What is Liquid Chromatography?
- Liquid Chromatography (LC) refer as one analytical method by which mixture of compounds are separated by using liquid mobile phase and a stationary phase that stay fixed.
- In this technique, the sample mixture are passed by a column which contain stationary material, and the separation occur due to different interaction of each molecule with it.
- The process can define as a method where the solutes distribute between two phase – one is mobile phase (liquid) and the other one stationary phase (solid or liquid supported on solid).
- The compounds that having stronger interaction with stationary phase move slower, while others elute faster – that cause separation of components.
- The technique are widely used in biochemistry, environmental analysis, pharmaceutical, and food industry etc., for qualitative and quantitative purpose.
- In LC, the solvent (mobile phase) move continuously by column under certain pressure, sometimes by gravity or by pump in case of HPLC (High Performance Liquid Chromatography).
- Based on polarity and adsorption characteristic, compounds are divided during the process which depend on type of stationary phase used.
- The common stationary phase are silica gel, alumina, or polymer-based material while the mobile phase consist of single solvent or mixture of solvents like methanol, water, acetonitrile etc.
- Separation efficiency depend by factors like flow rate, temperature, solvent polarity, and particle size of column packing material.
- In the early stage, the method was developed as simple column chromatography but later modified into advanced system like HPLC and UHPLC which give more accuracy, reproducibility, and high sensitivity.
- During analysis, a detector used at the end of column to monitor the eluted compounds by measuring absorbance or refractive index or fluorescence (depending on detection system).
- Retention time (tR) of each compound used for identification purpose and the peak area or height used for quantitative estimation.
- It’s important that the system remain stable, because any air bubble or improper packing of column prevail the resolution badly.
- Such as, for proteins or peptides, Reverse-phase LC are often used, whereas for ionic or polar compounds – Ion exchange LC or Normal-phase LC might be used.
- The whole principle based on differential partitioning between phases, that means solute distribution ratio control the separation process basically.
- Sometimes temperature variation (25°C to 60 °C) or gradient elution applied to improve the resolution or shorten the analysis time.
- In LC, mobile phase pressure can vary (low in normal LC, high in HPLC) which influence how fast the sample pass through column.
- The result obtained in form of chromatogram, which display peaks representing different analytes – their number, height and area give information about sample composition.
- The method not only give qualitative but also quantitative detail, even trace level compounds can detect.
- However, care must be taken by preparing sample properly, because impurities, particles, or improper solvent mixing cause error or damage of column.
- The process have become essential part of chemical and biological laboratories, they are used for separation of amino acids, nucleotides, drugs, pesticides etc.
- In modern laboratories, LC often coupled with Mass Spectrometry (LC–MS) for precise identification of unknown molecules.
- It’s an efficient, accurate and versatile technique, though the cost of instrument and maintenance are high.
- By understanding interaction of solute–stationary–mobile phase, better optimization of separation can be achieved and more pure fraction collected.
- Therefore, Liquid Chromatography remain one of the most used analytical method for separation and purification of chemical mixtures in both research and industrial fields.
Types of Liquid Chromatography
1. Normal Phase Liquid Chromatography–
This type was characterized by polar stationary phase and non-polar mobile phase, separation was achieved mainly by adsorption and polarity differences.
Silica gel and alumina are often used as stationary matrix, while solvents like hexane, chloroform are used as mobile phase (sometimes mixtures).
Polar analytes were retained longer, nonpolar compounds elute earlier, and selectivity was controlled by solvent strength, gradient sometimes applied.
2. Reverse Phase Liquid Chromatography –
This mode was described as the most widely used LC, where nonpolar stationary phase (C18, C8) are used and polar mobile phase (water + organic solvent) is used.
Columns packed with C18 (Octadecylsilane) are commonly used, and water with acetonitrile or methanol is mixed to make mobile phase, gradient elution is often employed.
Hydrophobic interactions are exploited, retention increase with analyte nonpolarity, and peptides/proteins, small drugs are frequently analyzed by this mode.
3. Ion Exchange Liquid Chromatography –
It was termed as separation based on charge, with cation-exchange and anion-exchange resins carrying opposite charges.
pH and ionic strength of buffer are critical, salt gradient or pH change are used to elute bound ions, careful buffer selection is required.
Proteins, peptides, nucleotides and amino acids are often fractionated by ion exchange, the method is powerful for charged biomolecules but sample must be desalted sometimes.
4. Size Exclusion / Gel Filtration Chromatography–
Separation was performed by size exclusion, porous beads were used and molecules are sieved by size.
Large molecules are excluded from pores and elute earlier, small molecules enter pores and elute later, no strong chemical interaction is expected so gentle conditions are maintained.
It was widely used for desalting, buffer exchange and determination of molecular weight, columns packed with porous polymers or agarose gels are typical.
5. Affinity Liquid Chromatography–
Highly selective binding was exploited by immobilized ligand on matrix (antibody-antigen, enzyme-substrate), target was captured specifically.
After washing, elution was done by changing pH, ionic strength or by adding competing ligand, this give high purity but ligand immobilization cost and stability must be considered.
6. Hydrophobic Interaction Chromatography (HIC)–
This technique was applied for proteins under moderate salt conditions where hydrophobic patches interact with moderately hydrophobic stationary phase.
Binding is increased by high salt, and elution is performed by decreasing salt or by adding organic modifier, it is complementary to reversed-phase, and gentle on proteins (not denaturing usually).
7. Chiral Liquid Chromatography–
Enantiomers were separated by chiral stationary phases or by use of chiral mobile-phase additives, stereoisomer separation is enabled.
Specialized chiral selectors (polysaccharide derivatives, cyclodextrins) are used, it’s critical for pharmaceuticals where enantiomers have different activity, and method development can be lengthy.
8. Ion Pair Liquid Chromatography–
Ion pairing reagents were added to mobile phase to help retain ionic analytes on reversed-phase columns, neutral pairs form and retention is changed.
The choice of ion pair reagent (short chain alkyl sulfonates, quaternary ammonium) and its concentration strongly affect selectivity and sometimes MS detection is affected.
9. High Performance Liquid Chromatography (HPLC) –
All above modes are often operated under HPLC conditions, where high pressure pumps, small particle columns and sensitive detectors are used.
In HPLC, flow is controlled precisely, resolution and speed are improved, and detectors like UV, RID, FLD are connected, LC–MS coupling is also common.
10. Ultra-High Performance Liquid Chromatography (UHPLC) –
It was introduced to use sub-2 µm particles and higher pressures to achieve faster, higher resolution separations, instrumentation and column stability were needed.
11. Preparative Liquid Chromatography –
This scale was used for purification rather than analytical trace detection, large columns and higher load are applied to collect fractions for further use.
12. Flash Chromatography (medium pressure LC) –
It was used for rapid, low-cost separations in synthetic labs, cartridges and pumps of moderate pressure are used and fraction collection is simple.
13. Capillary LC / Nano-LC –
Very low flow rates and small columns were used to increase sensitivity for MS, peptides and proteomics work were often performed by them.
14. Multidimensional LC (2D-LC) –
Orthogonal separation mechanisms were coupled (for ex. strong cation exchange followed by RP), complex mixtures were resolved better, peak capacity was increased.
Choice of LC type depend by analyte properties (polarity, charge, size, chirality) and purpose (analytical vs preparative), method optimization is required and sometimes multiple modes are combined.
Some methods are named for mechanism (adsorption, partition, ion-exchange, size exclusion, affinity), practical setups are often hybrid and they are optimized by solvent, pH, temp (25 °C or 40°C) and flow.
Each LC type have advantages and limitations, they are selected based on sample nature and required purity, and often they are combined to prevail impurities (malapropism intended) or to gain orthogonal separation.
Principle of Liquid Chromatography
- The principle of Liquid Chromatography refer as separation of mixture components by differential distribution between mobile phase (liquid) and stationary phase.
- A dynamic equilibrium is established repeatedly between two phase as mobile phase flow through column, and analytes are partitioned differentially.
- Retention of compound are governed by its affinity toward stationary phase versus its solubility in mobile phase, so different migration rate occur.
- The process was often described by mechanisms like adsorption, partition, ion-exchange and size exclusion, each mechanism giving different retention behavior.
- In adsorption-type, molecules are adsorbed on solid surface and desorbed by solvent, therefore retention time vary with surface interaction and solvent strength.
- In partition chromatography, solute distribution is governed by partition coefficient between two liquid phases, one immobilized on support and other move as mobile phase.
- For ion-exchange, electrostatic attraction was exploited where charged solute are bound to oppositely charged groups on resin, and elution is achieved by salt or pH change.
- In size-exclusion (gel filtration), separation are produced by molecular size where large molecules are excluded from pores and elute earlier, while small ones enter pores and elute later.
- The retention time (tR) of each analyte is used for identification and peak area (or height) is used for quantification, detector signal are recorded as chromatogram.
- Mobile phase composition, flow rate, temperature and column packing characteristics are adjusted to optimize equilibrium and resolution, and gradient elution is often applied to change partitioning dynamically.
- Theoretical plates and plate height concept are used to express column efficiency where smaller particle size give more plates and better separation, but pressure increase as a trade-off.
- Band broadening occur by longitudinal diffusion, mass transfer resistance and eddy diffusion, and these contribute to peak shape deterioration if not controlled.
- The chromatogram produced are a time vs signal plot where peak position correspond to tR and area proportional to amount, sometimes peaks overlap and resolution parameter is used to judge separation.
- Equilibration between phases is reversible and continuous, and analytes are carried by flow while momentary adsorption or partition happen along column length.
- If interaction with stationary phase is too strong, very long tR and broad peaks are observed, whereas very weak interaction cause co-elution and poor separation.
- Temperature effect are important, sometimes small increase (25°C or 40 °C) reduce viscosity and change partition coefficients, and selectivity may change with temp.
- Mobile phase viscosity and pressure are interrelated, high pressure (as in HPLC/UHPLC) are used to push solvent through small particle columns, giving faster separations.
- Detectors (UV, FLD, RI, MS) are placed after column to transduce eluted analytes into measurable signals, and coupling to MS give structural information in addition to retention.
- Gradient elution (where solvent strength is changed over time) are applied to elute strongly retained analytes and to compress run time, but it require re-equilibration of column after run.
- Sample-solvent compatibility and sample cleanup are important because particulates or incompatible solvent can disturb equilibrium or damage column, and clogging is often a problem.
- The fundamental idea therefore remain differential partitioning / differential migration of solute between two phase, minute differences in affinity lead to macroscopic separation.
- Column packing uniformity and particle size distribution are critical, because poor packing give channeling and uneven flow, and they cause loss of resolution.
- The original concept was demonstrated by Mikhail Tswett using plant pigments, and chromatography principles were later generalized for many classes of compounds.
- Practical operation involve balancing resolution, speed and sample load where analytical LC aim for high resolution and prep-LC aim for high capacity, they are chosen by purpose.
- Optimization is done by changing solvent polarity, pH, ionic strength, gradient profile, flow rate and column type so that selectivity and efficiency are improved, sometimes trial-and-error is used.
- Thus, LC principle is simple but versatile — different components move at different speed because they partition unequally, and small tweaks often prevail (malapropism intended) large improvement in separation.
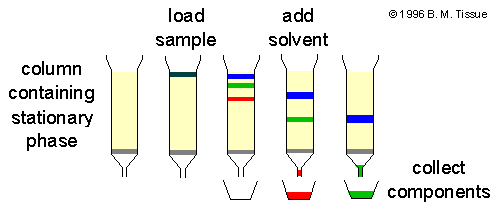
Liquid Chromatography Instrumentation

1. Mobile Phase – The liquid is used to move through the column, usually consist of solvent mixture (polar / non polar), buffers or modifiers are added for selectivity, sometimes gases are present, and composition is adjusted for resolution.
2. Pump – Pressure is generated by pump for forcing mobile phase by column, flow rate is controlled precisely but pulses may occur with reciprocating pumps, syringe or isocratic/gradient modes are used and they are chosen by method.
3. Injector – Sample is introduced into flow stream by manual or autosampler, injection volume (10 µL -100 µL or more) is selected depending on column ID and concentration, errors in injection will produce bad peaks.
4. Column– The heart of LC is the column, packed with particles (silica, polymer, bonded phases like C18) and length/ID decide efficiency and backpressure, stationary phase is termed as the medium which interacts with analytes.
5. Detector – The eluent is sensed by detector (UV-Vis, Refractive Index (RI), Fluorescence, Conductivity, MS) and signal is converted to chromatogram; sensitivity and selectivity are depended on detector type.
6. Data System / Recorder – The detector response is sent to software or chart recorder, peaks are integrated, retention time is measured, the processing is done by computer and reports are produced (PDFs, data files).
7. Column Oven – Column temperature is controlled by oven (25°C–60 °C or other), temperature influence viscosity and retention, good reproducibility is ensured by temperature control.
8. Waste Container – Effluent after detection is collected in waste bottle, fractions are collected by fraction collector when needed, waste disposal must be followed as lab rules say.
9. Degasser– Dissolved gases are removed by degasser (vacuum or helium sparging) to avoid bubble formation and baseline noise, sometimes operators forget degassing and baseline drift will prevail.
10. Guard Column – A short guard is placed before analytical column to trap particulates and contaminants, it is replaced often when backpressure rises, this protect the main column and extend lifetime.
Liquid Chromatography Protocol
- Sample preparation is done by filtering / diluting the mixture so that particles are removed and concentrations are within the instrument’s range.
- Mobile phase is prepared (solvent mixture, buffer, modifiers) and degassed or filtered so that bubbles or particulates don’t disturb flow.
- Instrument is primed: the pump, tubing, injector and column are checked, the system is equilibrated with mobile phase until baseline is stable, pressure and flow rate are set.
- Sample is injected into the LC system through injector (manual or autosampler) into flowing mobile phase, making sure that injection loop / volume is correct and sample is compatible.
- Separation occurs as the mobile phase carries analytes through the column (stationary phase) where interactions differ and thus retention times differ.
- Eluent exits the column and passes through detector where signal (absorbance / fluorescence / RI etc) is recorded as chromatogram (response vs time) for each component.
- Data processing is done: peaks are integrated, retention times noted, calibration (if quantitative) is applied, results are exported / reported.
- System shutdown / cleanup: flush column with appropriate solvents, turn off pump / waste disposal, clean injector and sample loop, record system use and log maintenance.
Rf Value
The Rf value, which stands for Retention factor, is a characteristic identification value used in chromatography. It is calculated as the ratio of the distance traveled by the analyte to the distance traveled by the solvent front. The Rf value is specific to each compound and can be used to analyze and identify different substances.
The formula for calculating the Rf value is:
Rf = Distance traveled by analyte / Distance traveled by solvent
In practice, the Rf value is determined by measuring the distance a particular compound travels on a chromatographic plate or column compared to the distance traveled by the solvent front. The distance traveled by the analyte is usually measured from the point of application to the center of the spot or peak, while the distance traveled by the solvent front is measured from the point of application to the leading edge of the solvent front.
The Rf value is a dimensionless quantity and typically ranges between 0 and 1. It is influenced by various factors such as the chemical nature of the analyte, the composition of the mobile phase, and the characteristics of the stationary phase. These factors determine the extent of interaction between the analyte and the stationary phase, which affects the analyte’s mobility and ultimately its Rf value.
The Rf value is useful in chromatographic analysis because it provides a means to compare and identify different compounds. Each compound will have a unique Rf value under specific chromatographic conditions. By comparing the Rf value of an unknown compound with the Rf values of known reference compounds, it is possible to tentatively identify the unknown substance.

For example, if substances A, B, and C are being analyzed, their Rf values would be calculated as follows:
Rf value of A = Distance traveled by substance A / Distance traveled by solvent (X)
Rf value of B = Distance traveled by substance B / Distance traveled by solvent (X)
Rf value of C = Distance traveled by substance C / Distance traveled by solvent (X)
By comparing these Rf values with those of known compounds, it becomes easier to identify and differentiate between different substances in a mixture.
Limitations of Liquid Chromatography
- High cost is involved for LC systems and consumables, the equipment, solvents, columns and detectors all add up in expense.
- Sample preparation is often extensive and time-consuming when complex matrices are used, many steps (filtration, extraction, cleanup) must be done.
- Some analytes are poorly compatible with LC, e.g., volatile compounds or very thermally unstable ones may not be suited and GC might be preferred.
- Resolution may be less than ideal for extremely complex mixtures where many components co-elute, retention time overlap or unresolved peaks happen
- Method development is arduous and requires expertise, many parameters (mobile phase, stationary phase, temperature, flow) need to be optimized and that takes time.
- The mobile phase is often high in viscosity (because it is liquid) which leads to slower separations or higher back-pressure and makes system demands greater.
- Sensitivity or detection of some compounds may be limited if detector/phase combination is not ideal; for example non-chromophoric species might not be detected well by UV detectors.
- In LC-MS combinations matrix effects (ion suppression/enhancement) may cause unpredictable response, reducing accuracy and precision of the results.
Advantages of Liquid Chromatography
- Wide applicability is offered by LC, many kinds of compounds (polar, non-volatile, thermally unstable) are able to be analysed which cannot by GC easily
- High sensitivity is achieved when using good detectors, trace amounts of substances can be picked up, and small sample sizes are enough.
- Good selectivity is provided by varied stationary/mobile phases which make separation of structurally similar compounds (like isomers) possible.
- Versatility in scale is allowed, both analytical and preparative work can be done by LC, from small sample amounts to larger quantities.
- Non-destructive testing of sample is possible in many cases so that sample can be recovered or further analysed after separation.
- Automation and high throughput can be achieved: many runs can be done with minimal operator effort once method is set up, boosting productivity.
Applications of Liquid Chromatography
- Environmental monitoring is enabled by LC where contaminants in water, soil, air and sludge are analyzed by LC-MS (liquid chromatography-mass spectrometry) for pollutants, herbicides, organometallics etc.
- In pharmaceutical / drug development the technique is used for analysing drug molecules, metabolites, impurities, bio-samples (blood, urine) and peptide/protein work, and it’s used by many labs for screening and validation.
- Food & beverage industry benefit by LC which helps in quality control, checking for adulteration, pesticide residues, flavour/aroma compound separation and measuring ingredient concentrations in food products.
- Forensic investigations are supported by LC when illegal drugs, toxins, illicit substances and their metabolites need to be detected in forensic samples, blood or urine, and LC-MS is often the method of choice.
- Chemical & petrochemical industry use LC for polymer/copolymer analysis, surfactants, additives, specialty chemicals, product quality and process monitoring in the manufacturing of plastics, solvents, insulating materials.
- Biotechnology / life sciences research use LC for biomolecule work like proteins, peptides, nucleic acids, carbohydrates, metabolomics and proteomics studies (especially when LC is coupled with MS) for deeper biological insight.
FAQ
What is liquid chromatography (LC)?
Liquid chromatography is a separation technique used to separate and analyze components of a sample based on their interactions with a stationary phase and a mobile phase. It involves passing a liquid sample through a column containing a stationary phase, where the different components separate based on their affinity for the stationary phase.
What are the different types of liquid chromatography?
There are several types of liquid chromatography, including high-performance liquid chromatography (HPLC), ion chromatography, size exclusion chromatography, affinity chromatography, and reversed-phase chromatography, among others. Each type utilizes different principles and stationary phases for specific applications.
What is the role of the mobile phase in liquid chromatography?
The mobile phase, typically a liquid solvent or a mixture of solvents, carries the sample through the column. It helps in the separation of components by interacting differently with the stationary phase and the sample components, leading to their differential elution.
What is the stationary phase in liquid chromatography?
The stationary phase is a material that is packed inside the column and interacts with the sample components to facilitate their separation. It can be a solid support with bonded functional groups or a liquid phase coated onto a solid support, depending on the type of liquid chromatography being employed.
How is the separation achieved in liquid chromatography?
The separation in liquid chromatography is achieved by exploiting the differences in chemical or physical properties of the sample components, such as polarity, size, charge, or affinity for the stationary phase. These differences cause the components to interact differently with the stationary phase, resulting in their separation as distinct peaks.
What are the typical detectors used in liquid chromatography?
Commonly used detectors in liquid chromatography include UV-Vis absorbance detectors, fluorescence detectors, refractive index detectors, and mass spectrometers. These detectors measure the physical or chemical properties of the eluted components and provide signals that can be used for qualitative or quantitative analysis.
How is liquid chromatography used in pharmaceutical industries?
Liquid chromatography is extensively used in the pharmaceutical industry for various purposes, including drug analysis, purity testing, impurity identification, quality control, and formulation development. It helps in ensuring the safety, efficacy, and quality of pharmaceutical products.
What are the advantages of liquid chromatography?
Liquid chromatography offers several advantages, such as high separation efficiency, versatility in separation modes, wide applicability to different sample types, and compatibility with various detectors. It allows for accurate quantification, sensitivity, and selectivity in compound analysis.
What are some common challenges in liquid chromatography?
Challenges in liquid chromatography can include method development and optimization, column selection, sample preparation, matrix interference, peak broadening, resolution limitations, and reproducibility issues. These challenges often require careful consideration and optimization to achieve reliable and accurate results.
How can liquid chromatography be coupled with other techniques?
Liquid chromatography can be coupled with various techniques to enhance its capabilities. For example, coupling LC with mass spectrometry (LC-MS) combines the separation power of liquid chromatography with the identification and structural information provided by mass spectrometry. Other common couplings include LC with UV-Vis spectrometry, fluorescence spectroscopy, or nuclear magnetic resonance (NMR) spectroscopy, among others, to gain additional information about the separated compounds.