What is the humoral immune response/What is humoral immune response?
- Humoral immunity, often termed antibody-mediated immunity, is a critical component of the body’s defense mechanism against pathogens and foreign entities present in extracellular fluids. This immunity operates within both the innate and adaptive branches of the immune system and is characterized by its specificity and adaptability.
- At its core, humoral immunity is orchestrated through a series of stages, namely the primary and secondary phases. The primary phase is initiated when the body first encounters an antigen, a surface protein typically found on the membranes of pathogens. Upon subsequent exposures to the same antigen, the secondary phase is activated, demonstrating the adaptive nature of this immune response.
- This immunity is facilitated by various immune cells, with the primary players being B-lymphocytes and plasma cells, also known as effector B cells. These cells are responsible for producing antibodies tailored against specific foreign agents or antigens. Furthermore, these antibodies can trigger the release of chemical mediators, such as interferons and complement proteins, amplifying the destruction of the antigen.
- Historically, vaccines have been designed to stimulate the humoral immune response by introducing attenuated or inactivated pathogens into the body. However, recent advancements, particularly highlighted during the COVID-19 pandemic, have seen the emergence of vaccines utilizing templates or mRNA sequences to activate the humoral immune response.
- It is essential to note that humoral immunity primarily targets pathogens proliferating in extracellular spaces. Some of these pathogens utilize the extracellular milieu as a conduit to traverse between cells. The term “humoral” is derived from the Latin word “humor,” referring to body fluids. This is apt, given that the immunity’s mechanism of action predominantly involves substances present in these fluids.
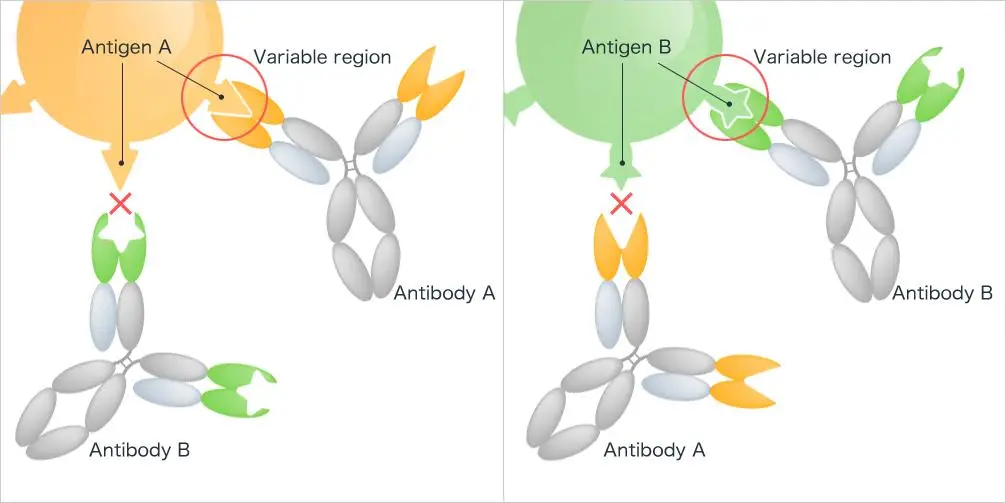
- The specificity of humoral immunity is evident in its tailored response against individual antigens. This specificity ensures that the body’s defenses are optimally directed against particular threats, enhancing the efficiency of the immune response. One of the hallmarks of humoral immunity is the production and secretion of antibodies by B cells, which play a pivotal role in neutralizing extracellular threats.
- Extracellular spaces, as deduced from numerous scientific investigations, are susceptible to invasion by a myriad of pathogens. Even pathogens that primarily reside within cells occasionally exploit the extracellular domain to migrate between cells. This underscores the significance of humoral immunity in safeguarding these spaces.
- In a broader context, humoral immunity encompasses the actions of macromolecules, including antibodies, complement proteins, and certain antimicrobial peptides, all of which are found in extracellular fluids. This immunity stands in contrast to cell-mediated immunity, which involves direct cellular responses to pathogens.
- The intricate study of the components constituting the immune system, their functionalities, and interactions is central to the field of immunology. Within this realm, humoral immunity is recognized for its role in antibody production and the associated processes, such as Th2 activation, germinal center formation, isotype switching, and the generation of memory cells. These processes collectively ensure that the body is equipped to neutralize pathogens, activate the complement system, and enhance phagocytosis, thereby effectively eliminating threats.
Humoral immunity definition
Humoral immunity, also known as the humoral immune response, refers to the branch of the immune system that produces antibodies in response to extracellular pathogens and foreign substances, primarily mediated by B-lymphocytes and plasma cells in the body’s extracellular fluids.
History
The evolution of our understanding of the immune system has been marked by groundbreaking discoveries and the contributions of pioneering scientists. One such figure in this historical narrative is Hans Buchner.
- Hans Buchner and the Humoral Theory: Hans Buchner, a German physician, is credited with the development of the “Humoral Theory” in 1890. This theory emerged from his meticulous studies on serum components, particularly focusing on their antibacterial properties. Buchner identified certain components in the blood serum and body fluids, which he termed “alexins.” He postulated that these alexins possessed inherent properties that enabled them to neutralize pathogenic microorganisms.
- Paul Ehrlich and the Concept of Complements: Subsequent to Buchner’s work, Paul Ehrlich introduced the concept of “complements.” He reinterpreted alexins as complements, describing them as soluble elements of the innate immune response. Ehrlich’s work underscored the versatility of complements, highlighting their involvement in both cell-mediated and humoral immune responses. His findings played a pivotal role in bridging the understanding between innate and adaptive immunity.
- Emil von Behring, Kitasato Shibasaburō, and the Discovery of Antitoxins: The concept of antitoxins was further elucidated by Emil von Behring and Kitasato Shibasaburō. Their collaborative research centered on bacteria responsible for diphtheria and tetanus. They discerned that upon exposure to bacterial toxins, the serum began to accumulate antitoxins. This enriched serum, they proposed, could be harnessed to immunize individuals who had not been previously exposed or immunized.
- Paul Ehrlich’s Contribution to Antibody Research: Paul Ehrlich’s research extended beyond complements. He delved into the role of antibodies in the immune response. Through his work on specific antibodies produced against plant toxins, namely ricin and abrin, Ehrlich posited that antibodies were instrumental in orchestrating the immune response against antigens.
- Anti-Diphtheria Antibody and Nobel Recognition: In a collaborative effort, Paul Ehrlich and Emil von Behring synthesized an antitoxin targeting the diphtheria toxin. This monumental achievement did not go unnoticed. In recognition of his invaluable contributions to medical science, Paul Ehrlich was conferred the Nobel Prize in Physiology or Medicine in 1908.
Characteristic pattern for Production of antibodies
The production of antibodies, a hallmark of the humoral immune response, follows a distinct and characteristic pattern. This process can be delineated into four sequential phases, each with its unique features:
- Lag Phase: This initial phase ensues immediately after the body is exposed to an antigen. During this period, the immune system recognizes and processes the antigen, but no detectable levels of antibodies are present in the bloodstream.
- Log Phase: Following the lag phase, the immune system enters the log phase. Here, there is a consistent and exponential increase in the concentration of antibodies in the bloodstream, indicating active synthesis and release of these molecules by B-lymphocytes and plasma cells.
- Plateau Phase: As the name suggests, this phase represents a state of equilibrium. The rate of antibody synthesis is counterbalanced by their catabolism, leading to a stabilization in the levels of circulating antibodies.
- Phase of Decline: In this final phase, the catabolic activities surpass the synthesis of antibodies. This results in a gradual reduction in the circulating antibody titers.
Antibodies
Antibodies, often referred to as immunoglobulins (Ig), are specialized proteins integral to the immune system. They play a pivotal role in the body’s defense mechanism against foreign invaders, such as pathogens.
- Origin and Biochemical Composition: The foundational concept of antibodies can be attributed to the pioneering work of Emil von Behring and Kitasato Shibasaburō. Biochemically, antibodies are glycoproteins and fall under the broader category of the immunoglobulin superfamily. While the terms “antibody” and “immunoglobulin” are frequently used synonymously, they essentially refer to the same molecular entities.
- Distribution and Function: Given their role in mediating humoral immunity, antibodies predominantly reside in various body fluids, including blood, secretions, and tissue fluids. Their primary function is to recognize and neutralize foreign antigens, thereby preventing potential infections. The therapeutic potential of antibodies is also being explored, with advancements in designing antibodies that can target intracellular antigens, offering potential strategies for addressing tumor antigens.
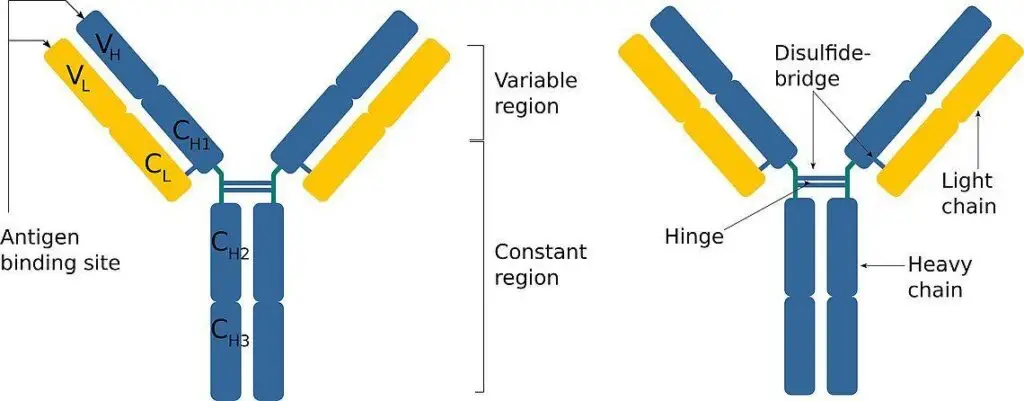
- Structural Attributes: Molecularly, antibodies exhibit a distinct Y-shaped configuration. This structure comprises four polypeptide chains: two heavy chains and two light chains. Each chain possesses an NH2 terminal and a C terminal. The NH2 terminal of all four chains, spanning a sequence of 100-110 amino acids, forms the Complementarity Determining Region (CDR) or the Hypervariable Region (HVR). This region is crucial for antigen recognition. The remaining segment of the molecule is termed the framework region or the constant region.
- Classification of Antibodies: Based on the variations in the constant region of the heavy chains, immunoglobulins are categorized into five primary classes: IgG, IgM, IgA, IgD, and IgE. Each class of immunoglobulin is tailored to combat specific types of antigens, reflecting the evolutionary adaptation of the immune system to diverse challenges.
In conclusion, antibodies are indispensable components of the immune system, offering a sophisticated line of defense against a myriad of external threats. Their intricate structure and diverse classifications underscore their versatility and specificity in safeguarding the body against potential infections.
Antibody production
Antibodies, also known as immunoglobulins, are specialized proteins produced and secreted by plasma B cells. These cells are characterized by markers such as CD-38, CD-79, and CD-138. The initial immunoglobulin produced upon antigen recognition is IgM.
Role of Antibodies in Adaptive Immunity: Antibodies are pivotal in the acquired immune response. They facilitate the identification and neutralization of antigens. Each antibody is tailored to recognize a specific antigen. The processes mediated by antibodies include:
- Agglutination and precipitation through the formation of antibody-antigen complexes.
- Priming for phagocytosis, which involves macrophages and other immune cells.
- Blocking viral receptors.
- Augmenting other immune responses.
- Initiating the complement pathway.
B Cell Activation and Pathways: B cell activation is a crucial step in antibody production. The naive B lymphocytes, upon encountering an antigen, undergo a signaling cascade initiated at their receptors. These receptors bind to unprocessed, hydrophilic antigens. There are two primary pathways for B lymphocyte activation:
- Helper T cell-independent pathway: Here, naive B cells develop B-cell receptors (BCRs) displayed on their surface. Upon encountering carbohydrate or lipid antigens, these B cells transition to an active state, undergo clonal proliferation, and produce plasma B cells responsible for synthesizing immunoglobulins.
- Helper T cell-dependent pathway: In this pathway, naive B cells, upon encountering proteinaceous antigens, internalize them through endocytosis. Post-processing, these antigens are presented on the B cell’s surface via MHC-II proteins. This presentation facilitates helper T cells’ recognition, which then secrete interleukins IL-4 and IL-6. IL-4 activates B cells, while IL-6 promotes their proliferation and differentiation.
B Cell Proliferation: B cell proliferation varies based on the activation pathway:
- In the helper T cell-independent pathway, B cell proliferation occurs without the need for IL-4 and IL-6 stimulation. This results in the production of plasma B cells but not memory B cells.
- In the helper T cell-dependent pathway, IL-4 and IL-6 are essential for B cell proliferation. This process produces both plasma B cells and memory B cells, which can last up to 10-20 years. Upon re-infection with the same antigen, these memory B cells swiftly stimulate antibody production.
Antibody-Antigen Interaction: The interaction between an antibody and its specific antigen is termed the antibody-antigen reaction. This highly specific chemical interaction was first detailed by Richard J. Goldberg in 1952, leading some to refer to it as “Goldberg’s theory.” Following this interaction, an agglutination process can occur, where cross-linking of antigens by antibodies results in a clumping appearance.
Significance of the Antibody-Antigen Reaction: The antibody-antigen reaction is fundamental to humoral immunity. By consistently initiating this reaction against foreign agents, the body fortifies its immune defenses, ensuring robust protection against potential threats.
Components of Humoral immunity
Humoral immunity, a critical arm of the adaptive immune system, is primarily mediated by antibodies. To fully grasp the intricacies of humoral immune responses, it’s essential to delve into its key components.
- Pathogens: Pathogens are harmful microorganisms distinct from the body’s normal flora. While many bacteria coexist harmoniously within us, pathogens are foreign entities that can cause disease. It’s crucial to differentiate between these harmful invaders and the benign microorganisms that typically reside in our systems.
- Antigens: Antigens are specific proteins or molecules present on the surface of pathogens. They can be proteins, glycoproteins, lipoproteins, polysaccharides, lipopolysaccharides, nucleic acids, or lipids. Not every antigen triggers an immune response. Those that do are termed immunogens, with proteins and polysaccharides being the most common. The active region of an antigen, responsible for binding to immune cells and antibody receptors, is known as the epitope.
- Haptens: Haptens are smaller molecules that, on their own, don’t present as complete antigens. However, when combined with carrier proteins, they can elicit an immune response. A classic example is urushiol from poison ivy, which becomes immunogenic when bound to skin proteins.
- Lymphoid Organs: These are specialized structures where immune cells develop, mature, and get activated. Primary lymphoid organs, like the red bone marrow and thymus gland, facilitate the formation and maturation of lymphocytes. Secondary lymphoid organs, including lymph nodes, spleen, and tonsils, are sites where lymphocytes are activated.
- Antigen-Presenting Cells (APCs): APCs play a pivotal role in the immune response by capturing pathogens, processing them, and presenting their antigens on their surface to T cells. This presentation is crucial for the activation of T cells, bridging innate and adaptive immunity.
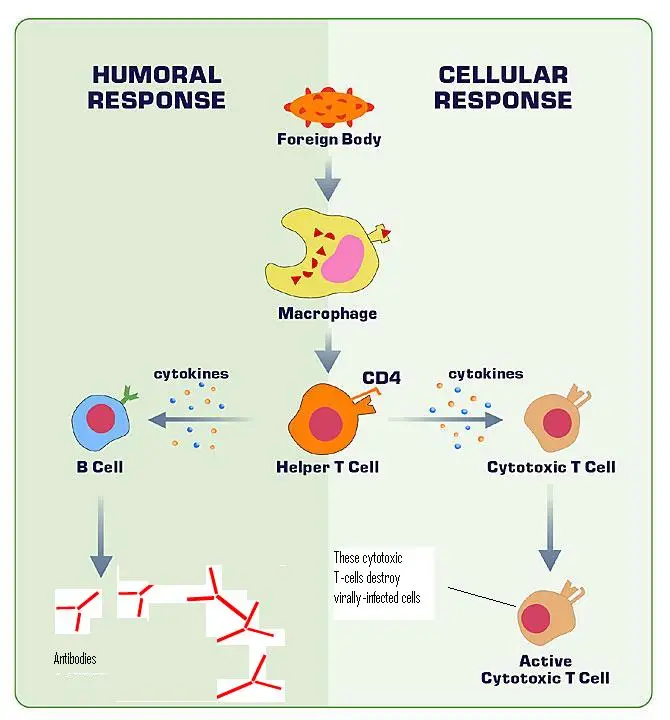
- T Cells: T lymphocytes, or T cells, are central players in both innate and adaptive immunity. While cytotoxic T cells directly target and destroy infected cells, helper T cells act as intermediaries, releasing cytokines to attract B cells and bridging the gap between the two immune systems.
- B Cells: B cells, or B lymphocytes, serve multiple roles. They can act as APCs, secrete cytokines, and most importantly, produce antibodies. Upon activation, B cells can differentiate into plasma cells, which produce antibodies, or memory cells, which provide long-term immunity against specific pathogens.
- Antibodies: Antibodies, or immunoglobulins, are the primary effectors of humoral immunity. They recognize and neutralize pathogens in various ways, such as direct binding, neutralization, or promoting phagocytosis. There are five main types of immunoglobulins: IgM, IgA, IgD, IgG, and IgE, each with a specific role in the immune response.
In conclusion, humoral immunity is a multifaceted system comprising various components, each playing a unique role in defending the body against foreign invaders. Understanding these components and their interactions is crucial for appreciating the complexity and efficiency of our immune system.
Phases/Humoral Immune response steps
Primary Phase/Primary humoral immune response
The humoral immune response, a pivotal component of the adaptive immune system, operates in distinct phases to ensure an effective defense against foreign pathogens. The primary phase, also known as the initial response, is characterized by a series of systematic and coordinated events that occur upon the body’s first encounter with an antigen.
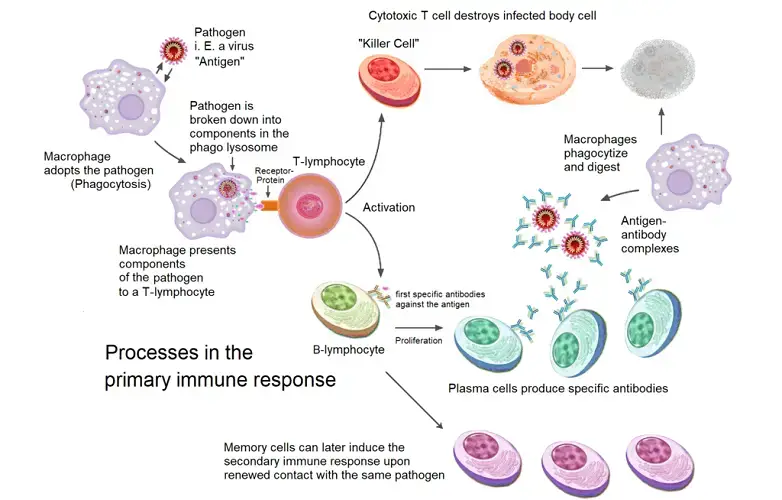
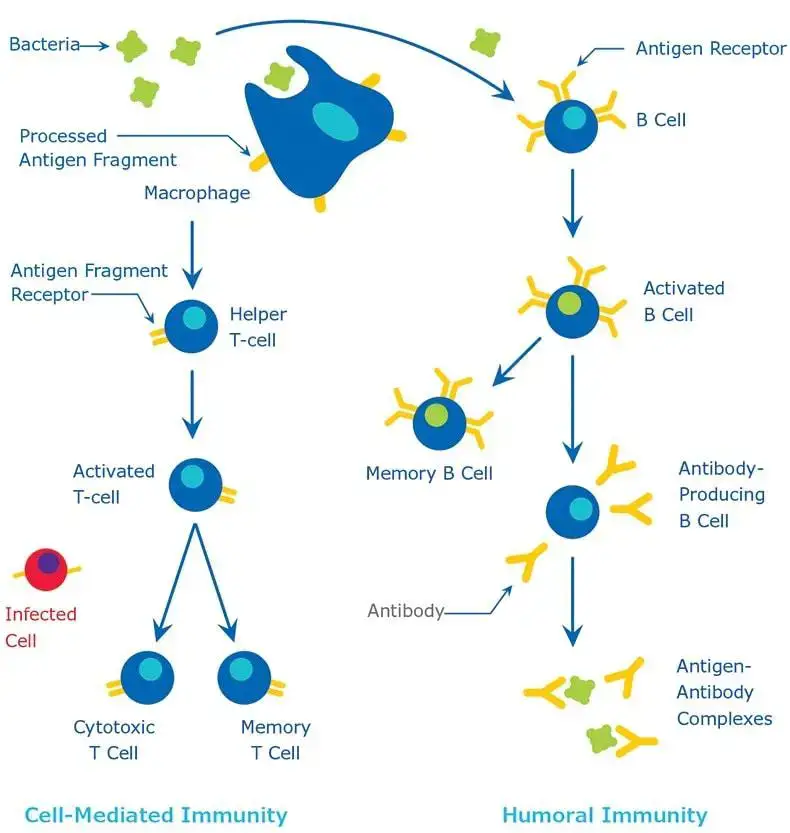
- Antigen Encounter and Processing: Upon the initial contact with a foreign pathogen, antigen-presenting cells (APCs) capture and internalize the antigen. These APCs then digest the antigen, converting specific fragments into major histocompatibility complex class II (MHC II) surface proteins.
- T Helper Cell Activation: The MHC II surface proteins, now presenting the antigen fragments, are recognized by T helper cells. This recognition is a crucial step, as it ensures that the immune system responds specifically to the foreign antigen.
- Cytokine Production: Upon recognizing the MHC II-antigen complex, T helper cells produce cytokines, signaling molecules that play a vital role in modulating the immune response.
- B Cell Activation and Differentiation: Influenced by the cytokines released by T helper cells, naïve B cells become activated. Once activated, these B cells undergo differentiation, giving rise to two primary cell types: plasma cells and memory B cells.
- Antibody Production: The newly formed plasma cells embark on their primary function: the production and secretion of antibodies. Initially, IgM antibodies are produced. However, if the pathogen persists even after peak IgM secretion, the immune system may also produce IgG or IgA antibodies to bolster the defense.
- Temporal Dynamics: The primary phase is not instantaneous. Following the initial exposure to an antigen, there’s a lag period, typically ranging from 7 to 10 days, before peak antibody levels are reached in the serum. This lag can vary based on the nature of the antigen; for instance, certain antigens might induce a response within hours, while others might take weeks. Factors influencing this period include the antigen’s dose, its mode of delivery (oral or parenteral), and its inherent properties.
- Antibody Dynamics: During this primary response, the concentration of antibodies in the serum rises for several weeks before gradually declining. While IgM levels tend to wane more rapidly, IgG levels persist for a more extended period, offering sustained protection.
In summary, the primary phase of the humoral immune response is a meticulously orchestrated series of events that ensure the body mounts an effective defense against novel pathogens. This phase lays the foundation for subsequent immune responses, ensuring that the body is better prepared for future encounters with the same antigen.
Secondary Phase/Secondary humoral immune response
The secondary humoral immune response, also referred to as the anamnestic or memory response, represents the immune system’s enhanced reaction to a previously encountered pathogen. This heightened response is facilitated by memory B cells, which are generated during the primary exposure to the antigen and can persist for extended durations, ranging from weeks to years.
- Activation of Memory B Cells: Upon re-exposure to the same antigen, memory B cells swiftly recognize the antigenic determinants of the microorganism. Their previous encounter with the antigen equips them with a heightened state of readiness, enabling a rapid and efficient response.
- Clonal Expansion and Differentiation: Upon antigen recognition, memory B cells undergo clonal expansion, producing a large number of highly-specific plasma cells. These plasma cells are tailored to produce antibodies that specifically target the encountered antigen.
- Enhanced Antibody Production: In the secondary response, there is a marked increase in the production of antibodies, surpassing the levels observed during the primary response by over a thousand-fold. While IgG is the predominant immunoglobulin produced, there is also a notable production of IgM, IgA, and IgE.
- Rapid Peak Response: One of the hallmarks of the secondary humoral immune response is the swift attainment of peak antibody levels, typically within a mere 3 to 5 days post-exposure. This contrasts with the longer lag period observed during the primary response.
- Persistence of Memory Cells: The enhanced and rapid secondary response can be attributed to the presence of antigen-specific memory cells, which persist post the initial antigen encounter. These cells undergo extensive proliferation, generating a vast array of specific B cells and plasma cells that mediate the secondary response.
- Qualitative Differences in Antibody Production: While the quantity of IgM produced during the secondary response is similar to that of the primary response, there is a significant increase in the production of IgG. Moreover, the produced IgG persists for a more extended period during the secondary response.
- Enhanced Antibody Affinity: Antibodies produced during the secondary response exhibit a higher affinity for the antigen, meaning they bind more tightly and are less likely to dissociate. This increased binding strength arises from somatic hypermutation, a process where changes occur in the DNA encoding the antigen-binding site, optimizing antibody-antigen interactions.
Fate of Antigen in Tissues
The fate of an antigen within the body is largely determined by its mode of introduction. This trajectory and eventual localization of the antigen play a pivotal role in shaping the immune response.
- Subcutaneous Administration: When antigens are introduced subcutaneously, they predominantly localize in the draining lymph nodes. The lymphatic system, which drains the interstitial fluid from tissues, captures these antigens and transports them to the lymph nodes. Here, specialized immune cells can recognize, process, and initiate an immune response against these antigens. Only a minuscule fraction of the antigen reaches the spleen, a secondary lymphoid organ, when introduced via this route.
- Intravenous Administration: Intravenous delivery of antigens paints a different picture. Such antigens are primarily found in systemic organs like the spleen, liver, bone marrow, kidneys, and lungs. The direct entry into the bloodstream allows these antigens to circulate widely and interact with various tissues. Interestingly, despite the extensive vascular network connecting them, lymph nodes exhibit minimal presence of these intravenously introduced antigens.
- Antigen Clearance: A significant portion of the antigens, regardless of their route of entry, undergo degradation. Reticuloepithelial cells, which form part of the body’s mononuclear phagocyte system, play a crucial role in this process. They engulf and break down these antigens, ensuring that the body is not overwhelmed. Post degradation, around three-quarters of these antigens are excreted via the urine, effectively eliminating them from the body.
Production of Antibodies
The intricate process of antibody synthesis is a testament to the body’s coordinated defense mechanism. This synthesis is orchestrated through the collaborative efforts of three primary cell types: macrophages, T helper cells, and B cells.
- Role of Antigen-Presenting Cells (APCs): Macrophages and dendritic cells, classified as APCs, are pivotal in presenting antigens to immunocompetent cells. For many T-cell-dependent antigens, including proteins and erythrocytes, macrophages are essential for processing the antigen before antibody production can commence. However, T-cell-independent antigens bypass this macrophage processing. Post-processing, macrophages and dendritic cells exhibit the antigen on their surface, either in its native form or as processed fragments.
- Macrophages and Optimal Antigen Exposure: Macrophages play a nuanced role in modulating the antigen dose exposed to lymphocytes, ensuring the optimal activation of immunological responses. Following macrophage processing, antigen fragments, in conjunction with class II MHC proteins, surface on the macrophages. This complex then engages specific receptors on helper T cells.
- T Cell Activation and B Cell Stimulation: Upon interaction with the antigen-class II MHC protein complex, helper T cells release cytokines. These cytokines, including Interleukin-2, Interleukin-4, and Interleukin-5, stimulate B cells to produce antigen-specific antibodies. The B cells, once activated, undergo clonal proliferation, differentiating into plasma cells. These plasma cells are the factories producing specific immunoglobulins or antibodies, which play roles such as neutralizing toxins and viruses and facilitating pathogen uptake by phagocytic cells.
- T-cell-independent Antigens: While helper T cells are indispensable for most antibody productions, certain chemicals, like polysaccharides, can activate B cells without T cell assistance. These are termed T-cell-independent antigens. However, these antigens prompt B cells to produce only IgM antibodies. The synthesis of other antibody types, such as IgG, IgA, and IgE, necessitates Interleukins 4 and 5, which are exclusively produced by T helper cells.
- B Cell Antigen Recognition: B cells employ their surface IgM as an antigen receptor, enabling them to recognize a plethora of antigens, from proteins to lipids and nucleic acids. This broad recognition spectrum empowers B cells to generate antibodies against a vast array of molecules. However, for protein fragments to be presented to helper T cells, they must be associated with class II MHC proteins.
Theories of antibody formation
The genesis of antibodies, essential components of the immune system, has been a subject of scientific curiosity and debate. Over time, two primary theories have emerged to explain the mechanism of antibody formation: the instructive theory and the selective theory.
A. Instructive Theory
This theory posits that an immunocompetent cell inherently possesses the capability to produce a myriad of antibodies. The antigen, upon introduction, instructs these cells to generate specific antibodies complementary to itself. Two sub-theories further elucidate this concept:
- Direct Template Theory:
- Proposed by Breinl and Haurowitz in 1930.
- Suggests that the antigen or its determinants act as a template, guiding the formation of the antibody. The resulting antibody molecule adopts a structure complementary to the antigenic template.
- Indirect Template Theory:
- Introduced by Burnet and Fenner in 1949.
- Proposes that antigenic determinants, upon entering antibody-producing cells, induce a hereditary change. This change involves the integration of the antigenic determinant’s genocopy into the genome, which is then passed on to progeny cells. However, this explanation has lost favor over time.
B. Selective Theories
These theories suggest that the body already possesses a repertoire of potential antibodies, and the introduction of an antigen merely selects the appropriate one. Three sub-theories further detail this concept:
- Side Chain Theory:
- Presented by Ehrlich in 1898.
- Argues that immunocompetent cells have surface receptors that can interact with antigens possessing complementary side chains. Upon antigen introduction, these receptors bind with the antigen, becoming inactive. To compensate, the cell overproduces similar receptors, which are then released as antibodies.
- Natural Selection Theory:
- Proposed by Jerne in 1955.
- Suggests that during fetal development, the body produces globulin molecules against all conceivable antigens. Upon antigen introduction, the antigen selectively binds to the most complementary globulin molecule, stimulating the production of the corresponding antibody.
- Clonal Selection Theory:
- Introduced by Burnet in 1957.
- Proposes that during fetal development, a vast array of immunologically competent cell clones, each with a unique antibody pattern, is produced. Each of these cells expresses membrane receptors specific to a particular antigen, determined before the cell’s exposure to the antigen. Upon antigen introduction, the cell with the matching receptor is activated, proliferates, and produces the corresponding antibody. This theory, widely accepted today, offers insights into immunological specificity, memory, and the ability to distinguish between self and non-self.
Factors affecting production of antibodies
The production of antibodies, a pivotal aspect of the immune response, is influenced by a myriad of factors. Understanding these determinants is crucial for both basic immunology and clinical applications. Herein, we delve into the significant factors that modulate antibody production:
- Genetic Factors:
- The genetic makeup of an organism dictates its response to antigens. Some are responders, producing antibodies upon exposure, while others, termed nonresponders, do not.
- The immune response (Ir) gene, located on the short arm of chromosome six, governs these variations.
- Age:
- Neonates and embryos exhibit an underdeveloped immune competence.
- Full immunological competence, marked by the maturation of lymphoid organs, is achieved by ages 5-7 for IgG and 10-15 for IgA.
- Nutritional Status:
- Malnutrition detrimentally affects both humoral and cell-mediated immunity.
- Specific deficiencies, such as those of amino acids and vitamins, can curtail antibody production.
- Route of Antigen Administration:
- The mode of antigen delivery plays a pivotal role in eliciting an immune response. Parenteral administration typically induces a more robust response compared to oral or nasal routes.
- Dose of Antigen:
- An optimal dose of antigen is essential for a maximal immune response. Extremely high or low doses can lead to immunological paralysis, a state of reduced immune responsiveness.
- Multiple Antigens:
- Concurrent administration of multiple antigens can lead to varied antibody responses due to antigenic competition. The precise formulation and proportion of these antigens are crucial for achieving desired outcomes.
- Adjuvants:
- Adjuvants are substances that enhance the immunogenicity of antigens by prolonging antigenic stimulation. Examples include Freund’s incomplete and complete adjuvants, aluminum salts, and other substances like silica particles.
- Immunosuppressive Agents:
- These agents dampen the immune response and are employed in scenarios like transplantation surgeries. Examples include:
- X-irradiation: Impedes antibody production post 24 hours of exposure.
- Radiometric Drugs: Specifically target B cells, inhibiting their replication.
- Corticosteroids: Diminish the reactivity of B and T lymphocytes by modulating interleukin synthesis.
- Antimetabolites: Hinder DNA and RNA synthesis, curtailing cell proliferation and differentiation.
- Antilymphocyte Serum (ALS): Targets circulating lymphocytes, sparing those in lymphoid organs.
- These agents dampen the immune response and are employed in scenarios like transplantation surgeries. Examples include:
Monoclonal Antibodies
Monoclonal antibodies (mAbs) are a class of antibodies derived from a single cell lineage, ensuring their homogeneity. Unlike polyclonal antibodies, which are produced by multiple plasma cell clones and are heterologous in nature, monoclonal antibodies are specific to a single antigenic determinant.
Origin and Distinction: Monoclonal antibodies originate from conditions like multiple myeloma, where a singular plasma cell clone produces antibodies against a specific antigenic determinant. This specificity contrasts with polyclonal antibodies, which arise from various plasma cell clones in response to an antigen.
Production Methodology: The pioneering method for monoclonal antibody production was introduced by Kohler and Milstein in 1975, earning them the Nobel Prize in 1984. Their technique involved the creation of hybridomas, which are formed by fusing myeloma cells with antibody-producing cells. These hybridomas, once established, can indefinitely produce monoclonal antibodies, proving invaluable for research and diagnostic purposes.
The process involves:
- Inoculating an animal, typically a mouse, with the desired antigen.
- Fusing the lymphocytes from the animal’s spleen with mouse myeloma cells that lack the enzyme hypoxanthine phosphoribosyl transferase (HPRT).
- Facilitating cell fusion using agents like polyethylene glycol.
- Culturing the fused cells in a medium (HAT media) that supports hybrid cell growth but inhibits the growth of parent cells.
- Screening the resulting cell clones for antibody production against the target antigen.
- Selecting and perpetually culturing the clones that produce the desired antibody.
Advancements and Therapeutic Applications: The original mouse-derived monoclonal antibodies posed challenges for therapeutic use in humans. Consequently, advancements led to the development of human monoclonal antibodies, including chimeric antibodies. These chimeric antibodies, composed of human constant regions and mouse variable regions, are being explored for leukemia treatment. Furthermore, they can be employed to target tumor cells either by delivering toxins, such as diphtheria, or through complement-mediated cytotoxicity.
Function of Antibodies
Antibodies, also known as immunoglobulins, play a pivotal role in the immune system’s defense mechanisms against pathogens and their associated products. These Y-shaped proteins are specifically tailored to recognize and neutralize foreign invaders, ensuring the host’s protection against various infections. Herein, we delve into the multifaceted functions of antibodies:
- Neutralization: One of the primary roles of antibodies is neutralization. By binding directly to pathogens or their toxins, antibodies can inhibit their ability to attach to and infect host cells. For instance, antibodies specific to bacterial toxins can obstruct the toxin’s interaction with host cells, thereby neutralizing its harmful effects. Similarly, antibodies can prevent viruses or bacteria from initiating infections by hindering their attachment to target cells.
- Opsonization: Opsonization is a process where antibodies coat pathogens, enhancing their recognition and uptake by phagocytic cells, such as macrophages and neutrophils. When antibodies bind to pathogens, the Fc region of the antibody interacts with Fc receptors on phagocytes. This interaction facilitates the engulfment and subsequent destruction of the pathogen by the phagocyte.
- Complement Activation: Antibodies can also activate the complement system, a series of proteins that work in tandem to destroy pathogens. Upon binding to pathogens, certain antibodies can trigger the complement cascade, leading to the formation of the membrane attack complex (MAC) that directly lyses certain bacteria and viruses. Additionally, some byproducts of the complement cascade, like C3b, can opsonize pathogens, further enhancing their phagocytosis.
- Diagnostic Utility: Beyond their physiological roles, antibodies serve as crucial tools in diagnostic medicine. The presence and concentration of specific antibodies, such as IgG, IgM, and IgA, in a patient’s serum can provide insights into the individual’s humoral immunity status. Techniques like radial immunodiffusion and immunoelectrophoresis are commonly employed to measure these antibodies, aiding in the diagnosis and monitoring of various diseases.
Humoral Immunity Examples
Humoral immunity, a cornerstone of the adaptive immune system, is characterized by the production of antibodies by B cells in response to foreign antigens. This mechanism has been instrumental in defending against various pathogens throughout history. Here, we elucidate two distinct examples of humoral immunity, spanning ancient and modern times.
1. Smallpox and the Advent of Vaccination
Historical records, including the discovery of smallpox lesions on the mummy of Pharaoh Ramses V, suggest the ancient prevalence of this deadly disease. Smallpox had catastrophic impacts, particularly decimating indigenous populations in the Americas and Australia. However, by the end of 1979, a global vaccination campaign successfully eradicated the virus, marking a monumental achievement in the annals of medicine.
The origins of vaccination can be traced back to Edward Jenner’s astute observation regarding the resistance of milkmaids to smallpox during its rampant spread. Prior to Jenner’s work, variolation, a practice involving the intentional introduction of pus from smallpox victims into healthy individuals, was employed as a rudimentary form of inoculation. While the underlying mechanisms of the secondary humoral immune response were not yet understood, the principle of inducing a mild infection to confer future protection was recognized.
Jenner’s groundbreaking experiment involved the inoculation of a young boy with pus derived from cowpox sores of a milkmaid. Remarkably, the boy exhibited immunity to subsequent smallpox exposure, laying the foundation for modern vaccination.
2. SARS-CoV-2 Vaccination: A Modern Marvel
The recent global challenge posed by the SARS-CoV-2 pandemic spurred rapid advancements in vaccine development. Unlike traditional vaccines employing weakened or inactivated pathogens, innovative approaches were necessitated due to limited knowledge about the virus.
The Oxford-AstraZeneca vaccine employs a viral vector strategy. Utilizing a genetically modified chimpanzee cold virus, known to elicit robust immune responses, the vaccine introduces the SARS-CoV-2 spike protein into the human body. This foreign protein triggers the primary humoral response, culminating in antibody production against the virus.
Conversely, the Pfizer-BioNTech and Moderna vaccines utilize messenger RNA (mRNA) technology. These vaccines deliver the genetic blueprint for the COVID-19 spike protein directly into cells. The cells then synthesize the spike protein, initiating the humoral immune response. Contrary to some misconceptions, mRNA vaccines do not alter human DNA. Instead, they harness the cellular machinery for protein synthesis, bypassing the transcription phase and directly initiating translation.
Differences Between Humoral and Cell-mediated Immunity – Humoral immunity vs cell mediated immunity
The immune system is a complex network of cells and proteins that defends the body against pathogens. It can be broadly categorized into two main types: humoral immunity and cell-mediated immunity. Each type has distinct characteristics and functions. Here, we delineate the differences between these two branches of the immune response.
| Characteristic Feature | Humoral Immunity | Cell-mediated Immunity |
|---|---|---|
| Antigen-specific antibodies produced | Yes | No |
| Triggered by … | B-cells/B cell lymphocytes | T-cells/T cell lymphocytes |
| Mediation via … | B-cells, T-cells, and Macrophages | Helper T-cells, Cytotoxic T cells, NK cell (killer T cells), and Macrophages |
| Action on/Target | Extraneous or extracellular microorganisms and their toxins | Intracellular microbes (e.g., tumor cells, viruses, bacteria, parasites) |
| Antigen-recognizing cell receptor | BCR receptors (mIgM+mIgD) *NOTE: ‘m’ stands for membranous. | TCR receptors |
| Accessory receptors involved | Igα, Igβ, CD40, CD21, Fe receptors | CD-2,3,4,8,28 & Integrin receptors |
| Antigen processing is needed | No (Unprocessed antigens are recognized.) | Yes (Antigens are first processed and then presented by MHC of antigen-presenting cells) |
| Major secretory cells | B-cells | T-cells |
| Major secretion | Antibodies (Antibody-mediated immunity) | Cytokines (signaling proteins) |
| Time of action/Spontaneity | Rapid | Delayed/Slow |
| Action on transplants and tumor cells | No | Yes |
Quiz
Which cells are primarily responsible for producing antibodies in the humoral immune response?
a) T cells
b) Macrophages
c) B cells
d) Dendritic cells
[expand title=”Show answer” swaptitle=”Hide answer”]
Answer: c) B cells
[/expand]
Which type of immunity is conferred by the production of antibodies?
a) Cell-mediated immunity
b) Innate immunity
c) Humoral immunity
d) Passive immunity
[expand title=”Show answer” swaptitle=”Hide answer”]
Answer: c) Humoral immunity
[/expand]
Which of the following is NOT a function of antibodies?
a) Neutralization of pathogens
b) Opsonization
c) Direct cell lysis
d) Activation of the complement system
[expand title=”Show answer” swaptitle=”Hide answer”]
Answer: c) Direct cell lysis
[/expand]
The primary immune response is characterized by the production of which type of antibody?
a) IgG
b) IgE
c) IgM
d) IgA
[expand title=”Show answer” swaptitle=”Hide answer”]
Answer: c) IgM
[/expand]
Which of the following is a characteristic of the secondary humoral immune response?
a) Slower onset than the primary response
b) Lower antibody concentration than the primary response
c) Production of memory B cells
d) Absence of IgG production
[expand title=”Show answer” swaptitle=”Hide answer”]
Answer: c) Production of memory B cells
[/expand]
Which type of cells present antigens to immunocompetent cells?
a) T cells
b) Antigen-presenting cells (APCs)
c) Plasma cells
d) Memory cells
[expand title=”Show answer” swaptitle=”Hide answer”]
Answer: b) Antigen-presenting cells (APCs)
[/expand]
Which antibody is primarily found in mucosal surfaces and secretions?
a) IgG
b) IgE
c) IgM
d) IgA
[expand title=”Show answer” swaptitle=”Hide answer”]
Answer: d) IgA
[/expand]
Which of the following is NOT a characteristic of monoclonal antibodies?
a) Produced by a single clone of cells
b) Heterogeneous in nature
c) Specific for a single antigenic determinant
d) Used in diagnostic and therapeutic applications
[expand title=”Show answer” swaptitle=”Hide answer”]
Answer: b) Heterogeneous in nature
[/expand]
The process by which antibodies enhance the phagocytosis of pathogens is called:
a) Neutralization
b) Opsonization
c) Agglutination
d) Activation
[expand title=”Show answer” swaptitle=”Hide answer”]
Answer: b) Opsonization
[/expand]
Which of the following is the primary function of the Fc region of an antibody?
a) Binding to antigens
b) Binding to complement proteins
c) Facilitating phagocytosis by binding to Fc receptors on phagocytic cells
d) Inducing direct cell lysis
[expand title=”Show answer” swaptitle=”Hide answer”]
Answer: c) Facilitating phagocytosis by binding to Fc receptors on phagocytic cells
[/expand]
FAQ
What is humoral immunity?
Humoral immunity refers to the component of the adaptive immune system that is mediated by secreted antibodies produced by B lymphocytes, which target extracellular pathogens and their toxins.
How is humoral immunity different from cell-mediated immunity?
While humoral immunity involves the production of antibodies by B cells to combat extracellular pathogens, cell-mediated immunity involves T cells that target and destroy infected cells or intracellular pathogens.
What cells are primarily responsible for humoral immunity?
B cells, or B lymphocytes, are the primary cells responsible for humoral immunity as they produce and secrete antibodies.
Why is it called “humoral” immunity?
The term “humoral” is derived from the Latin word “humor,” which means fluid. It refers to the components of the immune system found in bodily fluids, especially the antibodies present in the serum.
What are the main types of antibodies involved in humoral immunity?
The main types include IgM, IgG, IgA, IgE, and IgD, each having specific functions and locations in the body.
How does vaccination relate to humoral immunity?
Vaccination introduces a weakened or inactivated form of a pathogen or its components into the body, prompting the immune system to produce antibodies against it. This establishes a memory response, allowing the immune system to respond more rapidly and effectively upon future exposures.
What is the primary immune response?
The primary immune response occurs when the immune system encounters a pathogen or its antigens for the first time. It takes longer to develop and results in the production of memory cells and antibodies specific to that pathogen.
How does the secondary immune response differ from the primary response?
The secondary immune response is faster and more potent than the primary response. It occurs upon subsequent exposures to the same pathogen, utilizing memory cells produced during the primary response.
What are monoclonal antibodies?
Monoclonal antibodies are identical antibodies produced by a single clone of B cells. They are specific to a single antigenic determinant and are used in various medical and research applications.
Why is humoral immunity essential for our defense against pathogens?
Humoral immunity provides a defense against extracellular pathogens and their toxins by producing antibodies that can neutralize, opsonize, or lead to the destruction of these threats, preventing infections and diseases.
What is another name for the humoral immune response?
Another name for the humoral immune response is the “antibody-mediated immune response.” This is because this type of immunity is primarily mediated by antibodies produced by B cells in response to extracellular pathogens and their toxins.
The humoral immune response depends on which cells
The humoral immune response depends on B cells (B lymphocytes). These cells are responsible for producing and secreting antibodies that target extracellular pathogens and their toxins.
The humoral immune response is delivered by
The humoral immune response is delivered by antibodies, which are produced and secreted by plasma cells (differentiated B cells). These antibodies circulate in the blood and lymphatic system to neutralize and eliminate extracellular pathogens and their toxins.
References
- Janeway CA Jr, Travers P, Walport M, et al. Immunobiology: The Immune System in Health and Disease. 5th edition. New York: Garland Science; 2001. Chapter 9, The Humoral Immune Response. Available from: https://www.ncbi.nlm.nih.gov/books/NBK10752/
- Tanaka, T., Couser, W., & Nangaku, M. (2011). The Role of Humoral and Cell-Mediated Adaptive Immune Response. In (Ed.), An Update on Glomerulopathies – Etiology and Pathogenesis. IntechOpen. https://doi.org/10.5772/21937
- Metchnikoff, Elie (1905) Immunity in infectious disease. Cambridge University Press
- MLA style: Paul Ehrlich – Facts. NobelPrize.org. Nobel Prize Outreach AB 2022. Sun. 24 Jul 2022. https://www.nobelprize.org/prizes/medicine/1908/ehrlich/facts/
- Pier GB, Lyczak JB, Wetzler LM (2004). Immunology, Infection, and Immunity. ASM Press. ISBN 9781683672111.
- Goldberg, Richard J. (1952). “A Theory of Antibody—Antigen Reactions. I. Theory for Reactions of Multivalent Antigen with Bivalent and Univalent Antibody”. Journal of the American Chemical Society. 74 (22): 5715–5725. doi:10.1021/ja01142a045
- Janeway CA Jr, Travers P, Walport M, et al. Immunobiology: The Immune System in Health and Disease. 5th edition. New York: Garland Science; 2001. Chapter 9, The Humoral Immune Response. Available from: https://www.ncbi.nlm.nih.gov/books/NBK10752/