What is Leishmania donovani?
- Leishmania donovani is a significant intracellular parasite that belongs to the genus Leishmania, which comprises a group of hemoflagellate kinetoplastids known for their role in causing leishmaniasis. This organism is primarily responsible for visceral leishmaniasis, commonly referred to as kala-azar, the most severe form of leishmaniasis. L. donovani targets the mononuclear phagocyte system, infecting vital organs such as the spleen, liver, and bone marrow, leading to serious health consequences for infected individuals.
- The transmission of L. donovani occurs through the bites of sandflies, specifically those belonging to the genus Phlebotomus in the Old World and Lutzomyia in the New World. This species complex is notably prevalent across various tropical and temperate regions, including areas in Africa (especially Sudan), China, India, Nepal, southern Europe, Russia, and South America. Alarmingly, L. donovani is responsible for thousands of fatalities annually, affecting approximately 88 countries and placing about 350 million people at ongoing risk of infection, with an estimated 500,000 new cases reported each year.
- The discovery of L. donovani is attributed to two British medical officers: William Boog Leishman, who identified it in Netley, England, and Charles Donovan, who observed it in Madras, India, both in 1903. However, it was Ronald Ross who provided the correct taxonomic classification of this parasite. The life cycle of L. donovani is complex, requiring two distinct hosts for completion: humans, which serve as the definitive host, and sandflies, which act as the intermediate host. In some geographical areas, other mammals, particularly canines, function as reservoir hosts, facilitating the spread of the parasite.
- Within the human host, L. donovani exists in an amastigote form, characterized as small, spherical, and unflagellated. Conversely, in the sandfly vector, it takes on an elongated shape with a flagellum, known as the promastigote form. Unlike many other parasitic protists, L. donovani does not penetrate host cells directly; instead, it relies on the process of phagocytosis for entry into human cells. The whole genome sequence of L. donovani, derived from isolates in southeastern Nepal, was published in 2011, further enhancing the understanding of its biology and pathogenesis.
- Taxonomically, L. donovani sensu stricto is part of a species complex that includes the closely related L. infantum, which also causes visceral leishmaniasis. The former is primarily found in East Africa and the Indian subcontinent, while L. infantum is more prevalent in Europe, North Africa, and Latin America. The formal split of these species was established in 2007, although many references to L. donovani still encompass the entire complex (sensu lato). As of 2022, L. donovani is implicated in approximately 50,000 to 90,000 new infections globally each year, highlighting its persistent threat to public health.
Classification of Leishmania
| Domain: | Eukaryota |
| Phylum: | Euglenozoa |
| Class: | Kinetoplastid |
| Order: | Trypanosomatida |
| Genus: | Leishmania |
History and Distribution of Leishmania Donovani
The history and distribution of Leishmania donovani, the causative agent of visceral leishmaniasis or kala-azar, trace back over a century and reflect its significant public health impact globally. Understanding this history provides essential insights into its epidemiology and control.
- In 1900, Sir William Leishman first identified the parasite while examining spleen smears from a soldier who succumbed to “Dumdum fever,” a term historically associated with kala-azar, in Dum Dum, Calcutta.
- Subsequently, in 1903, Charles Donovan independently reported the same parasite in spleen smears from patients in Madras, leading to the naming of the organism as Leishmania donovani.
- The amastigote forms of the parasite observed in patient samples are termed Leishman-Donovan (LD) bodies, a crucial identification marker in diagnosing the disease.
- Visceral leishmaniasis poses a considerable public health challenge, with the World Health Organization (WHO) estimating around 500,000 new cases annually.
- Approximately 90% of these cases are concentrated in the Indian subcontinent, specifically in India and parts of Sudan and Brazil, underscoring the regional significance of this infection.
- The distribution of L. donovani encompasses endemic, epidemic, and sporadic forms:
- Endemic Regions: The parasite is consistently present in certain areas, particularly in parts of India, Brazil, and Sudan, where cases occur regularly.
- Epidemic Outbreaks: Significant epidemics have been reported, especially in India, Brazil, and Sudan, with notable outbreaks causing thousands of infections.
- Sporadic Cases: These occur in regions where the disease is not typically prevalent, including states like Tamil Nadu, Maharashtra, Karnataka, and Andhra Pradesh in India.
- The resurgence of kala-azar in India can be traced to the mid-1970s, when it began to escalate dramatically. By 1977, the disease had reached epidemic levels, accounting for over 110,000 cases primarily in the Bihar region, specifically in districts such as Muzaffarpur, Samastipur, Vaishali, and Sitamarhi.
- The epidemic expanded into West Bengal, with the first outbreak recorded in 1980 in Malda district.
- Currently, the disease maintains endemic status in 31 districts of Bihar, 11 districts in West Bengal, 5 districts in Jharkhand, and 3 districts in Uttar Pradesh.
- The epidemiological trends indicate that the infection is spreading beyond traditional areas, necessitating ongoing surveillance and research to understand the factors driving these changes in distribution.
Habitat of Leishmania Donovani
The habitat of Leishmania donovani is intricately linked to its lifecycle and the host organisms it infects. This intracellular parasite primarily resides within specific cells and environments that facilitate its survival and reproduction.
- The amastigote form, also known as the Leishman-Donovan (LD) body, is predominantly found within the reticuloendothelial system of the host.
- In humans, these intracellular amastigotes primarily inhabit macrophages located in various organs, including:
- Spleen: A major site where the immune response is initiated, providing an ideal environment for the parasite.
- Liver: The liver’s rich blood supply and immune cells offer a conducive habitat for infection.
- Bone Marrow: This site serves as a critical location for blood cell production, where the parasite can reside and evade the immune system.
- Amastigotes can also be located in lesser quantities in other areas such as:
- Skin: Though less common, skin involvement can occur in some cases of leishmaniasis.
- Intestinal Mucosa: The parasite may find a niche in the mucosal layers, although this is not its primary habitat.
- Mesenteric Lymph Nodes: These nodes are part of the immune system and can harbor the parasite during infection.
- Leishmania donovani is an intracellular parasite, not only infecting humans but also other mammalian hosts. Its ability to reside within host cells is crucial for its survival.
- In the context of the sandfly vector, the promastigote form of the parasite is found in the midgut. This form is elongated and flagellated, which aids in its motility and transmission.
- Additionally, promastigotes can be cultured artificially, allowing for further study and research on the parasite’s biology and behavior.
- The specific habitats of L. donovani allow it to evade the immune system effectively while utilizing host resources for replication. Therefore, understanding these habitats is essential for developing strategies to combat leishmaniasis and improve public health interventions.
Morphology of Leishmania donovani
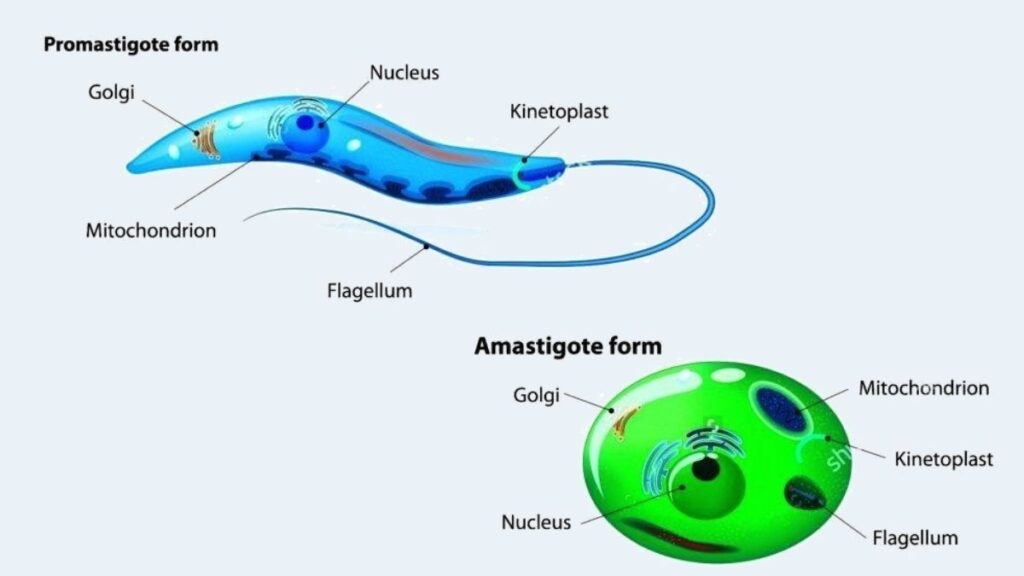
The morphology of Leishmania donovani is pivotal for understanding its life cycle, pathogenicity, and methods of identification. This parasite exhibits two distinct forms depending on its environment: the amastigote form found in mammalian hosts and the promastigote form present in sandflies and laboratory cultures.
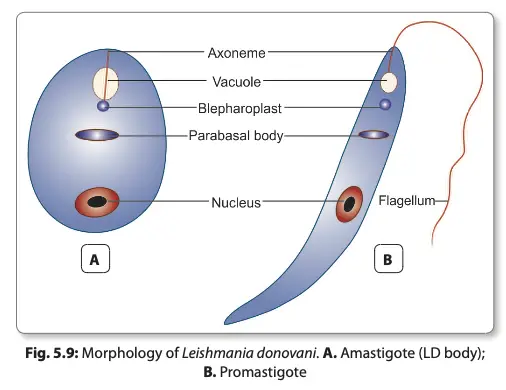
- Amastigote Form:
- The amastigote, or LD body, is generally ovoid or rounded, measuring approximately 2-4 µm in size.
- This form is intracellular, residing primarily within macrophages, monocytes, neutrophils, and endothelial cells in the host.
- When stained using Leishman, Giemsa, or Wright’s stain, the amastigote displays a pale blue cytoplasm, which is encapsulated by a limiting membrane.
- The large oval nucleus appears red, while a kinetoplast is positioned at a right angle to the nucleus, often stained red or purple.
- In well-prepared slides, the kinetoplast can be seen comprising a parabasal body and a small dot-like blepharoplast. These structures are interconnected by a delicate thread.
- An axoneme arises from the blepharoplast, extending toward the anterior tip of the amastigote cell.
- Additionally, a clear, unstained vacuole may be observed adjacent to the kinetoplast.
- Importantly, the amastigote lacks a flagellum, distinguishing it from the promastigote form.
- Promastigote Form:
- The promastigote represents the flagellated stage of the parasite, found in the midgut of the sandfly vector and in artificial cultures.
- Initially, promastigotes appear as short, oval, or pear-shaped forms but subsequently develop into long, spindle-shaped cells, ranging from 15-25 µm in length and 1.5-3.5 µm in width.
- A single nucleus is centrally located within the cell, with the kinetoplast positioned transversely near the anterior end.
- The flagellum is single and delicate, measuring between 15-28 µm in length.
- Staining with Giemsa or Leishman reveals a pale blue cytoplasm, a pink nucleus, and a bright red kinetoplast.
- A vacuole can be found near the base of the flagellum, which plays a role in the cell’s metabolic processes.
- The promastigote form does not possess an undulating membrane, differentiating it further from other flagellates.
- Both forms of Leishmania donovani exhibit distinct morphological characteristics that are crucial for their identification in laboratory settings. Understanding these differences enhances diagnostic capabilities and informs therapeutic strategies against leishmaniasis
Leishmania species
There are different species of Leishmania all of them are listed in below table;
| L. aethiopica | L. amazonensis |
| L. venezuelensis | L. waltoni |
| L. turanica | L. tropica |
| L. tarentolae | L. (Viannia) shawi |
| L. pifanoi (status disputed) | L. (Viannia) peruviana |
| L. (Viannia) panamensis | L. (Viannia) naiffi |
| L. mexicana | L. (Mundinia) martiniquensis |
| L. (Mundinia) macropodum | L. major |
| L. (Viannia) lainsoni | L. killicki (status disputed) |
| L. infantum | L. (Viannia) guyanensis |
| L. gerbili | L. garnhami (status disputed) |
| L. forattinii (status disputed) | L. (Mundinia) enriettii |
| L. donovani | L. chagasi (syn. L. infantum) |
| L. (Viannia) braziliensis | L. aristedesi (status disputed) |
| L. archibaldi (starus species) | L. arabica |
Life cycle of Leishmania donovani
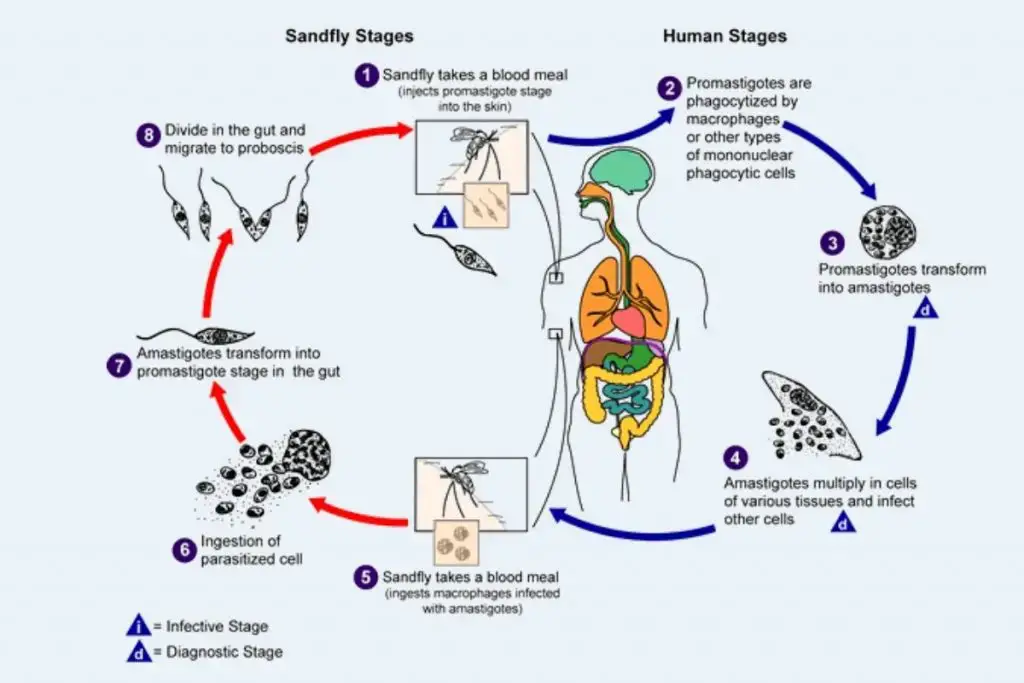
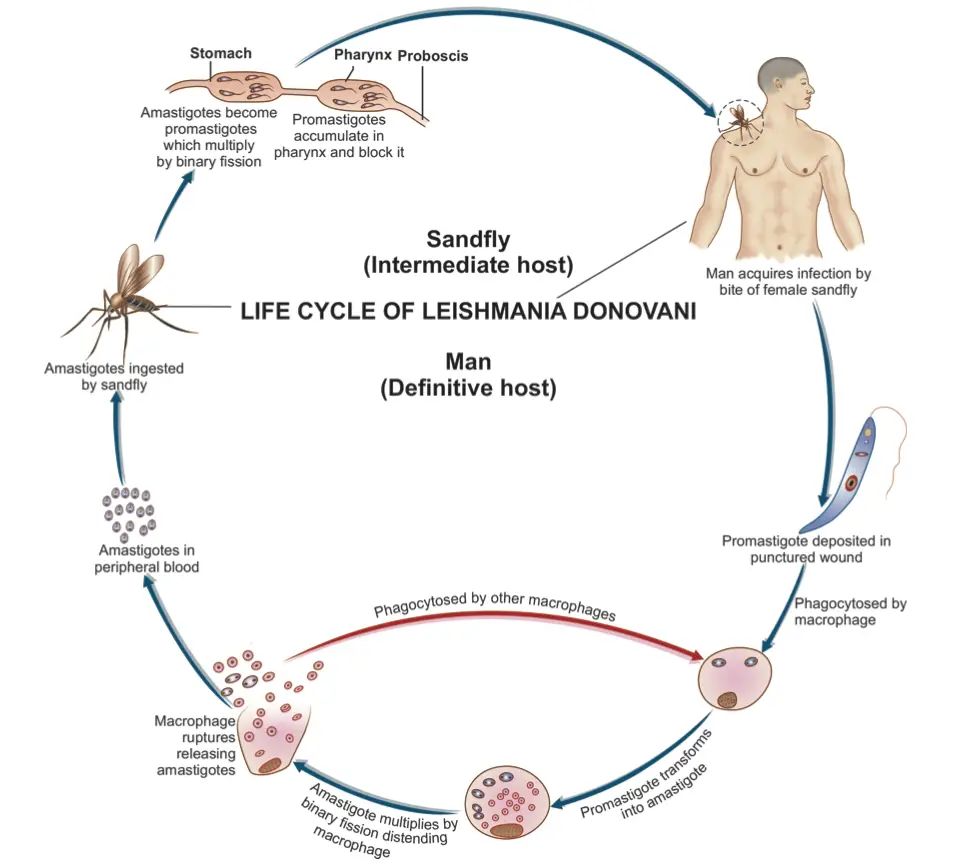
The life cycle of Leishmania donovani is complex and involves two distinct hosts: vertebrates (primarily humans) and the sandfly vector, specifically the genus Phlebotomus. This digenetic parasite exhibits a dual-phase life cycle, alternating between an intracellular phase in mammals and an extracellular phase in the sandfly, allowing it to propagate effectively in both environments.
- Life Cycle in Humans:
- The initial infection occurs when the sandfly bites a human, introducing L. donovani in its promastigote form.
- Once inside the human body, some promastigotes enter the bloodstream; however, many are quickly destroyed.
- Those that enter the reticuloendothelial system, which includes the liver, spleen, bone marrow, and lymph nodes, undergo transformation into amastigotes.
- The amastigote form is characterized by its ability to multiply through binary fission within the host’s cells, significantly increasing their population.
- As the number of amastigotes reaches critical levels (50 to 200 or more per host cell), the host cell ruptures, releasing numerous parasites into the surrounding tissue.
- These liberated amastigotes are then phagocytosed by new host cells, including neutrophils and monocytes (macrophages), which facilitates their dissemination through the bloodstream and contributes to systemic infection.
- The ongoing cycle of infection leads to progressive damage to the reticuloendothelial system, exacerbating the disease.
- Life Cycle in the Sandfly:
- The cycle continues when an uninfected sandfly bites a person with L. donovani infection, ingesting free amastigotes along with parasitized neutrophils and monocytes.
- Inside the midgut of the sandfly, the amastigotes undergo transformation into procyclic promastigotes, and subsequently into metacyclic promastigotes.
- This transformation is accompanied by a process of longitudinal binary fission, resulting in a significant increase in promastigote numbers, often filling the midgut’s lumen completely.
- Over a period of 6 to 9 days, the parasites proliferate extensively, migrating toward the pharynx and buccal cavity of the sandfly.
- Importantly, the salivary glands of the sandfly remain uninfected, which does not hinder the vector’s ability to transmit the parasites.
- When a heavily infected sandfly bites another host, the transmission of L. donovani is facilitated through the introduction of metacyclic promastigotes into the bloodstream, thus completing the life cycle.
Pathogenicity of Leishmania Donovani
The pathogenicity of Leishmania donovani is primarily manifested through its ability to cause visceral leishmaniasis, commonly known as kala-azar. This disease results from the invasion of the reticuloendothelial system by the parasite, leading to significant disruptions in various organs, particularly the spleen, liver, and bone marrow.
- Infection Mechanism:
- L. donovani invades macrophages, transforming into its amastigote form, which facilitates its survival and replication within these immune cells.
- The infection disseminates throughout the body as parasitized macrophages circulate, effectively spreading the pathogen to various tissues and organs.
- Impact on Major Organs:
- Spleen:
- The spleen is severely affected, becoming grossly enlarged due to the proliferation of amastigotes within the fixed macrophages, leading to a condition referred to as “blockade” of the reticuloendothelial system.
- The organ exhibits thickened capsules resulting from perisplenitis and becomes soft and friable, indicating a lack of fibrosis.
- Upon sectioning, the spleen’s cut surface appears red or chocolate-colored, attributed to engorged vascular spaces.
- Histologically, the reticulum cells are significantly increased, loaded with L. donovani bodies, while lymphocytic infiltration is minimal and plasma cells are abundant.
- Liver:
- The liver also shows enlargement, with heavily parasitized Küpffer cells and vascular endothelial cells, although hepatocytes remain unaffected.
- Liver function is generally preserved; however, prothrombin production may be decreased, leading to coagulation issues.
- The sinusoidal capillaries are dilated and engorged, and some degree of fatty degeneration is observable, giving a characteristic “nutmeg” appearance upon inspection.
- Bone Marrow:
- The bone marrow exhibits extensive infiltration by parasitized macrophages, potentially crowding hematopoietic tissues.
- This infiltration can result in severe anemia, with hemoglobin levels dropping to 5–10 g/dL due to both the infiltration and increased destruction of erythrocytes caused by hypersplenism.
- Spleen:
- Hematological Manifestations:
- Peripheral lymph nodes and lymphoid tissues, particularly in the nasopharynx and intestine, may become hypertrophic, though this is not consistently observed in Indian cases.
- Patients often present with leucopenia, characterized by marked neutropenia, and thrombocytopenia. This hematological profile suggests an autoimmune component, as antibodies against white blood cells and platelets may contribute to the observed pancytopenia.
Clinical Features of Kala-Azar
The clinical features of kala-azar, caused by Leishmania donovani, present a complex array of symptoms that emerge progressively, leading to significant morbidity. Understanding these features is essential for early diagnosis and treatment.
- Onset of Illness:
- The onset of kala-azar is typically insidious, often making initial identification challenging.
- Patients usually present with fever that can manifest in various patterns, including continuous, remittent, or irregular episodes.
- Splenomegaly:
- One of the hallmark signs of kala-azar is splenomegaly, which often develops early in the course of the disease.
- The enlargement of the spleen is progressive and can become massive, contributing to abdominal discomfort and altered blood cell dynamics.
- Hepatomegaly and Lymphadenopathy:
- While hepatomegaly also occurs, it is generally less pronounced than splenomegaly.
- Lymphadenopathy may be observed, but the extent and prominence vary among patients.
- Dermatological Changes:
- A distinctive feature of kala-azar is the alteration in skin texture and pigmentation.
- The skin may become dry, rough, and exhibit dark pigmentation, which is the origin of the term “kala-azar,” translating to “black fever.”
- Concurrently, hair may become thin and brittle, reflecting the systemic nature of the disease.
- Cachexia and Anemia:
- Significant cachexia is often present, characterized by severe weight loss and emaciation.
- Marked anemia is frequently noted, resulting from the infiltration of bone marrow by the parasite and the resultant effects on hematopoiesis.
- Bleeding Tendencies:
- Patients may experience epistaxis (nosebleeds) and bleeding from the gums, indicating potential coagulopathies associated with the disease.
- Prognosis and Complications:
- If left untreated, the prognosis for patients with kala-azar is dire, with most succumbing within approximately two years.
- Death is often due to intercurrent infections or complications such as dysentery, diarrhea, and tuberculosis, highlighting the weakened state of the immune system.
Post Kala-azar Dermal Leishmaniasis
Post-kala-azar dermal leishmaniasis (PKDL) is a condition that can develop in some patients following recovery from visceral leishmaniasis, also known as kala-azar. This sequel presents unique clinical features and is primarily observed in endemic regions such as India and East Africa.
- Incidence and Timing:
- Approximately 3–10% of individuals who recover from visceral leishmaniasis in endemic areas subsequently develop PKDL.
- This condition typically arises about one to two years after the initial systemic illness, indicating a delayed response to the underlying parasitic infection.
- Geographical Distribution:
- PKDL is predominantly observed in India and East Africa, with its manifestation differing notably between these regions.
- The characteristics of PKDL in these areas may vary, emphasizing the need for localized understanding and treatment strategies.
- Lesion Characteristics:
- PKDL is characterized by non-ulcerative skin lesions, which can manifest in several distinct forms:
- Depigmented Macules:
- These lesions often appear on the trunk and extremities, resembling tuberculoid leprosy.
- They typically lack significant inflammation and may be mistaken for other dermatological conditions.
- Erythematous Patches:
- Commonly found on the face, these patches exhibit a distinctive “butterfly distribution,” which refers to their bilateral presence across the cheeks and nose.
- The erythematous nature indicates some degree of inflammation, but these patches are generally not painful.
- Nodular Lesions:
- Both depigmented macules and erythematous patches may progress into painless, yellowish-pink granulomatous nodules.
- These nodules are non-ulcerating and can vary in size and distribution.
- Depigmented Macules:
- PKDL is characterized by non-ulcerative skin lesions, which can manifest in several distinct forms:
- Demonstration of the Parasite:
- The Leishmania donovani parasite can be isolated from the skin lesions associated with PKDL.
- This demonstrates that even after the systemic phase of the disease has resolved, the parasite can persist in a dormant or localized form, contributing to the pathogenesis of PKDL.
Laboratory diagnosis of Kala-azar
The laboratory diagnosis of Kala-azar, also known as visceral leishmaniasis, is crucial for timely and effective treatment. The diagnostic approach combines direct and indirect methods, utilizing various specimens and techniques to detect the presence of the Leishmania parasite or the body’s immune response to it.
Direct Evidence
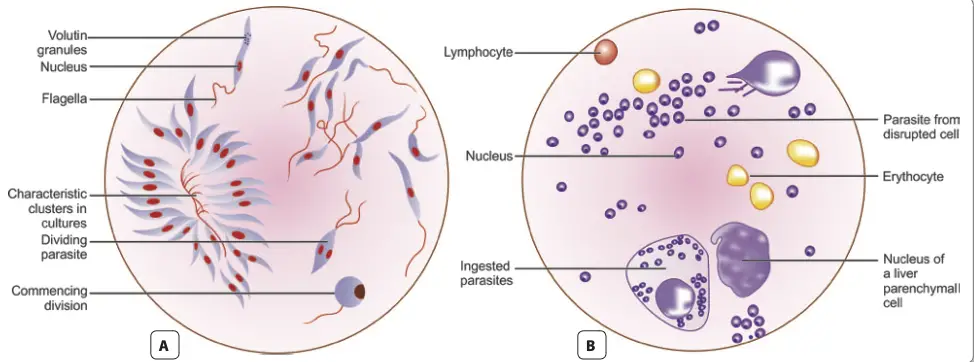
- Microscopy
- The microscopic identification of amastigotes within tissue aspirates is the gold standard for diagnosing visceral leishmaniasis.
- Specimen Collection: Common materials collected for microscopic examination include:
- Peripheral blood
- Bone marrow
- Splenic aspirates
- Enlarged lymph nodes
- Staining Techniques: Smears are stained using Leishman, Giemsa, or Wright’s stains and examined under an oil immersion objective.
- Amastigotes appear within macrophages, often in large quantities, and occasionally in extracellular forms.
- Peripheral Blood Smear:
- Amastigotes are found in circulating monocytes, and although the numbers may be limited, thicker blood films may improve detection rates.
- Examination of buffy coat smears is preferred, as they can show a diurnal periodicity with higher positivity during the day.
- Bone Marrow Aspirate:
- Considered the most common diagnostic specimen, collected via sternal puncture or iliac crest.
- The procedure involves using a sternal puncture needle to aspirate approximately 0.5 mL of marrow fluid after proper disinfection and anesthesia.
- Splenic Aspirates:
- Richer in parasites, making them more valuable for diagnosis; however, the procedure poses a risk of hemorrhage and is typically reserved for cases where bone marrow examination is inconclusive.
- Lymph Node Aspirates:
- Generally not useful for diagnosing Indian Kala-azar but may be relevant in other geographical contexts like African Kala-azar.
- Culture Techniques
- Tissue materials or blood can be cultured on NNN medium (rabbit blood agar).
- The medium consists of salt agar and defibrinated rabbit blood.
- Inoculation occurs in the water of condensation, with incubation at 22°C-24°C for 1-4 weeks.
- The culture is monitored weekly for the presence of promastigotes using high-power objective microscopy.
- Tissue materials or blood can be cultured on NNN medium (rabbit blood agar).
- Animal Inoculation
- Although not routine, animal inoculation may be employed using Chinese golden hamsters.
- The material is inoculated intraperitoneally or intradermally, and amastigotes can be detected in smears from developing ulcers or nodules.
Indirect Evidence
- Serodiagnosis
- Detection of Antigens: Low concentrations of antigens in serum can be identified using techniques like ELISA and PCR.
- Detection of Antibodies:
- Complement fixation test (CFT) was one of the earliest tests, using an antigen derived from human tubercle bacillus.
- Various serological tests have been developed to identify specific antibodies, including:
- Indirect immunofluorescent antibody test (IFAT)
- Counter immunoelectrophoresis (CIEP)
- ELISA and DOT-ELISA
- Direct agglutination test (DAT) for antileishmanial antibodies, which shows high sensitivity and specificity.
- Molecular Diagnosis
- Techniques like Western blotting and PCR aid in species identification, although PCR is typically limited to specialized laboratories.
- Non-specific Serum Tests
- Increased globulin content in serum leads to tests such as:
- Napier’s Aldehyde Test: A drop of formalin in serum reveals positive results through jellification, indicating hypergammaglobulinemia.
- Chopra’s Antimony Test: Serum diluted and overlaid with urea stibamine shows positive results through the formation of a precipitate.
- Increased globulin content in serum leads to tests such as:
- Skin Testing
- Leishmanin Skin Test (Montenegro Test): This delayed hypersensitivity test involves intradermal injection of killed promastigotes, with positive results indicated by induration after 48-72 hours. In active Kala-azar, this test may yield negative results.
- Blood Picture
- Complete blood count typically reveals normocytic normochromic anemia and thrombocytopenia, with leucopenia and a relative increase in lymphocytes and monocytes. The absence of eosinophils is noted, alongside hypergammaglobulinemia and altered albuminratios.
- Diagnosis of Post-Kala-Azar Dermal Leishmaniasis (PKDL)
- Diagnosis involves biopsy of nodular lesions, where amastigote forms can be demonstrated in stained sections or cultured. Immunodiagnosis is not applicable for PKDL.
Treatment of Kala-azar
The treatment of Kala-azar, also known as visceral leishmaniasis, involves a range of therapeutic options that are contingent on geographical factors and drug resistance. Effective management of this disease is crucial for patient recovery and public health outcomes. This expository overview delineates the primary treatment modalities available for Kala-azar, focusing on pharmacological interventions.
- Pentavalent Antimonial Compounds
- The primary treatment in many endemic regions is the use of pentavalent antimonial compounds, which have a high success rate for treating Kala-azar.
- Available Preparations:
- Sodium Stibogluconate: Contains 100 mg of sodium stibogluconate (SbV) per mL.
- Meglumine Antimonate: Contains 85 mg of sodium stibogluconate (SbV) per mL.
- Dosage:
- Administered at a daily dosage of 20 mg/kg through rapid intravenous (IV) infusion or intramuscular (IM) injection.
- The duration of treatment typically ranges from 20 to 30 days.
- Efficacy: Cure rates exceed 90% in most regions; however, resistance has been reported in Bihar, India, necessitating alternative treatments.
- Amphotericin B
- Amphotericin B is recommended as a first-line treatment in regions experiencing resistance to antimonial compounds, particularly in Bihar.
- Dosage:
- Administered at a dosage of 0.75–1.0 mg/kg on alternate days, totaling 15 infusions.
- Adverse Effects: Patients frequently experience fever and chills during the administration of amphotericin B, which is a common side effect of this treatment.
- Liposomal Amphotericin B:
- This formulation has been extensively utilized worldwide and is the only FDA-approved medication for visceral leishmaniasis.
- Dosage: Typically given at 3 mg/kg daily, allowing for higher doses to be administered safely, enhancing treatment efficacy without increased toxicity.
- Paromomycin
- Paromomycin is an intramuscular aminoglycoside antibiotic known for its antileishmanial properties.
- Dosage:
- Administered at a dosage of 11 mg/kg daily over a treatment period of 21 days.
- Paromomycin serves as an alternative option, especially in cases where other treatments may not be viable.
- Miltefosine
- Miltefosine represents the first oral medication approved for leishmaniasis treatment.
- Dosage:
- For patients weighing less than 25 kg, the recommended dosage is 50 mg daily for 28 days.
- For patients exceeding 25 kg, the dosage is increased to twice daily for 28 days.
- Its oral formulation provides a significant advantage in terms of patient compliance and convenience.
- Treatment of Post-Kala-Azar Dermal Leishmaniasis (PKDL)
- The treatment protocol for PKDL aligns with that for visceral leishmaniasis, emphasizing the use of the aforementioned pharmacological agents to ensure effective management of the condition.
Prophylaxis of Kala-azar
The following points outline the main strategies for the prophylaxis of Kala-azar:
- Early Detection and Treatment
- Prompt identification and management of all Kala-azar cases are essential to prevent further transmission.
- Early intervention can significantly reduce the incidence of the disease within affected communities.
- Integrated Insecticidal Spraying
- Implementing targeted insecticidal spraying programs aims to decrease the population of sandflies, which are the primary vectors of the disease.
- This method involves the application of insecticides in both indoor and outdoor settings, particularly in areas known to be endemic for Kala-azar.
- Destruction of Animal Reservoir Hosts
- In zoonotic forms of Kala-azar, controlling and eliminating the reservoirs, such as specific rodent populations, is crucial for reducing transmission rates.
- This approach may involve habitat modification and population control strategies to diminish interactions between reservoirs and human populations.
- Personal Prophylaxis
- Individuals can take proactive measures to protect themselves from sandfly bites. Recommendations include:
- Wearing thick, protective clothing that covers the skin.
- Utilizing bed nets, especially those treated with insecticides, to prevent nighttime bites.
- Installing window mesh screens to limit sandfly entry into homes.
- Applying insect repellents on exposed skin to reduce the likelihood of bites.
- Maintaining cleanliness in living environments to minimize potential breeding sites for sandflies.
- Individuals can take proactive measures to protect themselves from sandfly bites. Recommendations include:
- Absence of Vaccination
- Currently, there is no vaccine available for Kala-azar, making the emphasis on environmental and personal preventive measures even more crucial.
- Research continues in the quest for an effective vaccine, but until then, reliance on the strategies outlined above remains paramount.
FAQ on Leishmaniasis
1. What are leishmaniasis symptoms?
The symptoms of leishmaniasis are Breathing difficulty, Skin sores, which may become a skin ulcer that heals very slowly, Stuffy nose, runny nose, and nosebleeds, Swallowing difficulty, Ulcers and wearing away (erosion) in the mouth, tongue, gums, lips, nose, and inner nose.
2. What are leishmaniasis recidivans?
Leishmaniasis recidivans is a rare, cutaneous form of leishmaniasis, occurring in patients with a good cellular immune response. An unusual clinical variant of cutaneous disease caused by Leishmania tropica is leishmaniasis recidivans. Leishmaniasis recidivans typically recurs at the site of an original ulcer, generally within 2 years and often within the edge of the scar.
3. Where are leishmaniasis found?
leishmaniasis is found in parts of the tropics, subtropics, and southern Europe.
4. Can leishmaniasis be passed from dog to dog?
Transmission of L. infantum to dogs (and humans) is mainly through the bite of infected sandflies, but the parasite can also be transmitted vertically.
5. Can leishmaniasis be cured?
Leishmaniasis is a treatable and curable disease.
6. Can leishmaniasis be cured in dogs?
If a dog is severely affected by the disease, it will be difficult to cure him/her. But if he/she is in the early stages of the illness, there is a good chance of controlling the disease. However, with the therapies currently available, parasitological cure cannot be established.
7. Can leishmaniasis spread from dog to human?
No. There have been no documented cases of leishmaniasis transmission from dogs to humans. This information is not meant to be used for self-diagnosis or as a substitute for consultation with a health care provider.
8. Can leishmaniasis spread from human to human?
Transmission may occur from animal to sand fly to human. Humans can also transmit the parasite between each other through a blood transfusion or shared needles. In some parts of the world, transmission may also occur from human to sand fly to human.
9. How leishmaniasis is transmitted?
Leishmaniasis is transmitted by the bite of infected female phlebotomine sand flies.
10. How is leishmaniasis diagnosed?
Leishmaniasis is diagnosed by detecting Leishmania parasites (or DNA) in tissue specimens—such as from skin lesions, for cutaneous leishmaniasis (see instructions), or from bone marrow, for visceral leishmaniasis (see note below)—via light-microscopic examination of stained slides, molecular methods.
- Protozoa Definition, Classification, Characteristics, Structure, Diseases, Examples
- Amoeba Cell Characteristics, Structure, Movement, Nutrition, Reproduction, Disease, Habitat.
- Saini P, Kumar NP, Ajithlal PM, Joji A, Rajesh KR, Reena KJ, Kumar A. Visceral Leishmaniasis Caused by
- Leishmania donovani Zymodeme MON-37, Western Ghats, India. Emerg Infect Dis. 2020
- Aug;26(8):1956-1958. doi: 10.3201/eid2608.200557. PMID: 32687040; PMCID: PMC7392465.
- Davidson, R. N. (2017). Leishmaniasis. Infectious Diseases, 1059–1064.e1. doi:10.1016/b978-0-7020-6285-8.00123-4
- https://epgp.inflibnet.ac.in/epgpdata/uploads/epgp_content/S000035ZO/P000888/M020580/ET/1498559114MorphologyLeishmaniaQuad1.pdf
- https://en.wikipedia.org/wiki/Leishmania_donovani
- https://www.cdc.gov/dpdx/leishmaniasis/index.html
- https://www.biologydiscussion.com/protozoa-2/leishmania-donovani-habitat-morphology-and-life-cycle/49238
- https://www.biologydiscussion.com/parasitology/parasitic-protozoa/leishmania-donovani-haemoflagellates-parasitology/62145
- https://www.onlinebiologynotes.com/leishmania-donovani-morphology-life-cycle-pathogenesis-clinical-symptoms-lab-diagnosis-treatment-prevention-and-control/
- https://www.notesonzoology.com/parasitology/leishmania-donovani-with-diagram-zoology/4673