Iron-Hematoxylin Staining is a permanent staining method that is used for demonstrating different intracellular and nuclear structures of protozoans. It is the process in which hematoxylin is oxidized into hematein and then combined with ferric iron to form a dark ferric-lake complex that binds strongly with nuclear materials.
The staining gives a sharp contrast where the nucleus and other internal structures appear dark, while the cytoplasm appears lighter, so the identification of parasite morphologies becomes easy. It is the traditional stain that was used for most of the original descriptions of human intestinal protozoa, and it is still used because of its clarity and the permanence of the stained smears. This method is mostly regressive in nature, where the smear is overstained first and then differentiated to reveal the required structures.
It can stain fresh specimens, SAF-preserved specimens, or PVA-preserved specimens. It is the technique that is also used in histological studies for demonstrating fine structures like mitochondria, chromosomes, and muscle striations, but in diagnostic parasitology it is mainly used because it clearly shows the nuclear features of protozoans which is the major requirement for accurate identification.
Principle of Iron-Hematoxylin Staining
The principle of Iron-Hematoxylin staining is based on the interaction between oxidized hematoxylin and ferric iron which together form a stable iron-hematoxylin lake. It is the process in which hematoxylin is first converted into its active form hematein, and this oxidation is carried out rapidly because the ferric salts act as strong oxidizing agents. The ferric iron (Fe³⁺) also acts as the mordant, so it binds with the hematein to produce a dark dye-metal complex that attaches firmly to nuclear structures.
This complex behaves as a basic dye and gives an intense black coloration to the nucleus and other intracellular components, while the cytoplasm appears lighter. It is a regressive staining technique where the tissue is overstained at first and then differentiated carefully so that excess stain is removed while the essential nuclear detail remains. This strong ferric lake is highly resistant to acidic solutions, and it is the reason why iron-hematoxylin is used as a nuclear anchor when procedures involve acid reagents.
Purposes of Iron-Hematoxylin Stain
Iron-Hematoxylin Staining is used to study the morphology of protozoal parasites and for the identification of intestinal parasites from a given stool sample.
Requirements Iron-Hematoxylin Staining
- Hematoxylin dye source (extracted from logwood and later oxidized to hematein).
- Ferric iron mordant (ferric chloride or ferric ammonium sulfate) which also act as the oxidizing agent.
- Regressive staining step where sections are overstained before differentiation.
- Differentiation solution such as weak acid-alcohol or excess iron mordant depending on the method.
- Preparation steps based on the staining variant like mixing two solutions freshly in Weigert’s method or prior mordanting in Heidenhain’s method.
- Thin tissue sections when high cytological details are required.
- Washing or blueing step in water or weak alkaline solution.
- Properly fixed smear for protozoan study which may be fresh, PVA-preserved, or SAF-preserved.
Procedure of Iron-Hematoxylin Staining
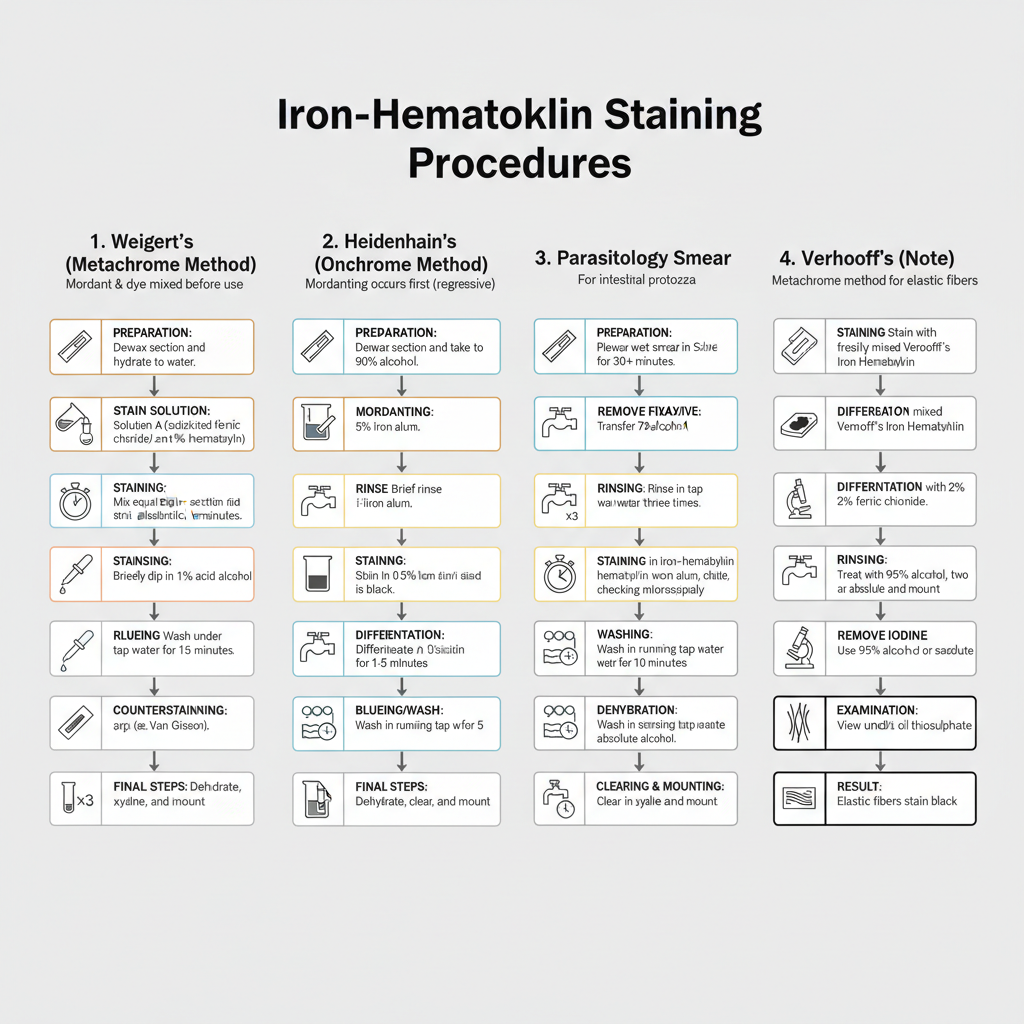
1. Weigert’s Iron Hematoxylin (Metachrome Method)
This method is used when an acidic counterstain is applied after the nuclear staining. It is the process where the dye and mordant are mixed just before staining.
Steps
- Preparation– The paraffin section is dewaxed and hydrated to water.
- Stain Solution– Equal volumes of Solution A (acidified ferric chloride) and Solution B (1% alcoholic hematoxylin) is mixed. This mixture is prepared fresh.
- Staining– The section is placed in this staining mixture for 20–30 minutes. Sometimes a longer period (1–2 hours) is needed depending on tissue.
- Rinsing– The slide is rinsed in water.
- Differentiation– It is dipped briefly in 1% acid alcohol (HCl in 70% ethanol). This removes excess stain.
- Blueing– The section is washed under tap water for about 10 minutes. It stabilizes the iron–hematoxylin complex.
- Counterstaining– The counterstain is added depending on the demonstration needed (Van Gieson, trichrome etc.).
- Final Steps– The section is dehydrated with alcohol, cleared in xylene, and mounted.
2. Heidenhain’s Iron Hematoxylin (Onchrome Method)
This is referred to as a regressive staining where the mordanting occurs first. It is used for studying fine intracellular details.
Steps
- Preparation– Dewax the section and take it to 90% alcohol through absolute alcohol.
- Mordanting– The section is kept in 5% iron alum (ferric ammonium sulfate). It is done for 30–45 minutes at 60°C or 12–24 hours at room temperature.
- Rinse– A brief rinse in water.
- Staining– It is stained in 0.5% hematoxylin for the same duration as mordanting. The tissue becomes jet black.
- Rinse– Rinsed briefly in water.
- Differentiation– Differentiation is done in 2–5% iron alum solution and checked microscopically. Saturated alcoholic picric acid diluted 2:3 (6%) is also used. This gives slower and more controlled differentiation.
- Blueing/Wash– The section is washed well in running water for about 5 minutes to remove iron alum.
- Final Steps– Dehydration, clearing, and mounting is done.
3. Iron–Hematoxylin Staining for Fresh Parasitology Samples
It is used for demonstrating intestinal protozoa in permanent smears.
Steps
- Smear Preparation and Fixation– A thin smear of stool sample is made and the wet smear is placed in Schaudinn’s fixative for at least 30 minutes.
- Removal of Fixative– The slide is transferred to 70% alcohol.
- Rinsing– Rinsed in tap water three times.
- Staining– Stained in iron–hematoxylin working solution for 4–5 minutes.
- Washing – Washed under smooth running tap water for 10 minutes.
- Dehydration– Treated with 95% alcohol for 5 minutes, then two changes of absolute alcohol for 5 minutes each.
- Clearing and Mounting– Cleared in xylene (two changes), then mounted using a permanent mounting medium.
- Examination– The smear is viewed under 100× and under oil immersion.
Note on Verhoeff’s Iron Hematoxylin– It is a metachrome method mainly used for elastic fibres. The stain solutions are mixed just before staining. Differentiation is carried out with 2% ferric chloride until only elastic fibres remain black. After this, 95% alcohol or sodium thiosulphate is used to remove iodine staining from the background.
Result and Interpretation of Iron-Hematoxylin Staining
Iron–hematoxylin stains give a characteristic black colour because the ferric iron mordant forms a very stable lake with hematein. It is the process where the tissue is first overstained and then differentiated, so the final appearance depends on how much dye is removed in the differentiation step. These methods are mostly regressive, and the shades of black and grey arise from controlled destaining.
General Results
Iron–hematoxylin produces a deep black or jet-black colour. It is the ferric lake that gives this colour. This reaction is different from alum hematoxylin where a blue or blue–purple colour is seen. In iron–hematoxylin, the black tone remains stable even after exposure to acidic counterstains.
Since these methods rely on overstaining, the background becomes dark first. After differentiation, only the desired parts retain black colour, and other tissue elements become lighter.
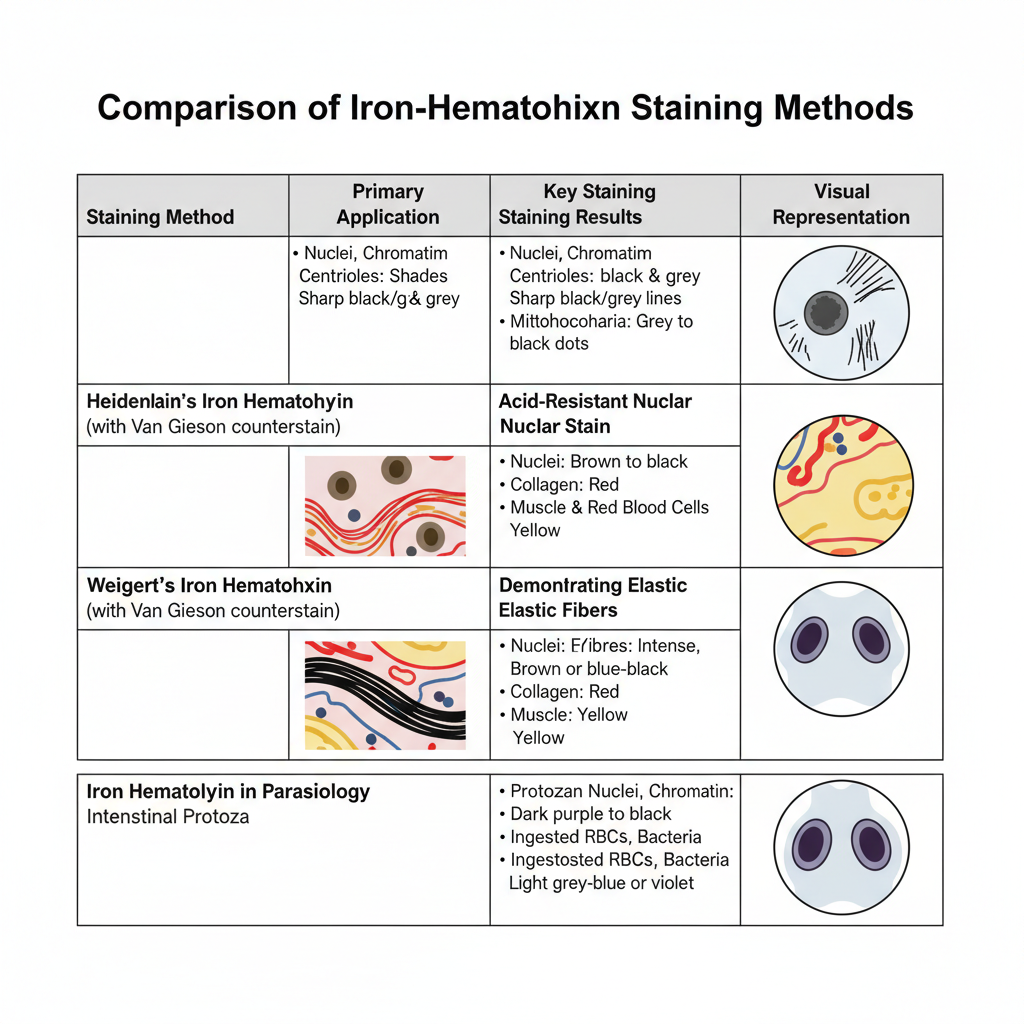
Heidenhain’s Iron Hematoxylin
Heidenhain’s method is used mainly for cytological details. The degree of differentiation determines the final appearance. It is the process where even very fine structures become visible in different shades of black and grey.
Main Results
- Cytological components (chromosomes, chromatin, nucleoli, centrioles, nuclear membrane, ground cytoplasm, muscle striations)– These appear in different shades of black and grey. The exact shade depends on the time of differentiation. It is useful because the contrast remains sharp for microscopic study and for monochrome photography.
- Red blood cells– These may appear black.
- Mitochondria– These are demonstrated clearly in grey or black shades.
- Myelin– Myelin is stained in this method and can be seen distinctly.
Weigert’s Iron Hematoxylin
Weigert’s method is mainly used as an acid-resistant nuclear stain. It is referred for use before staining methods that contain strong acids.
Main Results
- Nuclei– They appear black or brown to black. The iron–hematoxylin lake remains unaffected by acidic dyes, so nuclei stay dark.
- Background– It varies according to the counterstain. The purpose of using Weigert’s stain is to anchor the nuclei firmly while the cytoplasm and connective tissues take up the counterstain.
Example (Weigert’s IH + Van Gieson)
- Elastic fibres – black
- Nuclei – brown to black
- Collagen – red
- Muscle fibres and RBCs – yellow
This combination is widely used when nuclear preservation is needed in acidic trichrome stains.
Verhoeff’s Iron Hematoxylin
This method is specialised for demonstrating elastic fibres. It is the metachrome process where differentiation is done carefully using ferric chloride.
Main Results
- Elastic fibres– These are intensely black. This is the main purpose of the method. It is the process used for diagnosing vascular diseases involving elastic tissues.
- Nuclei– Brown or blue-black.
- Other connective tissue– The appearance depends on counterstain. If Van Gieson is used, collagen becomes red and muscle fibres/RBCs become yellow.
Results in Parasitology
Iron–hematoxylin is used for identifying intestinal protozoa in permanent stained smears. It is the process where very fine nuclear structures become visible.
Main Results
- Protozoan nuclear chromatin, chromatoid bodies, ingested RBCs, bacteria– These appear dark purple to black. Because these structures become sharply defined, this stain was earlier used for describing many protozoa in human faecal samples.
- Cytoplasm of trophozoites and cysts– It appears in violet, grey-blue, or light grey. This contrast between the dark nuclei and lighter cytoplasm helps in identifying Giardia trophozoites and cysts, and Entamoeba histolytica.
Uses of Iron-Hematoxylin Staining
- It is used as an acid-resistant nuclear stain, mainly when an acidic counterstain is applied after the nuclear staining.
- It is used with connective tissue stains such as Van Gieson stain, Masson’s trichrome, Gomori’s trichrome, and Movat pentachrome.
- It is used for high-resolution cytological study where chromosomes, chromatin, nucleoli, centrioles, and nuclear membrane are demonstrated in black and grey shades.
- It is used to show mitochondria, myelin, red blood cells and the cross striations of muscle fibres.
- It is applied for monochrome photomicrography because the black and grey contrast gives a clear image.
- It is used for staining elastic fibres in special stains like Verhoeff’s method.
- It is used for demonstrating myelin in nervous tissues with methods such as Weigert–Pal and Loyez.
- It is used in diagnostic parasitology for visualizing protozoal trophozoites and cysts with clear nuclear chromatin and chromatoid bodies.
- It is used to identify parasites such as Giardia and Entamoeba histolytica.
- It has been used for showing pigments like lead, copper and iron deposits in tissues.
Limitation of Iron-Hematoxylin Staining
- The stain has chemical instability because the ferric compounds cause rapid oxidation of hematoxylin, and the prepared solution loses staining ability very fast.
- Many solutions must be prepared freshly, and mixed working solutions such as in Weigert’s method remain usable only for a short time.
- These methods are regressive, and the differentiation step needs strict microscopic control, otherwise the section becomes overstained or over-differentiated.
- The procedure is long since some steps require many hours, and in Heidenhain’s method even overnight staining is needed.
- In parasitology, the fixation must be proper because poor fixation affects the staining result, and the fixative must be removed completely.
- Very thin sections are required to obtain the cytological details in these methods.
- Reagents like iron alum for differentiation should not be reused to avoid variations in concentration.
- The technique is difficult to standardise or automate due to chemical instability and the subjective decision needed during differentiation.
Advantages of Iron-Hematoxylin Stain
- It gives exceptional acid resistance because the iron–hematein lake remains stable even in strong acidic conditions.
- It is the required nuclear stain when acidic counterstains like Van Gieson or trichrome methods are used.
- The ferric compounds act both as mordant and oxidizer, so the stain becomes active very quickly after preparation.
- It shows very fine cytological details such as chromosomes, chromatin, nucleoli, centrioles, and mitochondria.
- It demonstrates muscle striations, myelin and red blood cells in clear shades of black and grey.
- It provides excellent contrast and is suitable for monochrome photomicrography.
- It is used in diagnostic parasitology for clear identification of protozoal structures like nuclear chromatin and chromatoid bodies.
- It works well even with certain non-mercury fixatives that do not perform well with some other stains.
- It stains elastic fibres black in methods like Verhoeff’s stain.
- It is used for staining myelin in specialized methods such as the Loyez method.
FAQ
What is Iron-Hematoxylin Stain?
Iron-Hematoxylin Stain is a type of histological stain used to highlight nuclei and cytoplasm in tissue samples. It is a modification of the classic Haematoxylin and Eosin (H&E) stain.
How does Iron-Hematoxylin Stain work?
Iron-Hematoxylin works by binding to acidic structures within the cell, such as the DNA of the nucleus, and staining them blue or blue-purple. The stain is then counterstained with eosin, which stains cytoplasm and other structures pink or red.
What is the difference between Iron-Hematoxylin and Haematoxylin and Eosin (H&E) stain?
Iron-Hematoxylin is a modification of the H&E stain, where an iron compound is added to the haematoxylin solution to improve the staining of nuclei and other acidic structures. The improved staining of nuclei makes it easier to distinguish between different types of cells and structures.
What kind of samples can be stained with Iron-Hematoxylin?
Iron-Hematoxylin can be used to stain a variety of biological samples, including tissues, cells, and cell cultures. It is commonly used in histology and pathology for analyzing tissue samples for disease diagnosis and research purposes.
How long does Iron-Hematoxylin staining take?
The staining process for Iron-Hematoxylin can take anywhere from 30 minutes to several hours, depending on the type of sample being stained and the specific staining protocol being used. The staining process typically consists of multiple steps, including fixation, deparaffinization, rehydration, and staining, with each step taking a certain amount of time. The staining itself usually takes anywhere from 5 to 30 minutes, depending on the strength of the stain solution and the desired intensity of staining. The tissue samples must then be washed and dehydrated, which can take an additional 15-30 minutes, before they are mounted and ready for observation under a microscope.
- Avwioro, G. O. (2011). Histochemical uses of haematoxylin—A review. Journal of Pharmacy and Clinical Sciences, 1(1), 24–34.
- DakoCytomation. (2004). Dako guide to special stains. DakoCytomation.
- Emge, D. J. (n.d.). H&E staining troubleshooting. Northwestern University Robert H. Lurie Comprehensive Cancer Center.
- Henderson, T. (2025, November 7). Understanding the differences between histology stains: A comprehensive guide. Contract Laboratory.
- Hooshyar, H., Rostamkhani, P., Arbabi, M., & Delavari, M. (2019). Giardia lamblia infection: Review of current diagnostic strategies. Gastroenterology and Hepatology from Bed to Bench, 12(1), 3–12.
- Leica Biosystems. (2025). An introduction to routine and special staining. Leica Biosystems Knowledge Pathway.
- Llewellyn, B. D. (2013). Hematoxylin formulae. StainsFile.
- Mokobi, F. (2023). Iron-hematoxylin staining. Microbe Notes.
- Myers, R. (2025). The basic chemistry of hematoxylin. Leica Biosystems Knowledge Pathway.
- Sampias, C., & Rolls, G. (2025). Hematoxylin & Eosin (H&E) staining intro: Procedures & more (H&E staining overview: A guide to best practices). Leica Biosystems Knowledge Pathway.
- StainsFile. (n.d.). Weigert’s iron hematoxylin. STEMCELL Technologies.
- Synnovis Group LLP. (2018, June 4). Haematoxylin – the story of the blues.
- Titford, M. (2005). The long history of hematoxylin. Biotechnic & Histochemistry, 80(2), 73–78.
- Wikipedia contributors. (n.d.). H&E stain. Wikipedia.