What is Ion Exchange Chromatography?
- Ion Exchange Chromatography (IEC) is a specialized form of liquid chromatography that facilitates the separation and purification of molecules based on their ionic charge. This technique hinges on the reversible interaction between charged particles in a sample and oppositely charged groups affixed to a stationary phase within a column. It’s a method that finds extensive application in the biotechnological field, particularly in the purification of proteins, enzymes, and other biomolecules with distinct charges.
- The foundational principle of IEC lies in the selective attraction and retention of ions through their exchange with ions pre-bound to the stationary phase. This phase is imbued with either positively or negatively charged groups, making it suitable for attracting and binding ions of the opposite charge present in the sample mixture. This selective binding is the crux of the separation process.
- IEC is broadly categorized into two types: cation-exchange chromatography and anion-exchange chromatography. Cation-exchange chromatography involves a stationary phase with negatively charged groups, designed to attract and bind positively charged ions (cations). Conversely, anion-exchange chromatography employs a stationary phase with positively charged groups to capture negatively charged ions (anions). The choice between cation-exchange and anion-exchange chromatography depends on the specific charge characteristics of the target molecules.
- This chromatographic method is not only pivotal in the purification and analysis of various biomolecules but also plays a significant role in water quality assessment and other analytical applications. The process involves the elution of bound molecules by introducing a solution with a higher concentration of competing ions or by altering the pH levels to weaken the ionic interactions, facilitating the release of the desired molecules.
- Overall, Ion Exchange Chromatography stands out for its effectiveness in separating charged molecules, its adaptability to a wide range of applications, and its relatively straightforward and cost-effective implementation, making it an invaluable tool in both research and industrial contexts.
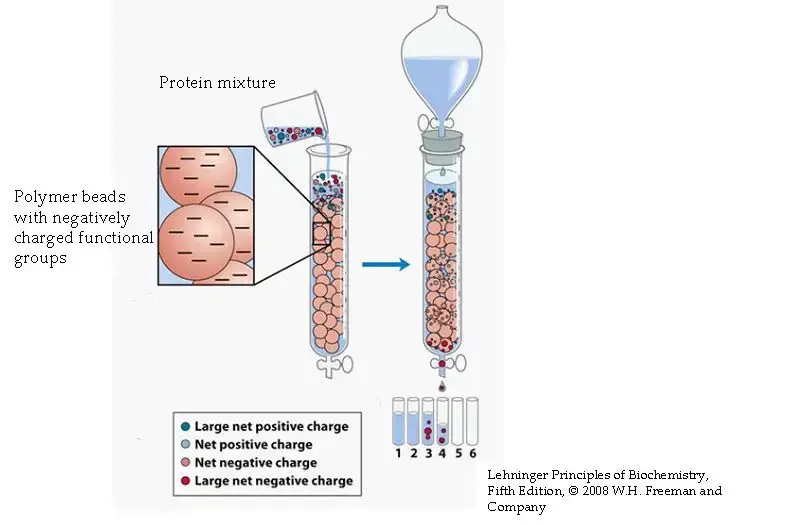
Definition of Ion Exchange Chromatography
Ion exchange chromatography is a separation technique that separates ions and polar molecules based on their affinity to ion exchangers. It involves the reversible exchange of ions between the target ions in the sample solution and ions attached to an ion exchanger.
Working Principle of ion exchange chromatography
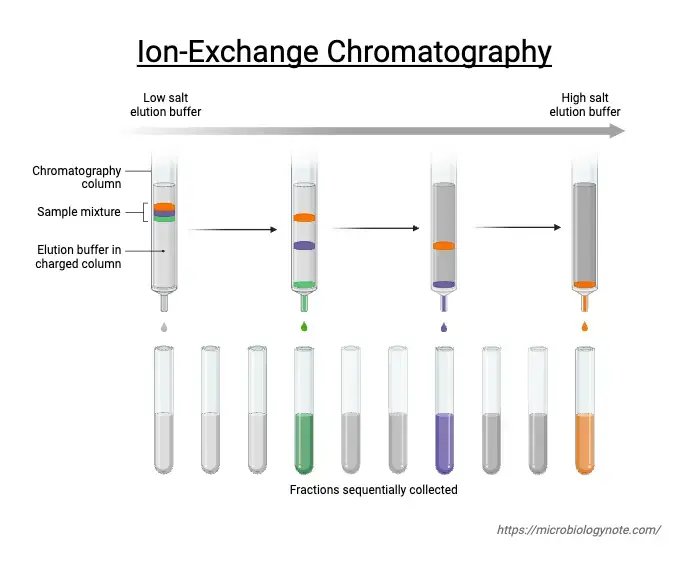
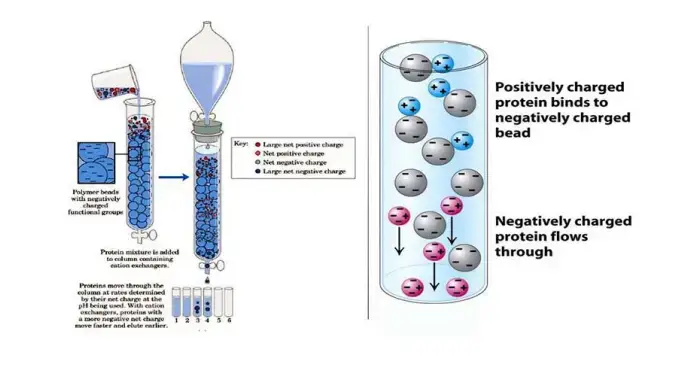
Ion exchange chromatography operates on the principle of attraction between oppositely charged molecules. Here’s how it works:
The stationary phase in ion exchange chromatography is composed of an ion exchanger, which is essentially a matrix with covalently bonded charged groups. These groups can either carry a positive or negative charge. When immersed in a liquid solution, these charged groups attract ions of the opposite charge, forming what’s called an “ion cloud” around the matrix.
In this setup, ions in the solution can swap places with ions attached to the matrix without altering the matrix itself. This exchange is reversible, allowing for separation of ions based on their affinity for the charged groups on the stationary phase.
Ion exchange chromatography is widely used in scientific research and industry to separate and purify substances such as proteins, peptides, amino acids, and nucleotides based on their ionic properties. Its versatility and effectiveness make it a valuable tool in biochemical and biotechnological processes.
This method is crucial in analytical chemistry for its ability to separate ions based on their charge, providing valuable insights into the composition and structure of substances in a wide range of applications.
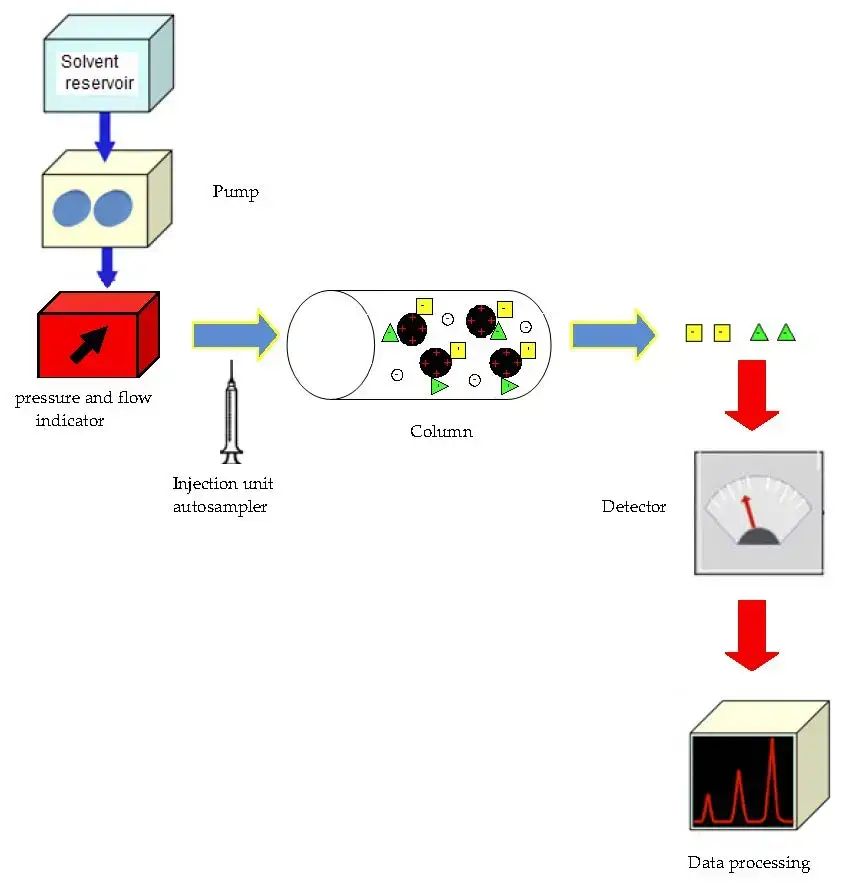
Instrumentation of ion exchange chromatography
Ion exchange chromatography (IC) employs a sophisticated setup to separate and analyze ions based on their charge. The key components of an IC system are as follows:
- Pump: The heart of the IC system, responsible for delivering a steady flow of eluent through the system’s components. The pump ensures that the mobile phase moves through the injector, column, and detector at a constant rate, which is crucial for maintaining consistent separation conditions.
- Injector: This component allows for the introduction of the sample into the chromatography system. Injection valves facilitate this process, accommodating liquid samples directly and solid samples dissolved in a suitable solvent. The injector is designed to handle a wide range of sample volumes, from 0.1 to 100 mL, with precision and under high pressures, up to 4000 psi.
- Columns: The choice of column material varies based on the application and can include stainless steel, titanium, glass, or inert plastics like PEEK. Column dimensions also vary, with diameters ranging from 2mm to 5cm and lengths from 3cm to 50cm, tailored to different analytical and preparative needs.
- Guard Column: Positioned before the main separation column, the guard column acts as a filter to remove particulates that could otherwise clog the main column, thereby extending its lifespan and maintaining its efficiency.
- Suppressor: This component enhances the sensitivity of ion detection by reducing the background conductivity of the eluent. Suppressors, often membrane-based, transform the ionic eluent into water, which improves the measurement of the target ions’ conductivity.
- Detectors: The electrical conductivity detector is a common choice in IC systems. It measures the conductivity of the eluent, which changes as different ions elute from the column, allowing for their detection and quantification.
- Data System: For basic analyses, a pre-programmed computing integrator may suffice. However, more complex analyses require advanced data processing capabilities, provided by data stations or minicomputers, which offer greater control and data management features.
Protocol
- Preparation of the Protein Mixture: Begin by adding 0.2 mL of the protein extract to a vial. Use a vortex to ensure the mixture is thoroughly dissolved. To remove any foam that may have formed, centrifuge the tube at maximum speed for 2 minutes using a microcentrifuge.
- Setting Up the Chromatography Column: Secure the chromatography column in an upright position on a stand. Remove the top cap first, followed by the bottom cap, to allow the buffer to drain out into a waste container. This step ensures the resin within the column settles properly.
- Equilibration of the Column: Introduce 1 mL of equilibration buffer into the column and allow it to drain completely. Repeat this step with another 1 mL of the buffer to ensure the column is fully equilibrated.
- Loading the Protein Extract: Gently apply the prepared protein extract onto the column, taking care to not disturb the settled resin.
- Washing the Column: To remove any unbound proteins, wash the column four times with the equilibration buffer. Collect 2 mL of the wash in labeled collection tubes to analyze later, if necessary.
- Elution of Bound Proteins: Implement a salt gradient for elution, starting with the equilibration buffer, followed by an elution buffer, and finally a high-salt buffer. Apply 1 mL of each buffer sequentially, collecting 1 mL fractions of the eluate in separate 2 mL tubes. Monitor the fractions for any changes in color, which may indicate the presence of proteins.
- Determination of Protein Concentration: Assess the protein concentration in each collected fraction, typically through spectroscopic methods, to identify the fractions containing the target proteins.

Protein concentration Calculating
Determining the concentration of proteins in various fractions is a crucial step in biochemical analysis and purification processes. This can be achieved through a colorimetric assay using a dye-based reagent, such as the hypothetical RED660 reagent mentioned. The procedure involves the following steps:
- Preparation of Samples: Begin by accurately labeling 10 tubes to correspond to the different fractions from which you wish to determine the protein concentration. Into each tube, transfer 50μL of the elute from each respective fraction.
- Reagent Preparation: Ensure the RED660 reagent is well mixed before use. This can be done by gently inverting the bottle several times, avoiding the creation of bubbles, which could affect the accuracy of the measurements.
- Reagent Addition: To each labeled tube containing the sample elute, add 1mL of the RED660 reagent. It’s important to ensure that the reagent is thoroughly mixed with the sample, which can be achieved by vortexing the tubes immediately after addition.
- Incubation: Allow the tubes to sit for 5 minutes at room temperature. This incubation period permits the reagent to react with the proteins in the sample, resulting in a color change that is proportional to the protein concentration.
- Spectrophotometer Calibration: While the samples are incubating, prepare the spectrophotometer. Turn it on and set it to the appropriate wavelength that corresponds to the maximum absorbance of the RED660-protein complex. Typically, the wavelength for dye-based protein assays falls within the visible range, but the exact value should be determined based on the reagent’s specific properties.
- Baseline Correction: To ensure accurate measurements, it’s essential to zero the spectrophotometer with an appropriate blank. This blank usually consists of 1mL distilled water or the same volume of the RED660 reagent used in the sample tubes, placed in a clean cuvette. Adjust the spectrophotometer until the absorbance of the blank is set to zero.
- Measuring Absorbance: After the incubation, transfer the reaction mixture from each tube to separate cuvettes. Measure and record the absorbance of each sample. The absorbance is directly related to the protein concentration, with higher absorbance values indicating higher concentrations of protein.
Procedure
The process of purifying proteins through chromatography involves several critical steps, each designed to ensure the successful separation of desired protein molecules from a complex mixture. The following outlines a general procedure for protein chromatography:
Sample Preparation
- Desalting: The initial step involves the desalting of the protein sample. This is crucial to remove excess salts that could interfere with the protein’s interaction with the chromatography resin, affecting the separation efficiency.
Resin Equilibration
- Resin Washing: Begin by thoroughly washing 100-200 grams of chromatography resin with water. This step is essential to remove any fine particles or impurities that might be present in the resin. Stirring the resin and then aspirating the supernatant can effectively accomplish this.
- Resin Equilibration with Buffer: After washing, the resin needs to be equilibrated with a specific buffer to prepare it for effective protein binding. For this purpose, use 500 mL of a 200 mM buffer solution. Gently stir the resin to create a slurry and then transfer it to a sintered glass column pre-filled with a small volume of water. To ensure complete equilibration, pass 10 mM Tris-HCl buffer through the column until the resin is fully conditioned and ready for the chromatography process.
Chromatography
- Sample Application: With the resin equilibrated, the next step is to apply the desalted protein sample to the column. This allows the proteins to interact with and bind to the resin based on their specific chemical properties.
- Elution Gradient Application: To elute (or wash off) the bound proteins from the resin, apply a gradient consisting of two 500 mL solutions ranging from 0-100 mM of a suitable elution buffer in 10 mM Tris-HCl. This gradient facilitates the selective elution of proteins by gradually changing the buffer conditions, which alters the interactions between the proteins and the resin.
- Fraction Collection: As the proteins are eluted from the column, collect them in separate fractions using a fraction collector. This allows for the isolation of different proteins based on their elution times.
- Analysis: After collection, it’s important to analyze each fraction for protein concentration and conductivity. This helps in identifying which fractions contain the target protein and provides insights into the purity and yield of the separation process.
This procedure, while general, highlights the essential steps involved in protein chromatography, from sample preparation to the final collection of purified proteins. Each step is crucial for the effective separation and purification of proteins, facilitating their further analysis or use in various biochemical applications.
Purification of serine peptidase from bovine whole brain tissue
The process of purifying serine peptidase from bovine whole brain tissue involves a series of meticulous steps designed to isolate this specific enzyme with high purity and activity. Here’s an overview of the procedure:
Preparation of Bovine Cytosolic Extract
- Homogenization: Begin by finely homogenizing 50 grams of bovine brain tissue in 200 mL of an ice-cold buffer solution (Buffer A), consisting of 100 mM Tris-HCl (pH 7.4), 5 mM dithiothreitol (DTT), and 0.5 mM EDTA to protect the integrity of proteins.
- Initial Centrifugation: Centrifuge the homogenate at 36,000 × g for 45 minutes. This step separates the mixture into a supernatant (S1) and a pellet (P1).
- Pellet Resuspension and Recentrifugation: Resuspend P1 in 100 mL of ice-cold distilled water and centrifuge again under the same conditions to obtain a second supernatant (S2). Discard the resulting pellet (P2).
- Ultracentrifugation: Combine the S1 and S2 fractions and subject them to ultracentrifugation at 100,000 × g for 45 minutes. The resulting supernatant (S3) is then aliquoted and stored at -20°C or -80°C for further use. The final pellet (P3) is discarded.
Ammonium Sulfate Precipitation
- First Saturation Step: Gradually add ammonium sulfate to 40 mL of S3 to achieve 45% saturation, adjusting the pH to 7.4 with 1 M NaOH, if necessary. Centrifuge to remove precipitated impurities, retaining the supernatant (S4) and discarding the pellet (P4).
- Second Saturation Step: Increase the saturation of S4 to 75% by adding more solid ammonium sulfate, again adjusting the pH as needed. The resulting pellet (P5), which contains the protein of interest, is kept after centrifugation.
- Resuspension and Dialysis: Resuspend P5 in a small volume of Buffer B (50 mM Tris-HCl pH 8.0, 5 mM DTT, 0.5 mM EDTA) and dialyze it against the same buffer to remove excess ammonium sulfate.
Partial Purification of Prolyl Oligopeptidase (POP)
- Column Equilibration: Prepare a DEAE-Sepharose column and equilibrate it with Buffer B.
- Sample Application and Washing: Apply the dialyzed extract to the column. Wash the column with Buffer B to remove unbound components.
- Elution: Elute the bound POP using a linear NaCl gradient in Buffer B. Collect fractions and assess them for total protein content and POP activity.
- Column Regeneration: After elution, regenerate the DEAE column with a high concentration of NaCl followed by NaCl-free Buffer B.
Post-DEAE Fraction Assay
- Substrate Preparation: Prepare a stock solution of the substrate Z-Gly-Pro-MCA in DMSO and dilute it with Buffer A to the desired concentration.
- Reaction Setup: Mix the substrate with post-DEAE fractions and incubate at 37°C for 30 minutes.
- Termination and Control: Terminate the reactions with acetic acid. Include a negative control where acetic acid is added before the substrate.
- Fluorescence Measurement: Measure the fluorescence of the liberated MCA product using specific excitation and emission wavelengths to quantify the enzyme activity.
This detailed process facilitates the isolation of serine peptidase from bovine brain tissue, utilizing a combination of centrifugation, ammonium sulfate precipitation, and column chromatography to achieve purification, followed by enzymatic assays to confirm activity.
Resin Selection in Ion Exchange Chromatography
Resin selection is a crucial aspect of ion exchange chromatography as it directly influences the efficiency and effectiveness of the separation process. When choosing a resin, several factors need to be considered:
- Functional Groups: Ion exchange resins consist of solid matrices, such as polystyrene, cellulose, polyacrylamide, or agarose, with negatively or positively charged functional groups attached to them. The selection of the resin depends on whether anion or cation exchange is desired. Anion exchangers have negatively charged functional groups that attract positively charged ions, while cation exchangers have positively charged functional groups that attract negatively charged ions.
- Flow Rate: The flow rate of the sample and buffer through the resin is an important consideration. Some resins have a higher flow rate capacity than others, which can affect the separation efficiency and the time required for the chromatographic process.
- Strength of Ion Exchanger: Ion exchange resins can be classified as either strong or weak ion exchangers. Strong ion exchangers have a higher affinity for ions and can bind them more tightly. Weak ion exchangers have a lower affinity and can bind ions more loosely. The selection of the resin strength depends on the specific requirements of the separation and the desired binding/release characteristics of the target ions.
- Dimension and Binding Capacity: The dimension of the resin refers to the size and shape of the resin beads or particles. The choice of dimension depends on factors such as the scale of the separation (e.g., analytical or preparative) and the available equipment. Additionally, the binding capacity of the resin is important as it determines the maximum amount of ions or molecules that can be effectively bound to the resin during the chromatographic process.
- Protein Stability: If the sample to be separated contains proteins, their stability plays a significant role in resin selection. Some proteins may be more stable in the presence of anion exchangers, while others may be more stable in the presence of cation exchangers. The stability of the protein should be assessed to determine the most suitable resin type to ensure optimal separation and preservation of protein integrity.
It is important to carefully consider these factors when selecting a resin for ion exchange chromatography. The specific requirements of the separation, including the type of ions or molecules to be separated, the flow rate, resin strength, dimension, binding capacity, and the stability of the sample components, all contribute to the resin selection process. By choosing the appropriate resin, the efficiency and selectivity of the ion exchange chromatography can be maximized, leading to successful separations and accurate analysis of the target components.
Sample Preparation in Ion Exchange Chromatography
Sample preparation is an essential step in ion exchange chromatography to ensure successful purification and optimal column performance. Proper sample preparation helps to clarify the sample, remove impurities, and maintain the stability of the target molecule. Here are some key points to consider when preparing samples for ion exchange chromatography:
- Clearing the Sample: Samples should be clear and free from particulate matter to prevent column clogging and prolong the lifespan of the chromatographic medium. Centrifugation and filtration techniques are commonly used for sample clarification.
- Sample Stability: It is crucial to retain the biological activity of the target molecule during purification. Denaturation or precipitation of sample components can adversely affect column function. Therefore, it is important to determine the stability limits of the sample and work within those limits during purification.
- Stability Testing: Before starting the purification protocol, it is advisable to perform stability tests on the sample. This can include testing pH stability, salt stability, stability towards organic solvents like acetonitrile and methanol, temperature stability, and assessing proteolytic activity.
- Centrifugation: Centrifugation is an effective method for removing lipids and particulate matter from the sample. The centrifugation conditions may vary depending on the sample type, and higher speeds may be required for cell lysates or serum samples.
- Filtration: Filtration is another technique used to remove particulate matter from the sample. Cellulose acetate or PVDF membrane filters are commonly used, and the filter pore size should be selected based on the bead size of the chromatographic medium.
- Desalting: Desalting columns can be used to remove low molecular weight contaminants and transfer the sample into the desired buffer conditions. Desalting is especially useful for removing salts from proteins with a molecular weight greater than 5,000.
- Specific Sample Preparation Steps: Depending on the sample characteristics, specific preparation steps may be required. For example, fractional precipitation can be used to remove gross impurities such as lipids or bulk proteins. Precipitation techniques using agents like ammonium sulfate, dextran sulfate, or polyethylene glycol can selectively precipitate certain components from the sample.
- Resolubilization of Protein Precipitates: Proteins that have been precipitated may require resolubilization before further purification steps. The resolubilization conditions will depend on the specific protein and may involve the use of denaturing agents such as urea or guanidine hydrochloride. It is important to remove these denaturing agents to allow for protein refolding and maximize recovery.
- Buffer Exchange and Desalting: Buffer exchange steps using desalting columns are often performed before or between purification steps to remove unwanted contaminants and transfer the sample into the desired buffer for ion exchange chromatography.
- Optimization and Validation: It is essential to optimize the sample preparation steps for each specific sample and validate their effectiveness. Monitoring the sample quality and stability throughout the purification process is important to ensure successful ion exchange chromatography.
Applications of ion exchange chromatography
Ion exchange chromatography (IEC) is a versatile separation technique with a wide array of applications across various fields. Here are some of the key applications:
- Amino Acid Analysis:
- IEC is extensively used in the separation and analysis of amino acid mixtures, which is fundamental in biochemical research and clinical diagnostics.
- It allows for the detailed characterization of the 20 principal amino acids found in blood serum or resulting from protein hydrolysis.
- Water Purification:
- One of the most critical applications of IEC is in the purification and softening of water.
- The technique achieves complete deionization by exchanging cations in the water with hydrogen ions and anions with hydroxyl ions, resulting in pure water suitable for various uses, including drinking.
- Nucleic Acid Hydrolysis Products Analysis:
- IEC plays a significant role in studying the hydrolysis products of nucleic acids.
- This application is crucial in understanding the structure of nucleic acids and their function as carriers of genetic information, providing insights into genetic coding and expression.
- Trace Metal Collection:
- Chelating resins used in ion exchange chromatography are instrumental in collecting trace metals from seawater.
- This application is essential in environmental monitoring and marine research, aiding in the study of oceanic trace metal distributions and cycles.
- Geological and Lunar Research:
- IEC is also utilized in the analysis of lunar rock samples and the detection of rare trace elements on Earth.
- This application provides valuable data for geological and extraterrestrial studies, contributing to our understanding of the Earth’s crust and the lunar surface.
Precautions of ion exchange chromatography
- Sample Handling:
- Handle samples meticulously to avoid any contamination, which is vital for analyses requiring high sensitivity.
- Ensure that all labware and solutions used in sample preparation and introduction are clean and free from contaminants.
- Column Care:
- The chromatography column, packed with ion-exchange material, is sensitive to physical stress. Avoid dropping, hitting, or shaking the column to prevent damage to the stationary phase.
- Protect the column from sudden pressure changes or excessively high pressures that might compromise the integrity of the packing material.
- Hydrophobic Component Accumulation:
- Given the higher hydrophilicity of the ion-exchange groups, there’s a risk of hydrophobic components from the samples accumulating within the column. This can cause unwanted interactions and effects on the separation process.
- Periodically clean and regenerate the column according to the manufacturer’s instructions to remove any retained hydrophobic substances.
- Preventing Precipitation:
- Take measures to avoid precipitation within the column, which can clog the stationary phase and reduce column efficiency.
- Ensure that all buffers and samples are filtered and clear before introduction to the column.
- Buffer Temperature Sensitivity:
- Be aware of the temperature sensitivity of certain buffers, such as Tris-based buffers. Adjust the pH of these buffers to the desired level at room temperature, considering their pH can vary significantly with temperature changes.
- Sample Cooling During Ammonium Sulfate Addition:
- When adding ammonium sulfate, particularly during protein precipitation steps, keep the sample cold to reduce the risk of denaturation or other unwanted reactions.
- This precaution is essential for maintaining the structural integrity and functionality of biomolecules.
Advantages of ion exchange chromatography
- Versatility in Separating Charged Molecules:
- IEC is highly efficient in separating a wide range of charged particles, from large biomolecules like proteins to smaller molecules such as nucleotides and amino acids.
- Applicability to Both Analytical and Preparative Tasks:
- This method is not limited to analytical applications; it is also highly effective for preparative purposes, making it a versatile tool in research and manufacturing processes.
- Broad Range of Separable Molecules:
- IEC can be employed to separate almost any kind of charged molecule, providing a broad utility across biochemistry, molecular biology, and chemistry.
- Capability to Separate Inorganic Ions:
- Beyond organic molecules, IEC is also capable of separating inorganic ions, expanding its utility to fields like environmental analysis, geochemistry, and materials science.
Limitations of ion exchange chromatography
- Specificity to Charged Molecules:
- IEC is limited to the separation of charged species. Neutral molecules without any charge will not interact with the ion exchange resin and thus cannot be effectively separated using this method.
- Dependence on Buffer Conditions:
- The effectiveness of IEC is highly dependent on the buffer composition and conditions. The pH and ionic strength of the buffer can significantly affect the binding and elution of molecules, requiring precise control and optimization.
- High Concentration of Non-Volatile Salts:
- IEC often involves the use of high concentrations of non-volatile salts, which can complicate the downstream processing, analysis, and identification of separated components, particularly in mass spectrometry.
- Kinetic Limitations:
- While IEC provides high selectivity, its kinetic performance can be relatively poor in some bioseparations. The rate of interaction between the molecules and the resin might limit the resolution and speed of separation.
Examples in which ion exchange chromatography was used as a liquid chromatograpic technique for separation or purification of bioactive molecules from natural sources
Ion exchange chromatography (IEC) is a versatile technique used extensively for the separation and purification of bioactive molecules from natural sources. This technique is particularly effective for charged or ionizable molecules such as proteins, peptides, enzymes, nucleotides, DNA, antibiotics, and vitamins. Below are examples illustrating the application of IEC in extracting bioactive compounds from various natural sources:
- Nigella sativa Linn. (Black Seed):
- Proteins were extracted from water extracts of N. sativa, followed by centrifugation and dialysis. The DEAE Sephadex A50 column was used for separation, with proteins detected at various molecular masses.
- Olea europaea L. (Olive):
- Leaf and root extracts were analyzed using high-performance anion-exchange chromatography with pulsed amperometric detection, identifying various sugars and sugar alcohols.
- Soybean:
- Soybean proteins were extracted and separated using an anion exchange perfusion column, identifying significant soybean proteins such as 11S and 7S globulins.
- Cochlospermum tinctorium A. Rich.:
- Ethanol and water extracts from the roots were purified using DEAE-Sepharose column chromatography, focusing on neutral and acidic polysaccharide fractions.
- Hen Egg:
- Egg white proteins were separated using anion and cation exchange chromatography columns, identifying proteins like ovalbumin, ovotransferrin, and lysozyme.
- Phaseolus vulgaris (Common Bean):
- Proteins extracted from bean seeds were purified using a Q-Sepharose column, leading to the identification of an antifungal peptide.
- Sweet Dairy Whey:
- Whey proteins were separated using Q- and S-Sepharose anion- and cation-exchange resins, focusing on proteins like α-lactalbumin and β-lactoglobulin.
- Morus alba (Mulberry) Leaves:
- Lectins were extracted and purified using DEAE-Sephacel column chromatography, identifying specific lectins MLL 1 and MLL 2.
- Lycium ruthenicum Murr. (Black Goji Berry):
- Polysaccharides were extracted and purified using DEAE-cellulose column chromatography, resulting in the identification of glycoconjugate polysaccharides.
- Coprinus comatus (Shaggy Mane Mushroom):
- Polysaccharides were extracted from mushroom stipes and purified using DEAE-Sepharose CL-6B column chromatography, identifying various polysaccharides including trehalose and glucans.
- Physalis alkekengi var. francheti (Chinese Lantern):
- Polysaccharides were extracted using different enzymes and purified through DEAE anion-exchange chromatography, leading to the separation of various polysaccharide fractions.
- Ornithogalum caudatum Ait. (Star of Bethlehem):
- Water-soluble polysaccharides were extracted and purified using DEAE-Sepharose fast flow anion-exchange chromatography, focusing on the separation of these polysaccharides.
- Paecilomyces variotii (A Fungus):
- Tannase enzyme was purified from the fermentation supernatant using DEAE Sepharose column chromatography, demonstrating the application of IEC in enzyme purification.
- Castanospermum australe (Moreton Bay Chestnut):
- Alkaloids and glycosides were extracted from seeds and purified through multiple ion exchange chromatography steps, showcasing the technique’s ability to purify complex molecules.
FAQ
What is ion exchange chromatography?
Ion exchange chromatography is a separation technique that utilizes the reversible exchange of ions between a stationary phase (ion exchanger) and analytes in a sample based on their charge interactions.
How does ion exchange chromatography work?
Ion exchange chromatography works by using an ion exchanger, which contains charged functional groups. When the sample is passed through the column, analytes with opposite charges to the functional groups will interact and bind to the stationary phase. By manipulating the eluent conditions, the bound analytes can be selectively eluted.
What types of analytes can be separated using ion exchange chromatography?
Ion exchange chromatography can separate charged molecules, including ions, proteins, nucleotides, amino acids, and other polar compounds.
What are the two types of ion exchange chromatography?
The two types of ion exchange chromatography are anion exchange chromatography, where negatively charged analytes are separated, and cation exchange chromatography, where positively charged analytes are separated.
What factors should be considered when selecting an ion exchange resin?
When selecting an ion exchange resin, factors such as the charge of the analyte, flow rate, strength of the ion exchanger (weak or strong), dimensions of the resin, and binding capacity should be taken into account.
What is the role of buffers in ion exchange chromatography?
Buffers are essential in ion exchange chromatography as they provide the necessary pH conditions for optimal ion exchange interactions. Buffers help maintain the ionization states of analytes and control the elution of bound species from the stationary phase.
What are the advantages of ion exchange chromatography?
Some advantages of ion exchange chromatography include its effectiveness in separating charged particles, its versatility in analyzing various charged molecules, its applications in both analytical and preparative purposes, and its capability to separate inorganic ions.
What are the limitations of ion exchange chromatography?
Limitations of ion exchange chromatography include its inability to separate neutral or non-ionic molecules, its reliance on charged analytes, and the requirement for buffers, which can increase complexity and cost.
Can ion exchange chromatography be used for large-scale purification?
Yes, ion exchange chromatography can be used for large-scale purification in industrial settings. Preparative ion exchange chromatography allows for the isolation and purification of target molecules on a larger scale.
What are the common detection methods used in ion exchange chromatography?
The most commonly used detection method in ion exchange chromatography is the electrical conductivity detector, which measures changes in electrical conductivity caused by the presence of charged analytes. Other detection techniques such as UV-Vis spectroscopy and mass spectrometry can also be used depending on the specific analytes being analyzed.
References
- Janson, J.C., and Rydén, L. (1998). Protein Purification: Principles, High Resolution Methods, and Applications (2nd ed.). Wiley Inc.
- Scopes, R.K. (1994). Protein Purification: Principles and Practice. Springer.
- Cytiva (formerly GE Healthcare Life Sciences). Ion Exchange Chromatography Principles and Methods Handbook.
- Cytiva (formerly GE Healthcare Life Sciences). Ion Exchange Chromatography Columns Handbook.
- Gagnon, P. (2017). Ion Exchange Chromatography: Principles and Methods. Methods in Molecular Biology, 1485, 3-20.
- Wang, G., and Wu, S. (Eds.). (2019). Ion Exchange Chromatography: Methods and Protocols. Methods in Molecular Biology, 2045.
- Shukla, A.A., and Hubbard, B. (Eds.). (2018). Ion Exchange Chromatography of Proteins: Advances and Future Directions. ACS Symposium Series, 1293.
- Hearn, M.T.W., and Xiang, Y. (Eds.). (2006). Ion Exchange and Solvent Extraction: A Series of Advances. CRC Press.
- Belafi-Bako, K., and Nemestóthy, N. (Eds.). (2018). Ion Exchange: Theory and Application. IntechOpen.
- Gusev, A.I., and Markin, A.V. (Eds.). (2017). Ion Exchange Materials: Properties and Applications. CRC Press.