and proper speed are needed to obtain clean sections. In most preparations the sections form ribbons which is floated on a warm water bath and then mounted on slides for staining. The microtome knife may be steel, glass or diamond depending on the hardness of the sample and the resolution needed, and the sharpness is maintained carefully for good results.
In ultramicrotomes extremely thin slices for electron microscopy is produced by using glass or diamond knives and by very fine mechanical advancement of the block. The mechanism works with a calibrated feed, usually a micrometer screw, so that the thickness of each section is reproducible and calibration is checked often. Soft tissues from explants or callus, or other plant parts like plumule or radicle, are first fixed and embedded and then sectioned to preserve internal structures such as cells, nuclei and fibers, although some shrinkage may occur.
The main concept is that uniform mechanical advancement of the specimen against a sharp cutting edge produce slices of equal thickness. For EM samples the ultrathin sections are collected on grids and contrasted, and the process is delicate and depends on the operator’s skill. Consistent technique is required because it ensures reproducible results and prevents damage to the specimen or the knife.
Parts of a microtome
- Base – It gives stability and strong support to the whole instrument. It is generally made of heavy metal so that vibration is reduced during cutting.
- Knife / Blade – It is used for cutting the specimen into thin slices. Steel, glass, or diamond blades are selected depending on the hardness of the sample.
- Knife Holder – This part holds the knife firmly in a fixed position. The knife angle can be adjusted here for proper sectioning.
- Specimen Holder (Object Clamp) – The embedded tissue block is fixed on this part. It can be moved slowly toward the knife by rotating the handle or by an automatic system.
- Feed Mechanism – It moves the specimen forward by a fixed distance after each cut so that continuous and uniform slices are obtained.
- Thickness Gauge / Scale – It is used to set the thickness of sections in micrometers (µm). Even slight changes in the gauge affect the uniformity of slices.
- Operating Handle / Motor Drive – In manual microtomes the handle is rotated for cutting. Automatic and semi-automatic types use a motorized drive to move the specimen or knife.
- Stage Clamp / Block Holder – An additional clamp is sometimes provided for extra firm holding of the specimen block, especially in rotary or sledge microtomes.
- Waste Tray – Thin tissue sections and debris fall into this tray placed below the knife, helping to keep the instrument clean.
- Adjustment Screws – These screws are used for alignment and tightening of the knife, knife holder and specimen holder, ensuring smooth and accurate cutting.
Types of Microtome
Microtomes is generally classified into–
- Manual microtomes
- Semi-automatic microtomes
- Automatic microtomes
1. Manual Microtome
This type is operated by hand, and all stages of cutting is done manually. It is simple to use but careful handling is needed since thickness adjustment and cutting both depend on operator control.
There are present different types of Manual Microtome such as;
- Rocking Microtome– It is the oldest design and works by a rocking action due to cross arm presence. The tissue block moves in an arc towards a fixed knife (Heiffor knife). Thin sections come out in curved shape because the knife is slightly biconcave. It is cheap and light-weight and reliable but vibration may occur due to light structure.
- Rotary Microtome– It is most widely used in laboratories. The specimen block moves up and down by rotation of handwheel while knife remains horizontal. It gives thin ribbons (2–3 µm). It may be semi-automatic or fully automatic when block movement is motorized. It is expensive and not suitable for large tissue blocks, and knife position facing upward may cause accidental cut.
- Hand Microtome– It is used for rigid botanical materials. Thin plant sections can be made but animal tissues is difficult to cut in this method.
- Vibrating Microtome– It is used for unfixed or unprocessed tissues. A vibrating knife cuts the specimen immersed in fluid so tearing is reduced. Voltage control adjusts speed. It is used mostly in neurobiology and plant studies.
- Sledge Microtome– It is heavy and stable and used for large tissue blocks. Knife slides backward–forward horizontally against fixed block. Knife is wedge-shaped and long (about 24 cm). Vibration is minimized but operation is slow compared to rotary or rocking type.
- Sliding Microtome– In this type knife moves horizontally against stationary specimen placed on an inclined plane. It is suitable for paraffin embedded blocks and celloidin sections.
- Freezing Microtome– Used for frozen tissues. It is connected with CO₂ for cooling. Knife moves while tissue remains static. Each cut advances slightly. It is easy to use but thin sections are not always consistent.
- Cryostat Microtome– Sectioning occurs inside deep freeze chamber (–10°C to –40°C). Liquid nitrogen is used. It is used for rapid diagnosis, enzyme histochemistry and fluorescent antibody staining.
- Saw Microtome– A circular saw blade is used to cut very hard tissues like bone or teeth. It produces thicker slices of few millimeters since ordinary knives cannot cut these materials.
- Ultra-Microtome– It is used for ultrathin sections (40–100 nm) required for Transmission Electron Microscope (TEM). Knives are made of diamond, sapphire or glass. It is operated by stepping motor and microprocessor for even cutting.
2. Semi-Automatic Microtome
In this type some steps like block movement or thickness setting is motor-driven, but trimming and knife placement is manual. It reduces operator fatigue and improves uniform section cutting.
3. Automatic Microtome
It is fully automated. User inputs size and direction in interface and instrument cuts thin slices with high precision. It is used in research and diagnostic histopathology.
There are two types of Automatic Microtome;
- Computerized Microtome– It has thermostatic switch, cryo-scalpel, cryoplate and semiconductor freezing system. It can perform paraffin and frozen sectioning (1–25 µm). Cryo-plate and scalpel are maintained between 0°C and –40°C.
- Laser Microtome– Cutting is done by infrared laser beam in non-contact manner. Short pulses prevent heat damage. It slices tissues precisely (5–100 µm) and is suitable for delicate biological materials.
Types of Microtome Knife
Microtome knife is the cutting blade used for producing thin tissue sections. It is the process in which different knife materials and knife shapes are selected according to hardness of tissue, type of embedding, and thickness required.
Based on the Material Used for Construction
1. Steel Knife
It is made from high-quality carbon or tool-grade steel. The knife is hardened by heating so edge sharpness is improved. The steel used is rust-resistant. It is commonly applied for general histological sectioning.
2. Tungsten Knife
It is made from tungsten carbide and is non-magnetic. It is about 100 times harder than steel. The knife is brittle but highly wear-resistant. It may cut up to 30,000 serial sections of undecalcified bone embedded in methacrylate before re-sharpening.
3. Diamond Knife
Blade is prepared from gem-quality diamond. It is very expensive but extremely durable. It is used mainly for ultrathin sectioning in electron microscopy. It gives slices of few nanometers without distortion.
4. Sapphire Knife
It is produced from artificial sapphire (alumina monocrystal) under controlled heating. It is harder than glass or tungsten. Knife life is long but it can cut only small blocks because edge limit is around 11 mm, so not suitable for large tissues.
5. Glass Knife
It is used in ultramicrotomy when thin sections (40–120 nm) is required. It is known as Ralph knife. Cutting edge is formed across glass thickness. It is hard but brittle. The knife should be treated before use because long storage reduce sharpness.
6. Disposable Knife
These are stainless-steel blades with pre-sharpened edges. Used mostly in rotary microtomes and cryostat microtomes. Teflon-coated type is preferred for cryo sectioning. It replaces old reusable steel knives and reduces maintenance work.
7. Non-Corrosive Knife
It is made from heat-treated stainless steel containing about 12–15% chromium. It is used mainly in cryo-microtome. It is free from impurities and resistant to oxidation produced by moisture during freezing.
Based on the Profile / Shape of Knife
1. Plano-Concave Knife
One surface is flat while the other surface is concave. It is used for soft materials like celloidin-embedded tissues or foamy compounds. Knife is placed obliquely to object. It is not suitable for hard tissues due to thin fragile edge.
2. Biconcave Knife
Both surfaces are concave. The back portion is thicker than plano-concave type. It is used for harder materials where stability is required. Degree of concavity varies among knives.
3. Wedge Knife
It has a wedge-shaped extra-thick edge. It is more rigid than plano or biconcave knives. It is used for rigid tissues and for frozen or paraffin-embedded blocks. It cannot be ground to very fine sharpness but stability during cutting is excellent. Cutting plane is placed transverse to specimen.
4. Tool Edge Knife
It is designed for very hard materials. It has least sharpness but maximum stability. Edge is formed by grinding bevels on both sides of knife. The bevel encloses sharper angle than rest of blade, giving better control during sectioning.
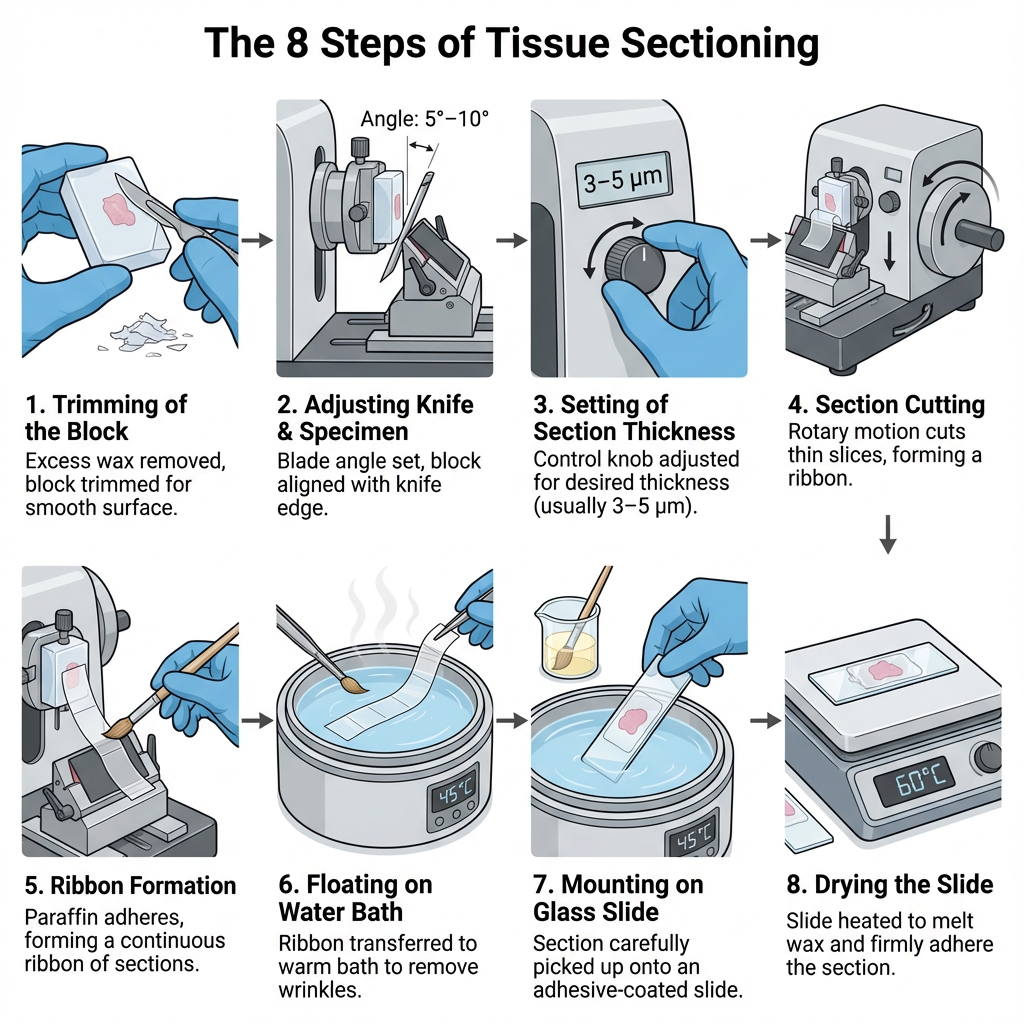
Steps of Tissue Sectioning
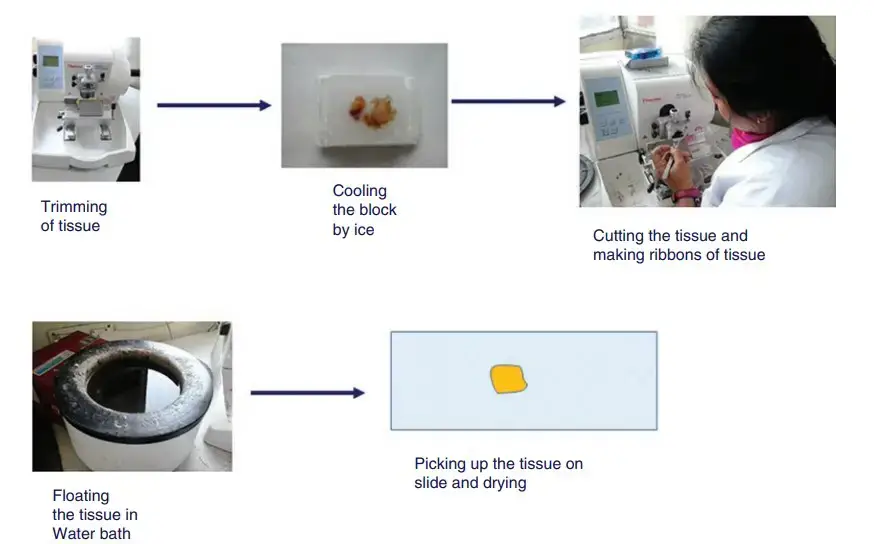
1. Trimming of the Block
The paraffin block containing the embedded tissue is first trimmed so that only the tissue surface remain exposed. Excess wax around the block edges is removed to give proper shape. A flat and even surface is needed for smooth sectioning.
2. Adjusting Knife and Specimen
The knife or blade is fixed firmly in the knife holder. Its angle (about 5°–10°) is adjusted for proper cutting. The tissue block is mounted in specimen holder with the surface facing knife edge. Alignment is checked by making small trial cuts.
3. Setting of Section Thickness
Thickness control knob is adjusted according to the required thickness. For paraffin sections it is usually 3–5 µm, while frozen sections is around 10–20 µm. If thickness is uneven it may produce tearing or compression during cutting.
4. Section Cutting
The block is moved toward the knife by rotating the handwheel in a rotary microtome. In each stroke a thin slice is cut and remains attached to previous slices forming a continuous ribbon. Cutting should be steady to maintain uniform thickness.
5. Ribbon Formation
The freshly cut sections adhere to each other because paraffin is slightly adhesive at room temperature. This forms a ribbon. The ribbon is guided gently using forceps or a fine brush to avoid folding or wrinkling.
6. Floating on Warm Water Bath
The ribbon is transferred to warm water bath kept at about 40–45°C, which is slightly below melting point of paraffin. It helps to flatten wrinkles and remove folds. The section spreads evenly on water surface.
7. Mounting on Glass Slide
A clean glass slide coated with adhesive material (egg albumin or Mayer’s albumin) is used. The selected flat section from the water surface is picked up carefully and placed at the center of slide.
8. Drying the Slide
Mounted slides are kept on hot plate or oven at about 60°C. The wax melts slightly and section adheres firmly to slide. After complete drying, the slides are ready for staining.
Uses of Microtome
Some of the common uses of microtomes include:
- It is used for preparing thin sections of paraffin-embedded tissues for routine histology and pathology, including biopsy and surgical specimens for disease diagnosis.
- It is used for producing frozen sections with freezing or cryo-microtomes, which is important for rapid intraoperative diagnosis, enzyme histochemistry and some immunofluorescence procedures.
- It is used for sectioning tissues in research areas like developmental biology, neuroscience, ophthalmology and botany where brain slices, eye tissues and plant organs are required.
- It is used for cutting ultrathin sections with an ultramicrotome for transmission electron microscopy and different high-resolution imaging techniques.
- It is used for sectioning hard specimens such as bone, teeth and wood by sledge, sliding or saw microtomes for biomedical, environmental and forensic studies.
- It is used for cutting fresh or softly fixed tissues (e.g. brain, nerve, spinal cord) with vibrating microtomes to preserve cell morphology for electrophysiology and imaging.
- It is used for obtaining reproducible thickness and smooth surfaces which improves staining quality, optical clarity and morphometric analysis.
- It is used for preparing large numbers of comparable sections from the same specimen or different samples in a high-throughput manner.
Advantages of Microtome
- It is used for producing very thin and reproducible sections (2–50 μm or even thinner in ultramicrotomes) which helps in getting better resolution and clear details under microscopes.
- It gives uniform thickness in many serial sections which is important for correct diagnosis, morphometry and different 3D reconstruction studies.
- It can be adapted with different types of microtomes such as rotary, sliding, cryo-, ultra-, vibrating and saw types to cut soft, hard, fragile, fatty or resin-embedded tissues and also some non-biological materials.
- It is designed in such a way that mechanical damage to delicate tissues is reduced, and vibrating or laser microtomes preserve tissue architecture and cell viability for functional studies.
- It is useful for processing large number of samples quickly with the same quality, and semi-automatic or automatic microtomes control section thickness and cutting speed.
- It reduces operator fatigue because hand-wheel movement and specimen advance is mechanized which also reduces repetitive-strain problems.
- It uses modern disposable blades and special blade holders which decreases sharpening time, lowers maintenance and reduces exposure to open cutting edges.
- It has strong heavy frames and stable mechanisms which minimize vibration and improve section quality and safety in routine histology work.
Limitations of Microtome
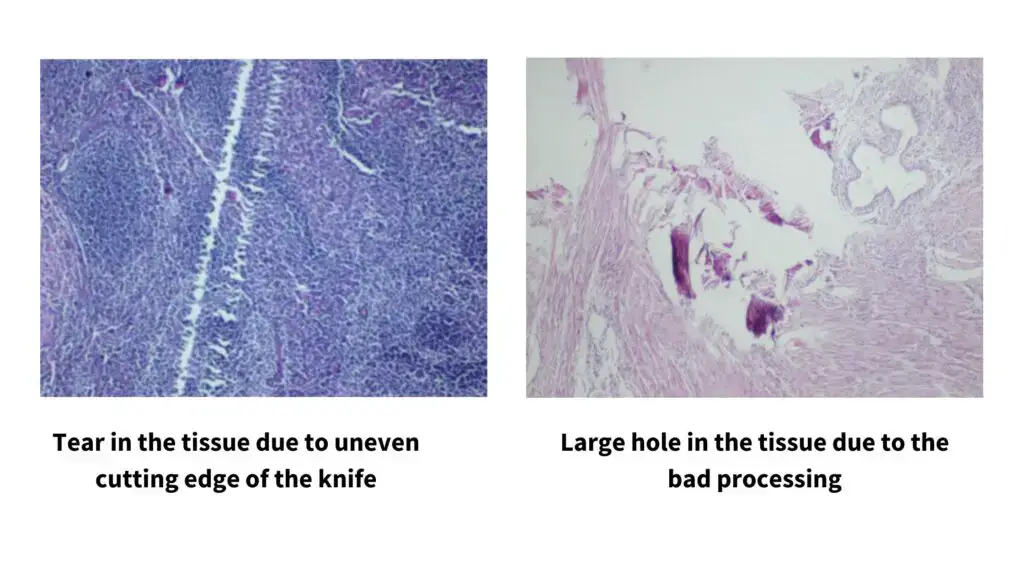
- It requires a skilled and well-trained operator because poor handling easily produce chatter, compression, folds or tearing of the sections.
- It has a limited range of section thickness, and routine work is usually in few micrometres, so very thick slabs cannot be produced with the same instrument.
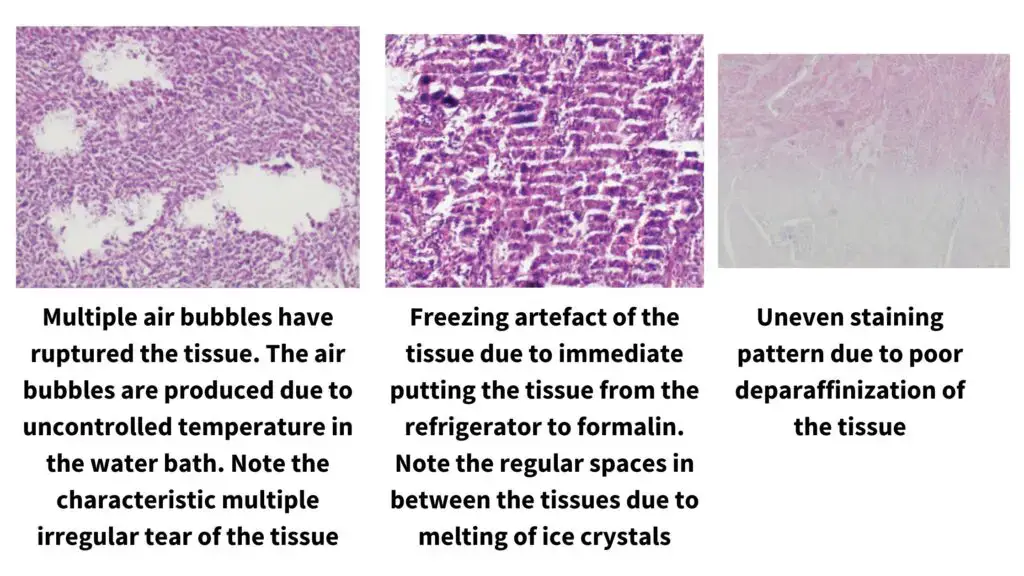
- It is difficult to cut hard, brittle, very fatty or poorly processed tissues, and these often show tearing, compression or knife marks even when suitable blades are used.
- It depends on fixation, embedding and freezing steps which may introduce shrinkage, cracking or distortion, and these artifacts cannot be corrected by the microtome.
- It is costly, and high-quality rotary, cryo- or ultramicrotomes have complex mechanisms that need time-consuming maintenance and repair.
- It is slow when large sample numbers are processed because cutting blocks, trimming, obtaining ribbons and handling sections take time if automation is limited.
- It has safety issues because very sharp exposed blades increase the risk of cuts, especially in manual or sliding microtomes without updated safety features.
- It may require a stable bench or controlled environment such as cryo-temperature, and some designs are bulky, noisy and not easily portable.
FAQ
What is a microtome?
It is an instrument used to cut very thin sections of biological tissues, and the sections is used for microscopic examination.
What are microtomes used for?
These are used for preparing thin sections of paraffin-embedded tissues, frozen tissues and resin blocks which is required for histology, pathology and different research studies.
What are the different types of microtomes?
Some of the main types are– rotary microtome, sliding microtome, sledge microtome, cryo-microtome, vibrating microtome, ultramicrotome and saw microtome.
What is the role of microtomes in histology?
It is used for obtaining uniform sections of tissues so that staining, diagnosis and cellular study can be done properly.
What is the ideal thickness for tissue microtomy sections?
It is usually 3–5 μm for routine paraffin sections, while special tissues may require slightly thinner or thicker sections depending on the study.
How do you prepare a paraffin block for sectioning?
The block is first faced to expose the tissue, and then it is cooled properly (often on a cold plate or ice surface) so that firm and smooth sectioning is possible.
What equipment is needed for paraffin sectioning?
A rotary microtome, paraffin-embedded blocks, disposable blades, forceps, water bath, slides and a warming plate are mainly required.
What are common problems during paraffin sectioning?
Some common issues are tearing, folds, compression, chatter, uneven thickness and poor ribbon formation.
What causes thick and thin sections during microtomy?
This occurs when section thickness control is not stable, or when the block and blade is not aligned properly, or the block is too warm or too hard.
Why do sections curl or fail to form ribbons?
It happens when the block temperature is inappropriate, or the blade edge is dull, or the clearance angle is not adjusted correctly.
What causes chatter in sections?
Chatter is produced when the tissue is too hard, the blade vibrates, or the cutting speed is uneven, and it appears as small parallel lines across the section.
How do I prevent wrinkles in tissue sections?
Wrinkles reduce when the block is cooled well, the blade is sharp, and the section is relaxed on warm water so that it spreads evenly.
How do you handle hard tissues during paraffin sectioning?
Hard tissues need proper decalcification or surface softening, and sometimes a slower cutting speed is used to avoid tearing and knife marks.
Can tissue microtomy be done on frozen samples?
Yes, it is done with a cryo-microtome where frozen tissues are sectioned at low temperature.
How do I clean and maintain my microtome?
It is cleaned by removing wax debris, wiping surfaces after use, oiling the moving parts if recommended, and keeping the instrument covered to protect it from dust.
- Mohammed, Faraz & Thapasum Fairozekhan, Arishiya & Mohamed, Shamaz. (2012). Microtomes and Microtome Knives – A Review and Proposed Classification. Annal Dent Univ Malaya. 19. 43-50.
- Dey, P. (2018). Tissue Microtomy: Principle and Procedure. In: Basic and Advanced Laboratory Techniques in Histopathology and Cytology. Springer, Singapore. https://doi.org/10.1007/978-981-10-8252-8_5
- McMillan, D. B., & Harris, R. J. (2018). Introduction. An Atlas of Comparative Vertebrate Histology, ix–xxix. doi:10.1016/b978-0-12-410424-2.00018-4
- https://nios.ac.in/media/documents/dmlt/HC/Lesson-09.pdf
- https://link.springer.com/chapter/10.1007/978-981-10-8252-8_5
- https://www.leicabiosystems.com/en-in/histology-equipment/microtomes/