What is Induced Fit Model?
- The Induced Fit Model is a concept that delves into the intricate nature of protein-protein interactions. Historically, Emil Fischer introduced the idea of the lock and key paradigm, which visualized these interactions as rigid and inflexible. In this model, the interacting interfaces of the two proteins have complementary shapes, and when they bind, there’s a minimal change in their conformation.
- However, the induced fit model offers a more dynamic perspective. It suggests that when proteins bind, there are significant conformational changes that allow proteins with varying shapes to interact even if they aren’t perfectly complementary in their unbound state. Imagine an enzyme and its ligand. When they interact, it’s akin to a hand sliding into a glove. The glove (enzyme) adjusts its shape to accommodate the hand (ligand). Initially, a weak complex forms, but as the interaction progresses, the structures rearrange, forming new interactions until a stable, high-affinity state is achieved.
- This model is an evolution of the lock-and-key theory, which was proposed a century ago. The induced fit theory, introduced by D. E. Koshland, Jr. in 1958, not only expands on the original idea but also sheds light on regulatory mechanisms, cooperative effects, and introduces new concepts of specificity. It posits that an enzyme’s active site isn’t perfectly shaped for substrate binding when it’s unbound.
- The structure of an enzyme’s active site is influenced by various factors. These include its interaction with cofactors or coenzymes, pH levels, ionic strength, temperature, and even enzymatic modifications like glycosylation and phosphorylation. The active site, a crucial binding region on the enzyme’s surface, is specifically designed for interactions. It comprises the catalytic site, which contains amino acid residues that facilitate the reaction, and the substrate binding site, which recognizes the ligand.
- The active site’s structure is fluid and can change based on the enzyme’s environment or substrate binding. This adaptability is why the model is termed “induced fit.” The enzyme’s active site undergoes minor changes to ensure the substrate fits optimally. This alteration in configuration catalyzes the reaction, reduces the activation energy barrier, and subsequently increases the reaction rate.
- In the induced fit model, both the substrate and the enzyme’s active site undergo changes until the substrate is fully attached. Once this attachment is complete, the final shape and charge distribution are established, prompting the enzyme to begin its catalytic action. It’s essential to note that initially, the enzyme’s active site and the substrate might not be entirely complementary, but they adjust to achieve a perfect fit.
- Therefore, the induced fit model provides a comprehensive understanding of enzyme-substrate interactions, emphasizing the dynamic and adaptable nature of enzymes. Besides, it underscores the importance of various factors in influencing enzyme activity and offers a logical progression of ideas, ensuring a clear and concise explanation of the topic.
Evidences Supporting Induced Fit Model
The Induced Fit Model has garnered significant attention in the realm of biochemistry, and various pieces of evidence support its validity. This model posits that proteins, including enzymes, are not static or rigid structures. Instead, they exhibit dynamic properties, allowing them to adapt and change in response to their environment.
Researchers have observed that different sections of an enzyme molecule can move during experiments. These movements vary in magnitude, with some being subtle and others more pronounced. Interestingly, these motions become especially noticeable when a substrate binds to the enzyme. This observation underscores the idea that the substrate and the enzyme’s active site are not perfectly complementary before they bind. Instead, the active site undergoes a deformation, adjusting its shape to fit the substrate more snugly upon binding.
Several pieces of evidence bolster the claims of the Induced Fit Model:
- X-ray Diffraction and Optical Rotational Analyses: These sophisticated techniques have provided compelling evidence for the structural modifications that occur in the catalytic site of enzymes. Such changes are consistent with the idea that the enzyme’s active site adapts its shape to accommodate the substrate.
- Catalytic Group Dynamics: The model explains the behavior of the catalytic group within enzymes. Specifically, it elucidates how this group remains separate, ensuring that non-substrates are not inadvertently processed by the enzyme. This selective processing is crucial for the enzyme’s specificity and efficiency.
- Transition State Formation: Another critical aspect explained by the Induced Fit Model is the formation of the transition state. This state arises before the actual conversion of substrates into products. The model provides insights into how enzymes facilitate this transition, further emphasizing their role in catalyzing biochemical reactions.
Therefore, the Induced Fit Model offers a comprehensive and detailed explanation of enzyme-substrate interactions. It emphasizes the dynamic nature of enzymes and their ability to adapt to substrates, ensuring efficient and specific reactions. The evidence supporting this model, derived from advanced analytical techniques, underscores its significance in understanding enzymatic functions and mechanisms.
Mechanism of Induced fit model
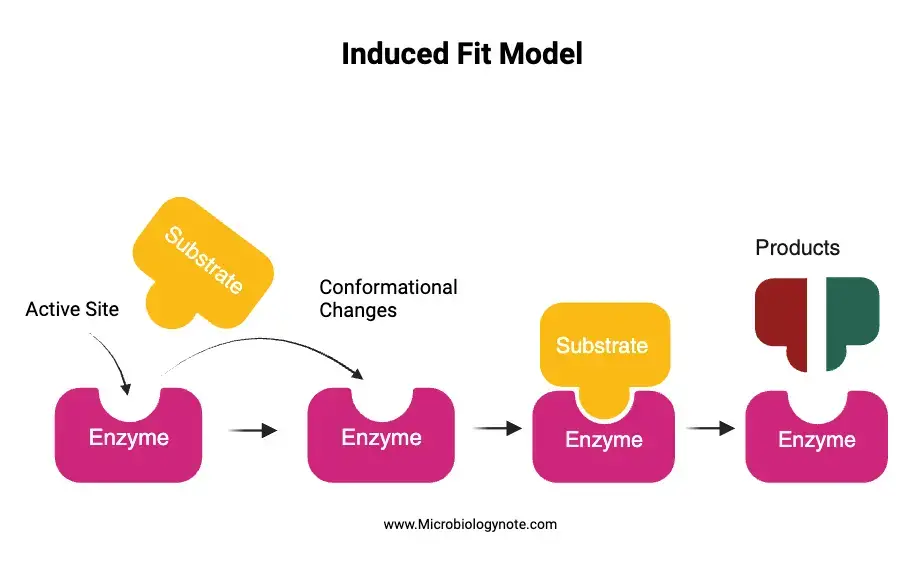
The Induced Fit Model provides a comprehensive understanding of enzyme-substrate interactions and the dynamic nature of enzymes. Here’s how the model works:
- Initial Contact with the Substrate: When a suitable substrate approaches the enzyme’s active site, it doesn’t fit perfectly. This is analogous to a key not fitting perfectly into a lock initially.
- Forces Attracting the Substrate: The substrate is drawn towards the enzyme’s active site due to various forces. These include electrostatic interactions, hydrogen bonds, and hydrophobic interactions, which are the same forces responsible for maintaining the tertiary structure of proteins during peptide chain folding.
- Binding Variability: It’s essential to note that enzymes don’t exclusively bind to a single substrate. Through allosteric binding, regulating molecules, either activators or inhibitors, can alter the enzyme’s conformation. This change affects the enzyme’s ability to catalyze reactions.
- Conformational Changes: The amino acid side chains constituting the active site undergo adjustments, positioning themselves in a manner that activates the enzyme’s catalytic function.
- Formation of the Enzyme-Substrate Complex: As the substrate continues to interact with the enzyme, the active site undergoes further conformational changes. This adaptation continues until the substrate forms a tight bond with the enzyme, resulting in the enzyme-substrate complex. At this juncture, the final shape and charge distribution of the complex are established.
- Catalysis: Once the enzyme-substrate complex is stable, the catalytic groups present at the active site engage with the substrate. This interaction facilitates the conversion of the substrate into two or more products.
- Release of Products: After the catalytic reaction, the products formed detach from the enzyme’s surface. This detachment allows the enzyme to revert to its original shape, ready to participate in subsequent reactions.
- Influence of Nonsubstrate Molecules: Molecules that aren’t substrates but are either too large or too small can still approach the enzyme’s active site. However, their interaction leads to a misalignment of the catalytic groups. As a result, despite being attracted to the active site, these molecules cannot catalyze reactions.
In summary, the Induced Fit Model emphasizes several key points:
- The precise orientation of catalytic groups is pivotal for enzyme functionality.
- The substrate induces significant alterations in the three-dimensional arrangement of amino acids at the enzyme’s active site.
- These structural changes, prompted by the substrate, ensure that the catalytic groups align correctly. In contrast, a nonsubstrate molecule won’t induce the necessary alignment, rendering the enzyme inactive for that particular molecule.
Therefore, the Induced Fit Model offers a detailed and sequential explanation of the dynamic interactions between enzymes and substrates, emphasizing the adaptability and specificity of enzymes in biochemical reactions.
Advantages of Induced Fit Model
The Induced Fit Model, a pivotal concept in enzymology, offers several advantages in understanding enzyme-substrate interactions. Here are the primary benefits of this model:
- Broad Specificity of Enzymes: One of the primary advantages of the Induced Fit Model is its ability to explain the broad specificity exhibited by enzymes. In simpler terms, this model illustrates how enzymes can recognize and bind to a variety of substrates. For instance, lipase enzymes, which are responsible for breaking down lipids, can bind to a diverse range of lipid molecules. This broad specificity ensures that enzymes can function efficiently in diverse physiological conditions and with various substrates.
- Catalysis Mechanism: Besides explaining enzyme specificity, the Induced Fit Model also sheds light on the mechanisms underlying enzymatic catalysis. When a substrate binds to an enzyme, the enzyme undergoes a conformational change. This change stresses the bonds within the substrate, making them more reactive. Therefore, the substrate’s bonds are more susceptible to breaking or forming, facilitating the catalytic process. This conformational change, induced by substrate binding, is pivotal in enhancing the reactivity of the substrate, leading to efficient catalysis.
- Enhanced Enzyme Efficiency: The Induced Fit Model suggests that enzymes are not just passive entities but can adapt their shape to fit substrates better. This adaptability ensures that enzymes can achieve maximum efficiency, even if the substrate isn’t a perfect match initially.
- Protection Against Unwanted Reactions: The model ensures that enzymes remain inactive until the appropriate substrate is present. This specificity prevents unwanted reactions from occurring, ensuring that only the correct reactions take place within a cell.
- Regulation and Control: The dynamic nature of the enzyme, as proposed by the Induced Fit Model, allows for better regulation. Enzymes can be modulated by other molecules, either enhancing or inhibiting their activity, leading to precise control over metabolic pathways.
- Flexibility in Evolution: The ability of enzymes to adapt their shape means they can evolve to accommodate new substrates. This flexibility has been crucial in the evolutionary process, allowing organisms to adapt to changing environments and dietary habits.
- Thermodynamic Stability: The induced fit ensures that the enzyme-substrate complex is thermodynamically more stable than when they are separate. This stability is crucial for the reaction to proceed efficiently.
- Minimization of Side Reactions: By ensuring a tight fit only when the correct substrate is present, the model minimizes the chances of side reactions, which can be wasteful or even harmful.
- Feedback Mechanisms: The dynamic nature of enzyme conformation in the Induced Fit Model allows for feedback mechanisms where the products of a reaction can influence the enzyme’s activity, leading to intricate control of metabolic pathways.
- Facilitation of Multi-step Reactions: Some enzymes facilitate reactions that involve multiple steps. The Induced Fit Model allows enzymes to undergo several conformational changes, each optimized for a particular step in the reaction sequence.
Limitations of Induced fit model
The Induced Fit Model, while providing a more dynamic perspective on enzyme-substrate interactions, does come with its set of limitations. Delving into these constraints offers a more comprehensive understanding of the model’s scope and its areas of improvement:
- Overlooking Catalytic Chemistry: One of the primary limitations of the Induced Fit Model is that it doesn’t account for the intricate chemistry of catalytic reactions. Enzyme catalysis is a complex process influenced by various chemical factors. For instance, electrostatic interactions play a pivotal role in stabilizing the enzyme-substrate complex. Additionally, enzymes often require cofactors, which are non-protein chemical compounds, to be catalytically active. Furthermore, the presence of proton donors and receptors is crucial for certain reactions. The Induced Fit Model, in its basic form, tends to overlook these nuances.
- Inadequate Representation of Highly Flexible Proteins: Proteins, especially enzymes, can exhibit a high degree of flexibility. This flexibility can manifest in various ways, such as movements in the protein backbone, rearrangements of specific domains, or transitions from a disordered state to an ordered one. The Induced Fit Model, in its current form, struggles to capture these intricate conformational changes. Therefore, for proteins that undergo significant structural alterations upon substrate binding, this model might not provide a complete picture.
- Lack of Emphasis on Proton Transfers: Many enzymatic reactions involve the transfer of protons. These transfers are crucial for the progression of the reaction and can influence the rate and direction of the reaction. The Induced Fit Model doesn’t delve deep into these proton transfer mechanisms, which can be a significant oversight for certain reactions.
Importance of Induced fit model
- Dynamic Interaction: Unlike the rigid lock-and-key model, the induced fit model underscores the flexibility of enzymes. It posits that enzymes are not static structures; instead, they can undergo conformational changes upon substrate binding, leading to a more precise fit.
- Enhanced Specificity: This model elucidates the broad specificity of enzymes. It suggests that while multiple substrates might initially bind to an enzyme, only the correct one induces the optimal conformational change, leading to catalysis.
- Catalytic Efficiency: The induced fit model provides insights into the catalytic power of enzymes. The conformational changes can stress and distort substrate bonds, making them more susceptible to catalysis. This dynamic adjustment ensures that enzymes can speed up reactions efficiently.
- Regulation and Allosteric Modulation: The ability of enzymes to change shape means they can be regulated more intricately. Allosteric modulators, which bind to sites other than the active site, can induce conformational changes that either enhance or inhibit enzyme activity.
- Adaptability: The induced fit model highlights the adaptability of enzymes. They can accommodate a range of substrates, and this flexibility allows them to perform diverse functions in the cell.
- Evolutionary Perspective: From an evolutionary standpoint, the ability of enzymes to adapt their shape for different substrates suggests a mechanism by which enzymes can evolve new functions. Over time, slight modifications in enzyme structure can lead to the ability to process new substrates.
References
- Prof. Dr. Daniel E. Koshland Jr. (1995). The Key–Lock Theory and the Induced Fit Theory. , 33(23-24), 2375–2378. doi:10.1002/anie.199423751
- Laddach, Anna & Chung, Sun Sook & Fraternali, Franca. (2018). Prediction of Protein-Protein Interactions: Looking Through the Kaleidoscope. 10.1016/B978-0-12-809633-8.20470-6.
- https://www.biologydiscussion.com/enzymes/theories-explaining-the-mode-of-enzyme-action/6128
- https://www.biologyonline.com/dictionary/induced-fit-model
- https://jackwestin.com/resources/mcat-content/enzyme-structure-and-function/induced-fit-model-of-enzymes
- https://www.mun.ca/biology/scarr/Induced-Fit_Model.html
- https://www.ahmadcoaching.com/2020/12/induced-fit-model-of-enzyme-action.html