Leaf Structure
The leaf is a vital plant organ responsible for photosynthesis, transpiration, and gas exchange. It is typically thin and flat to maximize its surface area, which enhances the absorption of light and carbon dioxide while allowing efficient release of oxygen and water vapor. The structure of a typical leaf consists of several key components:
- Blade (Lamina): This is the broad, flat, and green part of the leaf where most of the photosynthesis occurs. The blade is connected to the stem by the petiole.
- Petiole: The petiole is a slender stalk that attaches the leaf to the stem of the plant. Some leaves lack a petiole and are directly attached to the stem; they are called sessile leaves.
- Midrib: The central vein running down the middle of the leaf is known as the midrib. It provides support and is the primary route for transporting water, nutrients, and sugars between the leaf and the rest of the plant.
- Veins: Besides the midrib, the leaf has numerous smaller veins branching out across the blade. These veins serve as conduits for water, minerals, and nutrients throughout the leaf and also help in providing structural support.
- Epidermis: The leaf is covered by a thin, transparent outer layer called the epidermis. The upper epidermis is generally thin, while the lower epidermis has more stomata.
- Stomata: These are small openings, typically found on the lower surface of the leaf, through which carbon dioxide, oxygen, and water vapor are exchanged with the atmosphere. Stomata are essential for photosynthesis and transpiration.
- Guard Cells: Each stoma is flanked by two bean-shaped cells called guard cells. They control the opening and closing of the stomata, regulating the exchange of gases and water vapor between the leaf and the environment.
- Mesophyll: The interior tissue of the leaf is called the mesophyll. It is made up of two layers: the palisade mesophyll, located just beneath the upper epidermis and consisting of column-shaped cells with many chloroplasts for photosynthesis, and the spongy mesophyll, which lies beneath the palisade mesophyll and has loosely arranged cells with air spaces for gas exchange and storage of water and nutrients.
- Chloroplasts: These are specialized organelles found within the mesophyll cells and are the sites of photosynthesis. Chloroplasts contain chlorophyll, the pigment that captures light energy used to convert carbon dioxide and water into sugars and oxygen.
- Cuticle: The epidermis is covered by a waxy layer called the cuticle, which helps reduce water loss from the leaf’s surface.
The overall structure of a leaf allows it to efficiently capture light energy, absorb carbon dioxide, and carry out the process of photosynthesis while minimizing water loss during transpiration. Different plant species may have variations in leaf structure to adapt to their specific environments and ecological roles.
External Leaf Structure Observation Under the Microscope
Requirement
- Stereo Microscope: The first and foremost requirement is a reliable stereo microscope. Unlike conventional compound microscopes, the stereo microscope offers a three-dimensional view of the leaf’s surface. This depth perception is critical in comprehending the complex architecture and the spatial arrangements of various leaf features. With its dual eyepieces and zoom capabilities, the stereo microscope ensures a clear, magnified, and immersive experience, allowing us to appreciate the leaf’s beauty at a microscopic level.
- Pristine Leaf Specimen: The quality of the leaf specimen is of utmost importance. Selecting a healthy and fresh leaf that has not undergone decay or desiccation is crucial for accurate observations. A living leaf, plucked gently from its plant, guarantees the preservation of its original structures and maintains the integrity of its external features. This ensures that any observations made are a true representation of the leaf’s natural condition, devoid of artifacts that could arise from deterioration.
Additionally, to enhance the observation experience and obtain more comprehensive insights into the leaf’s external structure, supplementary materials may be useful:
- Microscope Slides and Coverslips: Preparing a thin leaf section on a microscope slide and covering it with a coverslip immobilizes the specimen for observation. This technique prevents any movement that may hinder a clear view and allows for a focused examination of specific regions of interest.
- Stains and Dyes: While not necessary, using certain stains or dyes can accentuate certain leaf structures, making them more distinguishable. For instance, staining the leaf with iodine solution can highlight the presence of starch, providing valuable information about the leaf’s energy reserves.
- Fine-tipped Forceps: Delicate forceps can aid in handling the leaf specimen with precision and care, minimizing any potential damage or alteration to its external features.
- A Light Source: Appropriate illumination is crucial for illuminating the leaf and enhancing visibility under the microscope. An adjustable light source, such as an LED lamp, can be directed to the specimen to achieve optimal contrast and clarity.
Observation 1 (leaf surface) Procedure
The observation of a leaf’s surface is a captivating journey into the intricate details and adaptations that enable plants to interact with their environment effectively. To embark on this exploration, follow this step-by-step procedure using a stereomicroscope:
- Preparation of the Leaf Specimen:
- Select a fresh and healthy leaf, preferably from a young plant, as it is likely to exhibit more distinct surface features.
- Gently pluck the leaf from the plant to ensure minimal damage.
- Handle the leaf with care, using fine-tipped forceps if necessary, to avoid any unnecessary bruising or tearing.
- Setting up the Stereomicroscope:
- Place the stereomicroscope on a stable surface in a well-lit area.
- Ensure that the microscope is properly aligned and adjusted for your comfort, with both eyepieces at the appropriate distance for your eyes.
- Positioning the Leaf Specimen:
- Lay the leaf on the stage of the microscope, ensuring it lies flat without any folds or creases.
- If the leaf is large, carefully trim it to fit the field of view while preserving important surface features.
- Initial Observation – Low Power:
- Start the observation at the lowest magnification (low power). This allows you to get an overview of the entire leaf and its major surface structures.
- Adjust the focus knob to bring the leaf into sharp focus. Take your time to ensure a clear image.
- Systematic Exploration – Increasing Magnification:
- Gradually increase the magnification by rotating the zoom or objective lens selector. Progress from low power to medium power and, if necessary, to high power.
- At each magnification level, take a moment to refocus and adjust the illumination to obtain the clearest image possible.
- Recording Observations:
- As you explore the leaf surface, make detailed observations of the various structures you encounter.
- Take note of patterns, textures, trichomes (hair-like structures), stomata, and any other prominent features.
- Use a notebook or a digital device to record your findings along with the corresponding magnification level.
- Extra Observations (Optional):
- If desired, apply gentle pressure to the leaf with a clean coverslip to observe any changes in surface features caused by slight compression.
- Use stains or dyes, if available, to highlight specific structures, such as using iodine to detect starch granules.
- Cleaning Up:
- Once you have completed your observations, carefully remove the leaf from the microscope stage.
- Clean any debris or residue from the microscope’s lenses and stage to maintain its optimal performance.
Observation 2 (stomata) Procedure
Stomata, the tiny pores found on the leaf epidermis, play a crucial role in regulating gas exchange and water movement in plants. To observe and estimate stomatal frequency on a leaf’s surface, follow this step-by-step procedure using either a compound microscope or a stereo dissecting microscope:
Requirements
- Clear Nail Polish: Used to create an impression of the leaf surface, capturing the stomata in a thin film for observation.
- A Leaf: Choose a healthy leaf of interest, ensuring it is flat and intact for the procedure.
- Compound Microscope (or Stereo Dissecting Microscope): Either type of microscope can be used, but a compound microscope will provide higher magnification and better resolution for estimating stomatal frequency.
- Tweezers: Used to carefully peel off the thin film of nail polish with the stomata impressions from the leaf surface.
- Microscope Glass Slide: To hold the thin film for observation.
- Microscope Cover Slip: Placed over the thin film on the slide to protect it and prevent distortion.
Procedure
- Application of Clear Nail Polish:
- On the upper or lower surface of the leaf (depending on the type of stomata you wish to observe), apply a thin, even layer of clear nail polish.
- Allow the nail polish to air-dry for about four hours to ensure it solidifies and captures the impressions of the stomata.
- Peeling Off the Film:
- Using tweezers, gently lift the dried nail polish film from the leaf’s surface. This film will contain the imprints of the stomata.
- Preparing the Microscope Slide:
- Carefully place the nail polish film on a clean microscope glass slide.
- To protect the film and ensure a clear view, position a microscope cover slip over the film gently.
- Observation Under the Microscope:
- Begin the observation at the lowest magnification (low power) to locate the stomata on the leaf’s surface.
- Gradually increase the magnification, progressing from low power to higher magnifications, such as 100x, for a closer examination.
- Focus the microscope carefully to get a clear view of the stomata and their surrounding structures.
- Count and record the number of stomata within a known area (e.g., a square millimeter) to estimate the stomatal frequency.
- Record Your Observations:
- While observing the stomata, take notes of their distribution, shape, and size.
- Record the stomatal frequency data obtained from the estimation to compare with other observations or scientific references.
By following this procedure and utilizing the appropriate microscope, you can gain valuable insights into the stomatal distribution and characteristics of the leaf under examination. The observation of stomata provides essential information about a plant’s adaptation to its environment and its ability to regulate water loss and gas exchange efficiently.
Leaf Structure Observations Under the Microscope
Requirements
- Maple Leaf (Fresh and Healthy): Select a fresh and healthy maple leaf as the subject of your observation. The choice of a maple leaf offers a diverse and fascinating array of structural features, making it an ideal candidate for in-depth examination.
- Microscope (Compound or Stereo): You will need a microscope to magnify the leaf’s structure and reveal the hidden wonders of its anatomy. Both compound microscopes and stereo microscopes can be used, depending on the level of detail and magnification you desire. Compound microscopes provide higher magnification, allowing for more intricate observations, while stereo microscopes offer a three-dimensional view of the leaf’s surface.
- Microscope Slides and Coverslips: Prepare clean and clear microscope slides to mount the leaf specimen. These slides provide a stable surface for observation and prevent damage to the microscope lenses.
- Tweezers: Use a pair of fine-tipped tweezers to handle the leaf specimen delicately. Tweezers enable precise manipulation of the leaf and facilitate the process of placing it on the microscope slide without causing damage.
- Water (Distilled or Deionized): Water droplets can be used to moisten the leaf surface gently, enhancing the visibility of certain structures during observation. Distilled or deionized water is recommended to avoid introducing impurities.
- A Small Brush (Soft-bristled): A soft-bristled brush, like a fine paintbrush, can be useful for removing dust or debris from the leaf’s surface before mounting it on the slide. This ensures a clearer and unobstructed view of the leaf’s structures.
Procedure for Observation of Leaf Structure
- Leaf Selection and Preparation:
- Choose a fresh and intact leaf from a healthy plant. Avoid leaves that show signs of damage or decay.
- Rinse the leaf gently under running water to remove any dirt or impurities.
- Simmering the Leaf (Optional Step):
- This step is optional and intended for specific research purposes. Simmering the leaf in water for an extended period (e.g., an hour and a half) can help soften its tissues, making it easier to dissect and observe specific structures. However, it is not necessary for general leaf structure observation and can be skipped for a more straightforward procedure.
- Softening and Handling the Leaf:
- If the simmering step is omitted, proceed directly to this step. Place the leaf on a plate or Petri dish.
- Add a small amount of distilled or deionized water to the plate to keep the leaf hydrated during the observation.
- Gently use the soft-bristled brush to remove the soft tissue from both sides of the leaf, exposing the underlying structures.
- Stabilizing the Leaf Vein:
- Identify the leaf vein (vascular bundles) running through the leaf.
- Carefully place the leaf vein between two hard surfaces, such as books or glass slides. This prevents the vein from twisting or curling during the observation.
- Microscope Observation:
- Position the stabilized leaf vein on the microscope stage.
- If using a stereo microscope, start with low magnification and gradually increase to explore the leaf vein’s three-dimensional structure and its surrounding tissues.
- If using a compound microscope, use low power to view the leaf vein and its cellular arrangements.
- Record Your Observations:
- As you observe the leaf structure, take detailed notes of the cellular organization, vein patterns, and any other interesting features you encounter.
- You may also capture images or sketches of the observed structures for documentation.
Compound Microscopy of Leaf Structure
Requirements for Compound Microscopy of Leaf Structure
- Compound Microscope: A high-quality compound microscope is the cornerstone of this endeavor. It provides multiple levels of magnification, allowing you to explore the leaf’s structure at varying depths, from low to high power. The compound microscope’s ability to reveal fine details through its lenses is vital for comprehensive leaf examination.
- Tweezers: Fine-tipped tweezers play a crucial role in delicately handling the leaf specimen during the preparation process. They help ensure that the leaf is positioned correctly on the microscope slide and prevent any damage that might compromise the observation.
- Needle: A fine needle serves as a valuable tool for precise manipulation during the preparation of the leaf specimen. It can be used to handle delicate structures and aid in the dissection process if needed.
- Glass Slides and Cover Slips: Clean and clear glass slides provide a stable platform for mounting the leaf specimen. Cover slips protect the specimen while maintaining a flat surface for optimal observation.
- Safranin: Safranin is a red dye used as a stain to enhance the visibility of certain cell structures in the leaf. It highlights cell walls and nuclei, aiding in the observation of leaf tissues with improved contrast.
- Glycerine: Glycerine serves as a mounting medium for the leaf specimen. Its refractive index closely matches that of glass, reducing image distortion and ensuring clear visualization of the leaf structures.
- Distilled Water: Distilled water is indispensable for various steps in the preparation process. It helps moisten the leaf specimen, aids in the transfer of the specimen to the slide, and is useful for diluting stains and other solutions.
- Watch Glass: A watch glass provides a convenient surface for mixing and preparing small amounts of solutions, such as safranin, before applying them to the leaf specimen.
Procedure for Compound Microscopy of Leaf Structure
- Leaf Preparation:
- Carefully fold the leaf to expose the lower surface.
- Using a pair of tweezers, gently peel off the lower surface of the leaf, which consists of the epidermal membranous transparent layer. Handle the peel with care to avoid damaging it.
- Placing the Peel in Distilled Water:
- Transfer the peeled epidermal skin into a watch glass containing distilled water. This helps to keep the peel hydrated and ready for the subsequent steps.
- Staining the Epidermal Peel:
- Move the epidermal peel from the watch glass with distilled water to another watch glass containing a few drops of safranin solution.
- Allow the peel to remain in the safranin solution for about 30 seconds to enable proper staining.
- Rinsing the Stained Peel:
- After the staining period, remove the epidermal skin from the safranin solution.
- Place it back into the watch glass with distilled water to remove any excess stain and avoid over-staining.
- Mounting the Epidermal Skin on a Glass Slide:
- Carefully place the rinsed epidermal skin onto a clean and dry microscope glass slide.
- Add a few drops of glycerine to the epidermal skin on the slide. Glycerine serves as a mounting medium, preserving the specimen and reducing image distortion.
- Cover Slipping the Specimen:
- Gently place a microscope cover slip over the epidermal skin on the slide. Ensure there are no air bubbles trapped under the coverslip for a clear view.
- Removing Excess Glycerine:
- Use blotting paper to carefully remove any excess glycerine from the slide, ensuring that the specimen is well-mounted and free of excess liquid.
- Microscope Observation:
- Place the prepared slide on the microscope stage and secure it in place.
- Begin your observation with low magnification and gradually increase to higher levels to explore different leaf structures in detail.
- Adjust the focus and lighting to obtain clear and sharp images.
- Recording Your Observations:
- Document your observations with detailed notes and, if possible, images or sketches of the observed leaf structures.
- Record the magnification used for each observation to maintain accuracy in your findings.
Observations
Upon observing the leaf structure under the microscope, a fascinating world of intricate details and functional adaptations is revealed, offering valuable insights into plant anatomy. The following unique observations can be made:
- Epidermal Cells: The epidermal layer of the leaf can be observed under the microscope, and its cells appear irregular in shape and arrangement. These outermost cells serve as a protective barrier for the leaf, safeguarding it from external environmental factors.
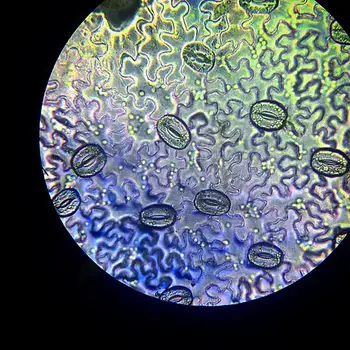
- Stomata (Leaf Spores): The most striking feature one will notice is the presence of stomata scattered between the epidermal cells. These stomata are bean-shaped structures that appear denser and darker under the microscope due to their distinct morphology and arrangement.
- Guard Cells: Stomata consist of two bean-shaped cells called guard cells, which can be differentiated from the surrounding epidermal cells. The guard cells are responsible for controlling the opening and closing of the stomata, thereby regulating the exchange of gases and water vapor between the leaf and the environment. Under higher magnification, the presence of a nucleus and chloroplasts within the guard cells becomes evident.
- Open and Closed Stomata: Under high magnification, students can observe variations between open and closed stomata. Open stomata are characterized by the gap between the two guard cells, allowing for gas exchange and transpiration. In contrast, closed stomata feature the guard cells lying more closely together, reducing the stomatal pore size and minimizing water loss during periods of low moisture.
- Trichomes (if present): Depending on the leaf type and species, trichomes, which are hair-like structures, may also be visible under the microscope. Trichomes serve various functions, such as reducing water loss, reflecting excess light, and deterring herbivores.
- Vascular Bundles: In some cases, with higher magnification, it may be possible to observe the leaf’s vascular bundles—bundles of xylem and phloem responsible for transporting water, nutrients, and sugars throughout the leaf and the rest of the plant.
- Palisade and Spongy Mesophyll: If the observation is conducted at an even higher magnification, the two distinct layers of the leaf’s mesophyll, namely the palisade and spongy mesophyll, may be discernible. The palisade mesophyll consists of densely packed, vertically arranged cells rich in chloroplasts, while the spongy mesophyll is characterized by loosely arranged cells with air spaces that facilitate gas exchange.
These observations open a window into the remarkable intricacy of leaf structure and its adaptation to the plant’s ecological niche. The presence and arrangement of stomata, guard cells, and various cell types enable plants to carry out photosynthesis, regulate transpiration, and thrive in diverse environments, underscoring the ingenuity of nature’s design.
Leaf Cross Section Under the Microscope
Requirements
- A Sharp Razor: A high-quality, sharp razor is crucial for obtaining a thin and precise cross section of the leaf. A well-honed razor ensures clean cuts, reducing damage to the leaf’s internal structures and enabling clear observation under the microscope.
- A Leaf: Choose a fresh and healthy leaf from a plant of interest. Select a leaf with a desirable size and thickness, as it will directly influence the quality of the cross section.
- Distilled Water: Distilled water serves various purposes during the procedure. It can be used to moisten the leaf’s surface, making it easier to obtain a thin cross section. Additionally, distilled water can be employed to clean the razor between cuts, preventing contamination and ensuring accurate results.
- A Compound Microscope: A compound microscope is indispensable for examining the leaf’s cross section in detail. The microscope’s capability to provide multiple levels of magnification enables researchers to explore different layers and cell structures of the leaf tissue.
- Glass Slides and Cover Slips: Clean and clear glass slides offer a stable platform for mounting the leaf cross section. Cover slips are used to protect the specimen while maintaining a flat surface for optimal observation.
Procedure
- Leaf Selection and Preparation:
- Choose a healthy leaf with desirable thickness and size for the cross-section. Leaves from young and actively growing parts of the plant are preferred.
- Gently rinse the leaf under running water to remove any surface dirt or impurities.
- Rolling the Leaf:
- Carefully roll the leaf along its length. This rolling action helps to create a cylinder-like structure, making it easier to obtain a thin, longitudinal section.
- Obtaining the Cross Section:
- Hold the rolled leaf firmly and use a sharp razor or microtome to cut through the leaf cylinder. Aim to create an extremely thin and almost transparent slice.
- Take your time to ensure precision while cutting to avoid damaging the leaf’s internal structures.
- Placing the Slice on a Microscope Slide:
- Gently lift the obtained thin leaf slice and place it onto a clean microscope glass slide.
- If necessary, use a dropper or pipette to add a small drop of water onto the slide near the leaf section. The water helps to preserve the specimen’s hydration and provides a clear view under the microscope.
- Covering the Slice:
- Optionally, you can place a cover slip gently over the leaf slice to protect it and prevent the specimen from drying out.
- Microscope Observation:
- Place the prepared slide on the microscope stage and secure it in place.
- Begin the observation at low magnification to locate the leaf’s cross-section and the different layers of the leaf’s internal structure.
- Gradually increase the magnification to explore specific cell structures, tissues, and features in greater detail.
- Recording Your Observations:
- Document your observations with detailed notes, recording the observed structures and any notable characteristics.
- If possible, capture images or make sketches to supplement your observations.
Observation
The observation of a leaf’s cross section under the microscope provides a captivating journey into the hidden world of plant anatomy, unveiling the intricate arrangements of cells and tissues that contribute to the leaf’s functionality. Here are the unique observations one can make:
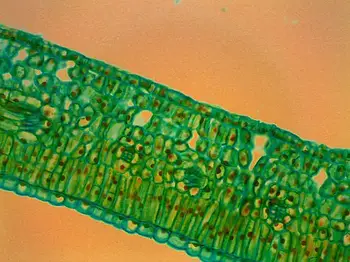
- Upper and Lower Epidermal Cells: At higher magnification, the microscope reveals the upper and lower epidermal cells of the leaf. These cells form the protective outer layer of the leaf, providing a shield against environmental factors and preventing excessive water loss.
- Mesophyll Layer: Sandwiched between the upper and lower epidermis lies the mesophyll layer. This crucial section contains two distinct types of cells: the palisade mesophyll and the spongy mesophyll.
- Palisade Mesophyll: In the mesophyll layer, one can observe elongated and tightly packed cells known as the palisade mesophyll. These cells are vertically arranged and rich in chloroplasts, responsible for conducting a significant portion of photosynthesis within the leaf.
- Spongy Mesophyll: Adjacent to the palisade mesophyll, the spongy mesophyll can be seen as spherical or ovoid cells. These cells are loosely arranged, creating air spaces or intercellular gaps between them. These spaces facilitate gas exchange, allowing for efficient carbon dioxide uptake and oxygen release during photosynthesis.
- Air Spaces: Within the spongy mesophyll, the presence of air spaces is evident. These spaces connect with stomata, tiny pores found on the leaf surface, enabling the exchange of gases between the internal leaf tissues and the external environment.
- Vascular Bundles (Veins): Depending on the quality of the cross section and the level of magnification, it may be possible to observe the leaf’s vascular bundles, commonly referred to as veins. These bundles consist of xylem and phloem tissues responsible for transporting water, nutrients, and sugars throughout the leaf and the rest of the plant.
- Cuticle: Although not visible in all cross sections, it is possible to observe the cuticle, a waxy layer covering the epidermal cells. The cuticle plays a crucial role in reducing water loss and protecting the leaf from pathogens and environmental stress.
Leaf Structure Under the Microscope Video
FAQ
What is leaf structure under the microscope?
Observing leaf structure under the microscope involves examining the intricate cellular arrangements, specialized tissues, and features within a leaf’s internal organization at various magnifications.
Why is it important to study leaf structure under the microscope?
Studying leaf structure provides valuable insights into plant anatomy, adaptations, and ecological roles. It helps understand how leaves perform essential functions like photosynthesis, gas exchange, and transpiration.
What are the primary components observed in a leaf cross section?
In a leaf cross section, you will typically observe the upper and lower epidermal cells, the mesophyll layer (comprising palisade and spongy mesophyll), and the vascular bundles (veins).
What is the difference between palisade mesophyll and spongy mesophyll?
Palisade mesophyll consists of elongated cells packed with chloroplasts responsible for most photosynthesis. Spongy mesophyll contains loosely arranged cells with air spaces for efficient gas exchange.
How are stomata observed under the microscope?
Stomata, the leaf’s tiny pores, can be observed in the epidermal layer as bean-shaped structures. They appear denser and darker under the microscope due to their unique morphology.
What is the role of the epidermal layer in a leaf?
The epidermal layer serves as a protective outer covering for the leaf, preventing water loss and providing defense against external environmental factors.
Why do leaves have air spaces in the spongy mesophyll?
Air spaces in the spongy mesophyll facilitate the diffusion of gases, enabling efficient exchange of carbon dioxide and oxygen necessary for photosynthesis and respiration.
How do vascular bundles (veins) contribute to leaf function?
Vascular bundles, composed of xylem and phloem tissues, transport water, nutrients, and sugars throughout the leaf and the rest of the plant, supporting various metabolic processes.
What techniques are used to prepare a leaf for microscopic observation?
Techniques like peeling the epidermal layer, obtaining cross sections with a razor, and staining with safranin can be used to prepare leaf specimens for microscope observation.
What can be learned from observing leaf structure under the microscope?
Observing leaf structure allows us to understand the leaf’s cellular and tissue adaptations, enabling photosynthesis, optimizing gas exchange, and maintaining water balance, which collectively contribute to the plant’s survival and growth.