- Alexander Fleming’s 1928 publication on penicillin is a landmark in the history of medicine. It was anticipated that as more antimicrobial compounds were found, infectious diseases would be eradicated by the use of these antimicrobials.
- Unfortunately, the rapid emergence of bacterial resistance to these antimicrobials quickly dampened this optimism and necessitated that physicians request the microbiology lab to test a patient’s pathogen against various concentrations of a particular antimicrobial to determine susceptibility or resistance to that drug.
- The original approach for assessing antimicrobial susceptibility was based on broth dilution methods , which remain the gold standard but are time-intensive to perform. This led to the creation of a disc diffusion method for determining bacterial sensitivity to antimicrobials.
- The majority of clinical microbiology laboratories in the United States had adopted the disc diffusion method for assessing bacterial sensitivity to antimicrobials by the beginning of the 1950s.
- Each laboratory changed the technique to meet its own requirements, including the use of various media, inoculum concentration, incubation period, incubation temperature, and antimicrobial drug concentration.
- Interpretation of susceptibility and resistance was solely dependent on the presence or absence of an inhibitory zone surrounding the disc, and two or three concentrations of the same antibiotic were frequently tested against the pathogen.
- Many researchers published variants of the approach, which resulted in various protocols and significant confusion. In 1956, W. M. M. Kirby and his colleagues at the University of Washington School of Medicine and King County Hospital established a single-disk approach for determining antibiotic susceptibility.
- Throughout the early 1960s, the lack of standards for determining bacterial susceptibility remained a challenge. Kirby and his colleague A. W. Bauer thoroughly evaluated the literature on susceptibility testing.
- They combined and revised all existing disc diffusion method descriptions and published their findings. This report prompted the World Health Organization to organise a committee in 1961 to lay the framework for the development of an uniform approach for assessing the susceptibility of a single antimicrobial disc.
- As a result, a standardised protocol for the disc diffusion susceptibility test, now known as the Kirby-Bauer disc diffusion test, was developed. Through a global consensus approach, the Clinical Laboratory Standards Institute (CLSI) is currently responsible for updating and amending the original Kirby and Bauer technique.
- This maintains procedure homogeneity and repeatability as bacteria acquire novel mechanisms of resistance and new antimicrobials are discovered to combat them.
- Their papers include interpretative recommendations regarding zone sizes. The CLSI document, Performance Standards for Antimicrobial Disk Susceptibility Tests; Approved Standard, Ninth Edition, is the current standard for susceptibility testing in clinical laboratories.
What is Kirby Bauer Disk Diffusion test?
The Kirby-Bauer disk diffusion test is a laboratory method used to determine the effectiveness of antibiotics against bacterial strains. The test is named after its inventors, Drs. Paul Kirby and Robert Bauer, who developed it in the 1960s.
In the Kirby-Bauer test, a bacterial culture is spread evenly over a solid growth medium, such as agar. Small paper disks containing different antibiotics are placed on the surface of the agar, and the plates are then incubated to allow bacterial growth. If an antibiotic is effective against the bacterial strain, it will create a zone of inhibition, a circular area around the disk where the bacteria do not grow because the antibiotic has diffused into the agar and inhibited their growth.
The size of the zone of inhibition is measured and compared to established interpretive criteria to determine the susceptibility or resistance of the bacteria to the antibiotic being tested. These interpretive criteria are established by regulatory agencies and professional organizations based on clinical data and laboratory standards.
The Kirby-Bauer test is a widely used method for antibiotic susceptibility testing because it is easy to perform and provides rapid results. However, it has limitations and may not accurately predict the clinical effectiveness of antibiotics in all cases. Therefore, the results of the Kirby-Bauer test should always be interpreted in conjunction with other laboratory and clinical information.
Purpose of Kirby Bauer Disk Diffusion test
The goal of the Kirby-Bauer disc diffusion susceptibility test is to detect the sensitivity or resistance of pathogenic aerobic and facultative anaerobic bacteria to various antimicrobial drugs in order to aid a physician in selecting appropriate treatment choices for a patient. The pathogenic bacterium is cultured on Mueller-Hinton agar with antimicrobial-impregnated filter paper discs present. The presence or absence of growth around the discs is a proxy for the compound’s capacity to suppress the organism.
Principle of Kirby Bauer Disk Diffusion test
The determination of bacterial resistance to antimicrobials is an integral aspect of the treatment of patient infections. The disc diffusion method developed by Kirby and Bauer has been standardised and is a viable alternative to broth dilution methods for laboratories who lack the resources to implement the more recent automated methods for broth microdilution testing.
When a 6-mm filter paper disc impregnated with a known concentration of an antimicrobial agent is placed on a Mueller-Hinton (MH) agar plate, water from the agar is rapidly absorbed by the disc. The antibiotic starts to disperse into the agar that surrounds it. Since the rate of diffusion through the agar is slower than the rate of antimicrobial extraction from the disc, the concentration of antimicrobial is highest closest to the disc and decreases logarithmically as the distance from the disc increases.
The rate of diffusion of the antibiotic through the agar is dependent on the drug’s diffusion and solubility properties in MH agar as well as its molecular weight. Compounds with a lower molecular weight diffuse at a faster rate than those with a higher molecular weight. The combination of these elements gives each antibiotic a distinct breakpoint zone size that indicates susceptibility to that antimicrobial agent. Prior to placing discs on the agar surface, if the agar plate has been infected with a suspension of the pathogen to be tested, growth of the bacteria and diffusion of the antimicrobial chemicals occur simultaneously.
In the presence of an antimicrobial agent, growth occurs when bacteria achieve a critical mass and are able to overcome the inhibitory effects of the antimicrobial agent. For most commonly recovered pathogens, the predicted time for a bacterial suspension to achieve critical mass ranges from 4 to 10 hours, depending on the species, the medium, and the incubation temperature.
Since the antimicrobial agent diffuses in three dimensions, the depth of the agar layer influences the extent of the zone of growth inhibition; therefore, a thin layer of agar will create a greater zone of inhibition than a thicker layer. A sharply-margined circle of bacterial growth surrounding the disc indicates the point at which critical mass is attained. The concentration of antimicrobial compound at this margin is referred to as the critical concentration and is roughly equivalent to the minimal inhibitory concentration determined by broth dilution susceptibility testing. Zone size measured in a disc diffusion test has no inherent significance.
Via in vivo testing of blood and urine, the interpretation of antimicrobial resistance and susceptibility is determined in order to calculate the achievable concentration of a certain antimicrobial that results in the clearance of an illness. This data is connected with zone sizes to produce interpretive standards. Clinical Laboratory Standards Institute Performance Standards for Antimicrobial Disk Susceptibility Tests: Approved Standards, Ninth Edition, contains the latest interpretation requirements.
Requirement
- Mueller- Hinton agar
- Antibiotic discs
- Cotton swabs
- Petri dishes
- 0.5 McFarland Turbidity standard
- Inoculum
- Forceps
- Metric ruler or caliper
As the zone of inhibition for these organisms is known, Staphylococcus aureus ATCC 25923 (Biosafety level (BSL) 2), Escherichia coli ATCC 25922 (BSL 1), and Pseudomonas aeruginosa ATCC 27853 (BSL 2) are recommended for quality assurance purposes. Due to the fact that the zone sizes for these creatures are known, they are suggested for usage in educational settings, although the use of unknowns should also be integrated. The zone sizes for these three species for quality control testing can be found on the package insert of any antimicrobial disc you purchase.
Antimicrobials are chosen based on the type of organism being examined and the origin of the isolate (blood, urine, wound, etc.). See the Interpretative Standards Tables for a list of recommended antimicrobials for this exercise.
Mueller-Hinton agar
For the following reasons, MH agar is regarded the ideal medium for regular susceptibility testing of nonsusceptible bacteria:
- Reproducibility from batch to batch is acceptable for susceptibility testing.
- It contains a low concentration of sulfonamide, trimethoprim, and tetracycline inhibitors.
- It supports adequate growth of the majority of nonselective pathogens.
- Many data and years of expertise have been gathered on susceptibility testing conducted with this media.
Please be aware that using media other than Mueller-Hinton agar may produce inaccurate results. This approach should only be used to evaluate aerobic or facultative bacteria that grow well on MH agar without supplementation. For fussy organisms, MH agar must be supplemented with additional nutrients, and this technique must be modified accordingly. In this basic procedure, neither the supplements nor the procedural changes are described. As prepared agar plates, MH agar can be acquired from Remel (Lenexa, KS), BD BBL (Franklin Lakes, NJ), or any other supplier of prepared agar plates. Follow the manufacturer’s storage instructions for prepared plates. Dehydrated media from firms like as Remel, BD BBL, or any other source of dehydrated media can also be used to produce MH agar. Prepare the medium in accordance with the manufacturer’s instructions.
Formula for Mueller-Hinton agar per liter of purified water
| Beef, Infusion from | 300.0 g |
| Casamino acid, technical | 17.5 g |
| Starch | 1.5 g |
| Agar | 17.0 g |
Mueller-Hinton agar Preparation Procedure
- Suspend the aforementioned ingredients in 1 litre of filtered water.
- Blend thoroughly The ingredients must be heated with constant stirring and brought to a boil for one minute to completely dissolve.
- Autoclave for 15 minutes at 121°C. Dispense as desired. Let to solidify at room temperature, then store between 4 and 8 degrees Celsius.
- From the date of preparation, Mueller-Hinton agar is stable for approximately 70 days.
- When the expiration date of 70 days approaches, each laboratory should test known strains of microorganisms against each antimicrobial agent being employed to confirm the purity and functionality of each batch of produced media.
More Information About Mueller-Hinton agar
- If the MH agar plates are prepared from dehydrated medium, they must be poured to a depth of 4 mm (approximately 25 ml of liquid agar for 100-mm plates and 60 ml of liquid agar for 150-mm plates, but in any case to a measured depth of 4 mm). Plates with insufficient depth will yield erroneous findings for susceptibility, since the antimicrobial agent will diffuse deeper than it should, resulting in bigger zones of inhibition. In contrast, plates poured to a depth more than 4 mm will produce falsely resistant results.
- After solidification, the pH of MH agar should fall between 7.2 and 7.4 at room temperature and should be measured when the medium is first made. If the pH is less than 7.2, certain medications (aminoglycosides, quinolones, macrolides) will appear to lose potency, while others may appear to have excessive action (tetracycline). If the pH is greater than 7.4, the reverse effect may occur.
- Excessive thymidine or thymine can reverse the inhibitory effects of sulfonamides and trimethoprim, resulting in zones of inhibition that are smaller and less defined, or none at all.
- The findings of aminoglycoside and tetracycline tests against Pseudomonas aeruginosa will be impacted by the wrong concentration of divalent cations (calcium and magnesium). A high concentration of cations will lower zone sizes, whereas a low concentration will increase zone sizes. Calcium excess will enhance the size of P. aeruginosa’s resistance zone to daptomycin. Excess zinc ions may diminish the carbapenems’ zone of inhibition against P. aeruginosa.
- At least weekly, MH agar should be checked with recognised organism strains to ensure that the medium and discs are functioning as planned.
Antibiotic susceptibility disks
- Antimicrobial discs are available from all respectable vendors, including Remel and BD BBL. They come in spring-loaded cartridges containing 25 or 50 discs and can be ordered individually or in quantities of 10 cartridges.
- For results to be reproducible, proper storage of these drives is necessary. Cartridges containing commercially made paper discs should be stored at 8°C or frozen at -14°C in a freezer that does not thaw automatically.
- Let the discs to reach room temperature before removing the protective wrapping. Once the cartridges have been opened, they should be stored in a container containing desiccant for no more than one week.
- Semiautomatic disc dispensers are provided from Remel and BD BBL, among others. Be cautious that disc cartridges from one company may not be compatible with those from another.
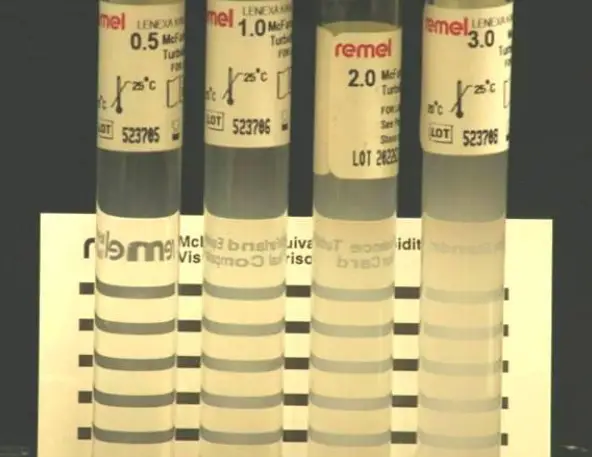
McFarland standard
McFarland standards are suspensions of barium sulphate or latex particles used to visually compare bacterial density (Fig. 1). Companies such as Remel or BD BBL offer commercially prepared standards for purchase. They frequently contain a Wickerham card, a little card with parallel black lines. A 0.5 McFarland standard corresponds to an E. coli suspension containing between 1 x 108 and 2 x 108 CFU/ml.
In-house preparation of a 0.5 McFarland standard is possible as described below.
- With continual stirring, add a 0.5-ml aliquot of 0.048 mol/liter BaCl2 (1.175% wt/vol BaCl2 • 2H20) to 99.5 ml of 0.18 mol/liter H2SO4 (1% vol/vol).
- Check the correct density of the turbidity standard by measuring absorbance with a spectrophotometer that features a 1-cm light path and a matched cuvette. For the 0.5 McFarland standard, the absorbance at 625 nm must be between 0.08 and 0.13.
- Put 4- to 6-ml aliquots of the barium sulphate suspension into screw-cap tubes of the same size as those used to standardise the bacterial inoculums.
- Close the tubes tightly and store them in the dark at room temperature.
Use of the McFarland standard in the Kirby-Bauer procedure.
- Prior to use, vigorously stir the barium sulphate standard using a mechanical vortex mixer and examine for a consistently murky appearance. Change the standard if there are big particles. If you are utilising a latex-based standard, do not use a vortex mixer; instead, mix by inverting the standard gently.
- During the “preparation of the inoculum” stage, as the student adds bacterial colonies to the saline, he or she should compare the resulting suspension to the McFarland standard. This is accomplished by comparing the appearance of the lines through both suspensions while holding the standard tube and the inoculum tube side-by-side and no more than 1 inch from the face of the Wickerham card (with enough light present). The tubes should not be held flush against the card. If the bacterial suspension appears lighter than the 0.5 McFarland standard, additional organisms from the culture plate should be added to the test tube. If the suspension appears denser than the 0.5 McFarland standard, extra saline must be introduced to the inoculum tube to dilute the solution to the correct density. In some instances, it may be simpler to start over than to continue diluting an unusable bacterial suspension.
Protocol / Procedure of Kirby Bauer Disk Diffusion Susceptibility Test
Preparation of Mueller-Hinton plate
- Let an MH agar plate (one per organism to be examined) to reach room temperature. To prevent condensation, it is better to keep the plates in the plastic sleeve while they warm.
- If liquid is visible on the surface of the agar, invert the plate and leave the lid slightly ajar to let the excess liquid to drain and evaporate. Plates may be dried at 35°C in an incubator or at ambient temperature in a laminar flow hood (usually 10 to 30 minutes).
- Label each MH agar plate for each organism to be examined appropriately.
Preparation of inoculum
- Touch four or five isolated colonies of the organism being tested with a sterile inoculating loop or needle.
- The organism is suspended in 2 ml of sterile saline.
- Create a smooth suspension by vortexing the saline tube.
- Modify the turbidity of this suspension to meet the 0.5 McFarland criterion by adding more organisms if the solution is too clear or by diluting it with sterile saline if it is too opaque.
- Within 15 minutes of preparation, use this suspension.
Inoculum preparation
Test organisms must be in the log phase of growth for results to be considered legitimate. It is suggested that subcultures of the organisms to be tested be established the day before. Never employ excessive inoculum densities. Never inoculate plates with undiluted overnight broth cultures or any non-standardized inocula. If the organism is difficult to directly suspend into a smooth suspension, the growth method should be utilised to prepare the inoculums. Nonetheless, the organisms advised in this process all yield suspensions with minimal trouble that are smooth. If necessary, consult the Clinical Laboratory Standards Institute publication for the inoculum growth technique method.
Inoculation of the MH plate
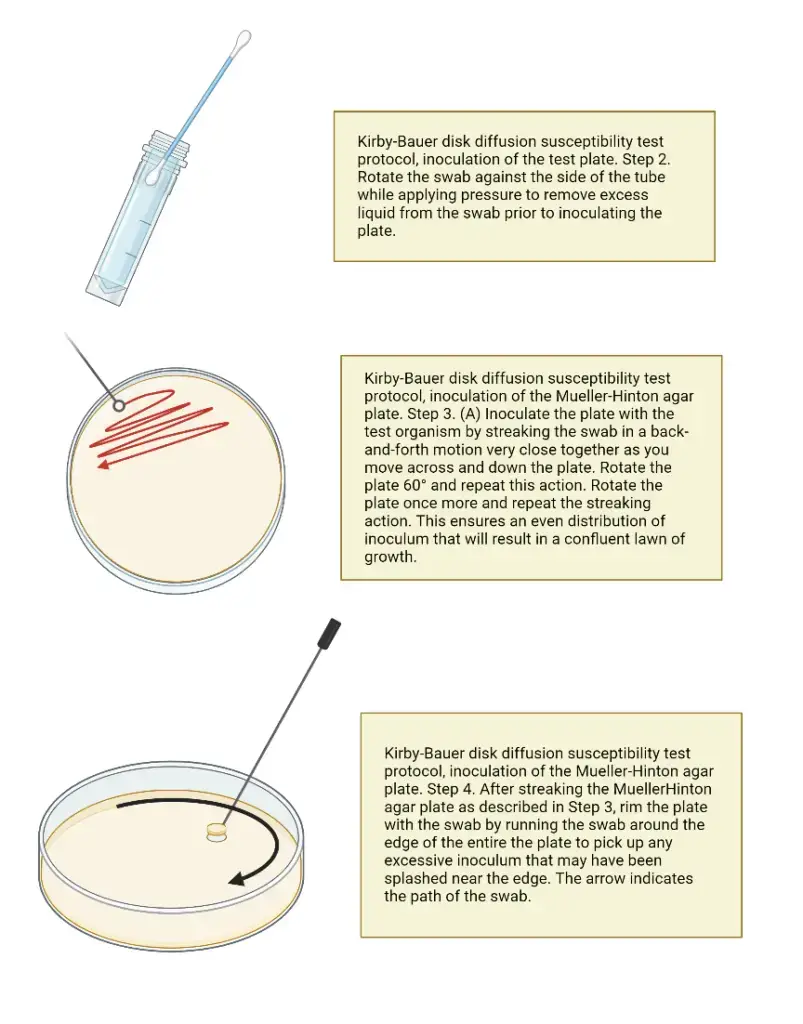
- Place a sterile cotton swab in the inoculum tube.
- To remove excess fluid, rotate the swab against the tube’s side (above the fluid level) while applying firm pressure. The swab should not be saturated with water.
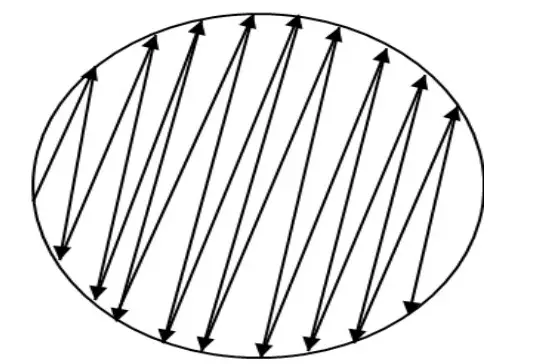
- Inoculate the dried surface of an MH agar plate by streaking the swab three times across the entire agar surface; rotate the plate about 60 degrees between each application to ensure a uniform distribution of the inoculum.
- Apply the swab to the plate’s rim to absorb any surplus liquid.
- Dispose of the swab in a suitable container.
- Let the surface of the agar plate to dry at room temperature for at least 3 to 5 minutes, but no longer than 15 minutes, with the lid slightly ajar, before moving to the next step.
Placement of the antibiotic disks
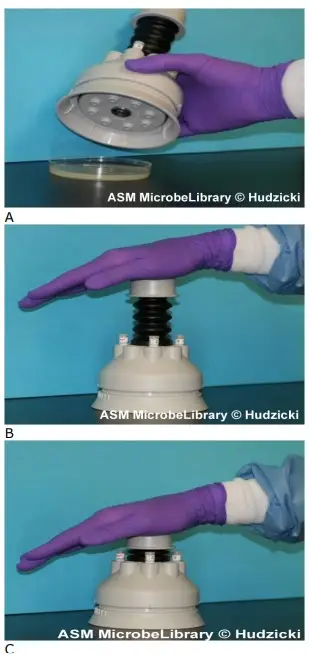
- Put the appropriate antimicrobial-impregnated discs on the surface of the agar using either forceps or a multidisk dispenser to dispense each disc individually. (See steps a-d for the use of the multi-disk dispenser or steps e-g for the positioning of individual discs using forceps.
- To utilise a multidisk dispenser, place the inoculated MH agar plate on a level surface with the lid removed.
- Position the dispenser over the agar plate and firmly depress the plunger once to dispense the discs onto the plate’s surface.
- Remove the dispenser from the plate and, using forceps that have been cleaned with an alcohol pad or flambéed with isopropyl alcohol, touch each disc to the agar surface to ensure complete contact. This should be completed prior to replacing the lid of the petri dish, as static electricity may cause the discs to move on the agar surface or cling to the top.
- Do not relocate a disc once it has made contact with the agar surface, even if it is not in the correct spot, because some of the medicine begins diffusing instantly upon contact with the agar.
- To put discs one by one on the agar plate using forceps, position the MH plate on the given template. Clean the forceps with a sterile alcohol pad and allow them to dry naturally, or immerse the forceps in alcohol and ignite them.
- Carefully remove one disc from the cartridge using the forceps.
- Remove a portion of the lid from the petri dish. Put the disc on the plate over one of the dark spots on the template and gently press the disc with the forceps to ensure that it is in full contact with the agar surface. Replace the cover to minimise the agar surface’s exposure to room air.
- Continue placing one disc at a time onto the agar surface, as instructed in stages f and g above, until all discs have been placed.
- Once all discs are in place, replace the lid, invert the plates, and place them in an incubator with air at 35 degrees Celsius for 16 to 18 hours. Incubate Staphylococcus against oxacillin or vancomycin or Enterococcus against vancomycin for 24 hours prior to reading.
Disk placement
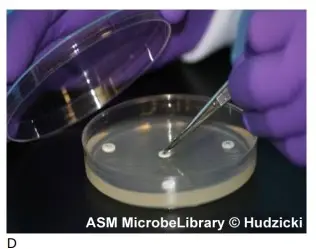
Not closer than 24 mm (centre to centre) should discs be placed on the MH agar plate. No more than 12 discs should be placed on a 150-mm plate, and no more than 5 discs should be placed on a 100-mm plate. The semiautomatic disc dispensers, which store 16 and 8 discs, respectively, may not maintain the recommended 24 mm center-to-center spacing. The template supplied in this approach maintains the recommended 24 mm center-to-center spacing and permits up to eight discs to be placed on the plate. Avoid positioning discs close to the plate’s edge, as the resulting zones may not be perfectly round and will be difficult to measure. Each disc must be pushed with forceps to ensure full contact with the agar surface; otherwise, uneven zone forms may result. If the surface of the agar is disturbed in any manner (disc penetrating the surface, visible lines owing to excessive pressure during inoculation, etc.), the zone’s shape may be altered. Before printing the template for use in your microbiology lab, ensure that the circle’s diameter corresponds to the size of the Mueller-Hinton agar plates you use (100 mm). If necessary, the “reduce” or “enlarge” feature on a photocopier can be used to alter the template’s size. You can also create your own template by tracing a circle around an MH agar plate on a piece of paper. Add the placement marks based on the number of discs you intend to utilise during your lab session, maintaining the recommended distance as outlined in the preceding section.
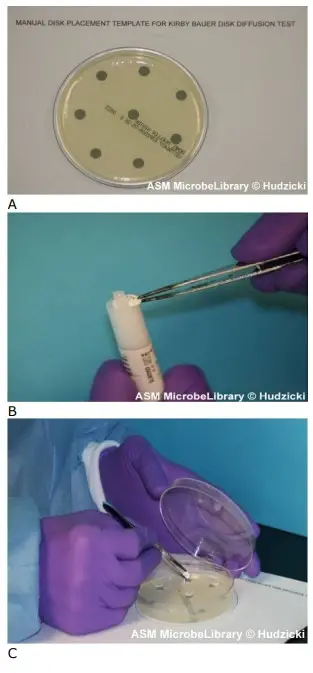
Incubation of the plates
The required temperature range is 35°C 2°C. Remember that at temperatures above 35 degrees Celsius, methicillin-resistant Staphylococcus may not be detectable. Plates should not be incubated in CO2, since this will decrease the pH of the agar and cause errors due to the improper pH of the medium.
Results are available after 18 hours of incubation, unless you are testing Staphylococcus or Enterococcus against oxacillin or vancomycin, or against vancomycin. Before reporting vancomycin or oxacillin, read the findings for the other antimicrobial discs and re-incubate the plate for a total of 24 hours.
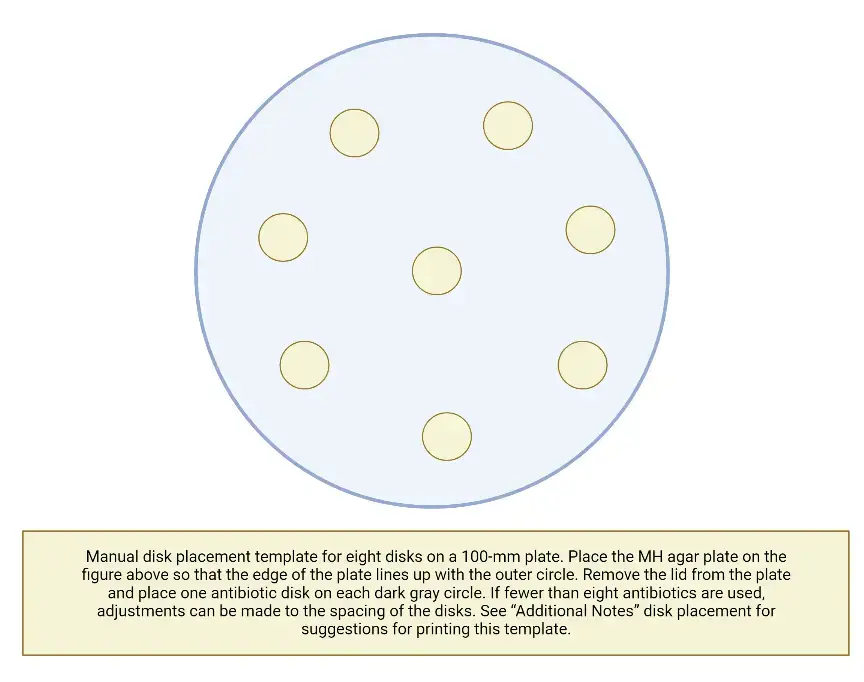
- Using a ruler or calliper, measure the zone sizes to the closest millimetre following incubation; include the disk’s diameter in the measurement.
- Always round up when measuring zone diameters to the nearest millimetre.
- The measurements are taken visually while observing the back of the petri dish. Hold the plate a few inches above a black, nonreflective surface that is lighted by reflected light.
- See the plate with a direct, vertical line of sight to eliminate parallax, which could lead to misreading.
- Note the zone size on the recording sheet.
- If the positioning of the disc or the size of the zone prevents you from reading the zone’s diameter, measure from the centre of the disc to a point on the zone’s circumference where a definite edge is present (the radius) and multiply that measurement by 2 to calculate the zone’s diameter.
- Up to the edge of the disc, growth is indicated as a 0 mm zone.
- Organisms that swarm, such as Proteus mirabilis, must be measured differently than species that do not swarm. Disregard the thin veil of swarming and measure the outside edge of a zone of inhibition that is otherwise evident.
- Separate, distinct colonies inside a blatant inhibitory zone should not be deemed swarming. These colonies are either mutant organisms that are more resistant to the tested treatment, or the culture was not pure and they represent a distinct organism. If repeated testing confirms that the phenomenon persists, the organism must be considered resistant to the medicine.
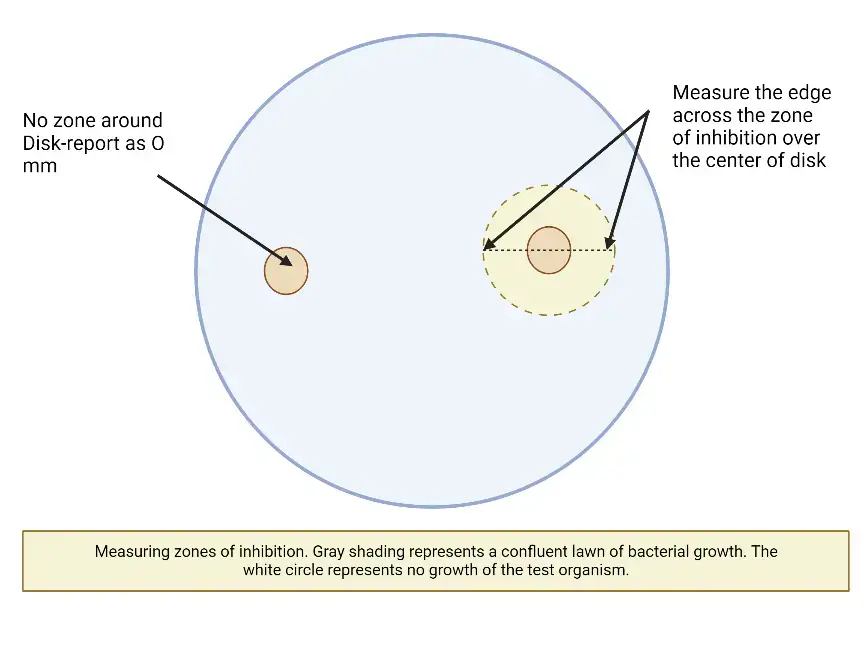
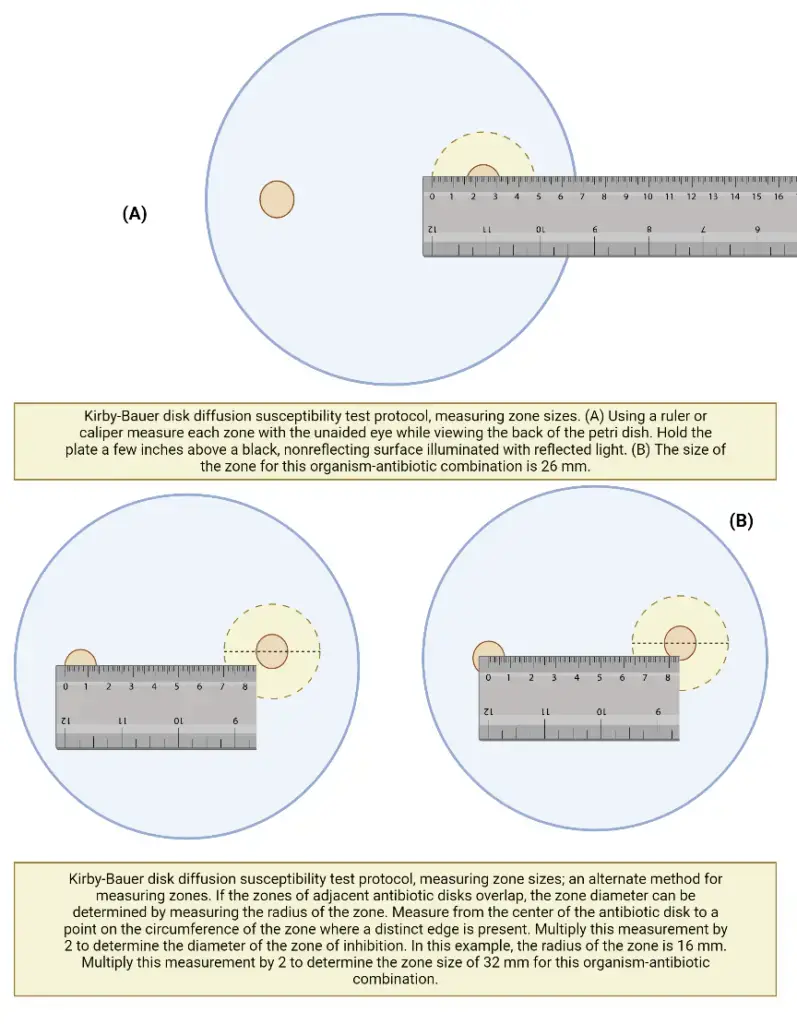
Measuring zone sizes
- If the plate was correctly infected and all other requirements are met, the zones of inhibition should be evenly circular, and a confluent growing lawn should be observed.
- If distinct colonies are visible across the entire plate, the inoculum was insufficient and the test must be redone.
- The zone edge should be regarded as the area where there is no visible growth that can be recognised with the naked eye. Do not use a device with magnification to see zone edges.
- When measuring the zone of inhibition for swarming organisms (e.g., Proteus spp. ), disregard the thin veil of swarming development in a zone of inhibition that is otherwise visible.
- With trimethoprim and the sulfonamides, antagonists in the medium may permit little growth; thus, ignore slight growth (20% or less of the lawn of growth) and measure the more visible margin to calculate the zone diameter.
Interpretation and Reporting of the Results
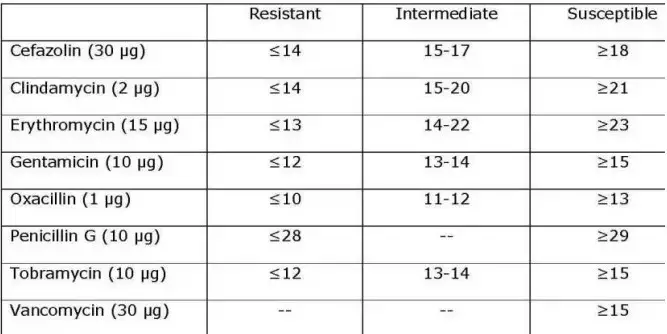
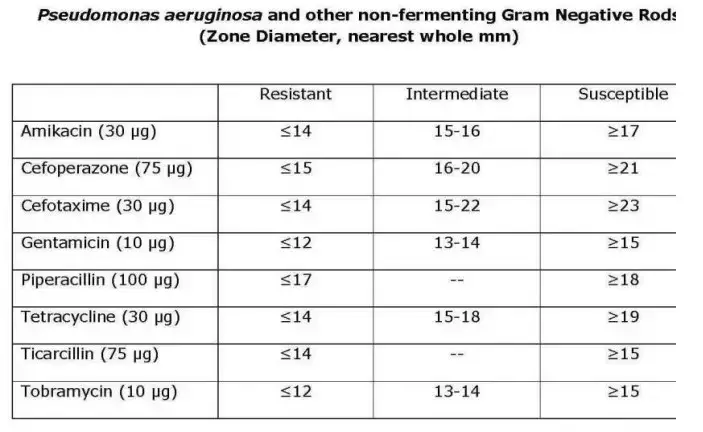
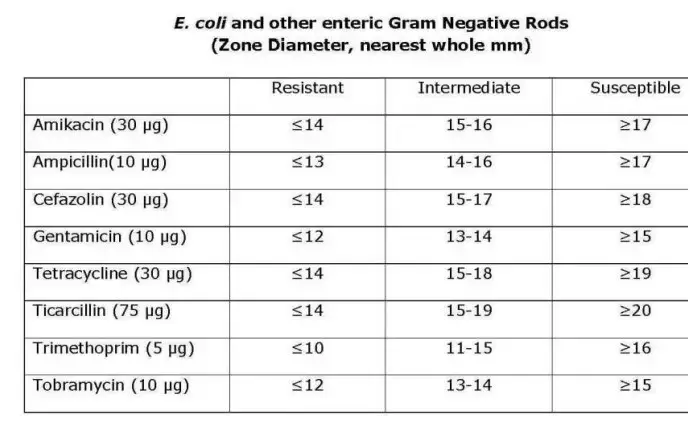
- Determine the sensitivity or resistance of the organism to each drug tested using the established CLSI criteria. Notice that various creatures use different charts. The following are abbreviated charts relevant to the species and antimicrobial discs recommended by the author.
- Using the interpretation chart, note on the recording sheet whether the zone size is susceptible (S), intermediate (I), or resistant (R) for each drug.
- The susceptibility results of the Kirby-Bauer disc diffusion test are solely reported as susceptible, intermediate, or resistant. Physicians are not informed of zone sizes.
Recommended Antimicrobial Disks and Interpretative Zone Sizes
This battery of discs is recommended for use in an educational setting since it reduces the number of antimicrobials required to test all three types of organisms. Educators may select additional medications from the CLSI charts based on the circumstances.
Advantages
- This test is used to determine the optimal antibiotics for treating an infection.
- It is useful for monitoring antimicrobials and selecting the most effective antibacterial agents.
- It can be performed without specialised equipment and interpreted by all medical personnel.
- This test is less expensive to do.
Limitations
- Not all sluggish or fussy creatures can be accurately tested.
- According to CLSI guidelines, it is not considered a gold standard test because it is only used for screening the susceptibility pattern of the organisms.
Applications of Kirby Bauer Disk Diffusion Susceptibility Test
The Kirby-Bauer disk diffusion susceptibility test is widely used in clinical microbiology for the following applications:
- Selection of appropriate antibiotics: The test helps in selecting the most appropriate antibiotic treatment for bacterial infections. It provides information about the susceptibility or resistance of bacterial strains to various antibiotics, which can guide physicians in selecting the most effective treatment.
- Monitoring antibiotic resistance: The test is used to monitor the emergence and spread of antibiotic-resistant bacterial strains. By testing bacterial isolates from different patients, the test can help identify the prevalence and distribution of antibiotic-resistant strains in a population.
- Quality control of antibiotic production: The test is used to ensure the quality of antibiotic products. It can be used to test the potency and purity of antibiotics produced by pharmaceutical companies to ensure they meet regulatory standards.
- Research: The test is used in research to study the mechanisms of antibiotic resistance and to develop new antibiotics. The test can help identify new compounds with antimicrobial activity and determine their efficacy against bacterial strains.
Overall, the Kirby-Bauer disk diffusion susceptibility test is a valuable tool in the diagnosis and management of bacterial infections. It provides important information about the susceptibility of bacterial strains to antibiotics and helps guide the selection of appropriate treatment.
FAQ
What is Kirby Bauer Disk Diffusion Susceptibility Test?
The Kirby Bauer Disk Diffusion Susceptibility Test is a laboratory test used to determine the effectiveness of antibiotics against bacterial strains.
How is the test performed?
In this test, paper disks containing different antibiotics are placed on a bacterial culture spread over a solid growth medium. The plates are then incubated to allow bacterial growth. If an antibiotic is effective against the bacterial strain, it will create a zone of inhibition, a circular area around the disk where the bacteria do not grow because the antibiotic has diffused into the agar and inhibited their growth.
What is the purpose of the test?
The purpose of the test is to determine the susceptibility or resistance of bacterial strains to different antibiotics. This helps guide physicians in selecting the most appropriate antibiotic treatment for bacterial infections.
What are the limitations of the test?
The test has limitations and may not accurately predict the clinical effectiveness of antibiotics in all cases. Therefore, the results of the Kirby-Bauer test should always be interpreted in conjunction with other laboratory and clinical information.
What are the factors that can affect the test results?
Factors that can affect the test results include the age of the bacterial culture, the type and concentration of the antibiotic, the size of the disk, and the thickness of the agar medium.
How is the test interpreted?
The size of the zone of inhibition is measured and compared to established interpretive criteria to determine the susceptibility or resistance of the bacteria to the antibiotic being tested.
How long does it take to perform the test?
The test typically takes 24 to 48 hours to perform.
What type of bacterial strains can be tested with the Kirby Bauer test?
The Kirby-Bauer test can be used to test a wide range of bacterial strains, including gram-positive and gram-negative bacteria.
How is the test used in antibiotic development?
The Kirby-Bauer test is used in antibiotic development to identify new compounds with antimicrobial activity and determine their efficacy against bacterial strains.
How does the test contribute to the fight against antibiotic resistance?
The test is used to monitor the emergence and spread of antibiotic-resistant bacterial strains, which helps guide the development of new antibiotics and the selection of appropriate antibiotic treatments.