What is Gel Electrophoresis System?
- Gel electrophoresis system is considered as one of the common laboratory apparatus which is used to separate biological molecules like DNA / RNA / proteins etc. In this system, molecules are separated based on their size, charge and shape, which makes the method highly specific and reliable in analysis.
- The gel medium (mostly agarose or polyacrylamide) is prepared as a semi, solid medium that acts as a sieve restricting the movement of larger molecules.
- An electric current is passed by the power supply unit, and charged molecules move through the gel according to their net charge (negative ones move toward anode).
- The system generally has few main components, gel casting tray, electrophoresis tank, power supply, buffer solution and combs for well formation.
- The DNA, RNA, protein, etc. samples are placed in small wells on a gel, and the dye may be added to monitor the migration path during the run.
- While electrophoresis is performed, voltage and buffer composition are kept at appropriate levels; otherwise band smearing or overheating can be the main problems that result in indistinct separation.
- The separated fragments are thus photographed under UV light after nucleic acid has been stained with dyes like Ethidium bromide or SYBR Safe.
- For protein analysis, the instrument is employed with SDS, PAGE, where sodium dodecyl sulfate uniformly imparts a negative charge to all protein molecules.
- It’s largely utilized in genetics, biochemistry, and molecular biology labs, for instance, to measure fragment length, purity checking, or PCR products confirmation, and so forth.
- Sometimes the phrase “gel electrophoresis unit” is also referred to, which both mean almost the same instrument set, up that functions on the same principle of migration by an electric field.
- The apparatus should be handled with care; leakage, uneven gel or bubble formation can disrupt the migration pattern greatly.
- The system is made for different scales, mini, midi, or large format, depending on the source of the experiment (research, teaching or diagnostic use).
- Thus, Gel Electrophoresis System can be considered as the total gear which makes it possible for charged biomolecules to move and separate through gel medium under the influence of an electrical field.
Definition of Gel Electrophoresis System
Gel electrophoresis is a laboratory technique that uses a gel as a medium to separate and analyze biomacromolecules such as DNA, RNA, and proteins based on their size and charge. An electric field is applied to move the molecules through the gel, with smaller or differently charged molecules migrating faster and farther than larger ones. It is a widely used method in molecular biology and biochemistry for various applications, including DNA fragment analysis, protein separation, and molecular characterization.
Principle of Gel Electrophoresis
The method relies on the movement of ions/molecules through a porous gel medium when an electric field is applied.
The biological molecules in a gel matrix (such as agarose or polyacrylamide) are separated by size, charge, and in some cases, shape.
The sample (DNA, RNA, protein) is put in wells at one end, and because of their net negative charge (for nucleic acids) the fragments move towards the positive electrode (anode).
Smaller molecules are able to move at a higher speed and hence can travel a longer distance through the gel network, while larger molecules are limited/restricted more strongly by the gel pores.
The gel is a molecular sieve, the pore size being determined by gel concentration (higher percent = smaller pores) and that, in turn, determines the resolution of fragments.
The migration rate is also influenced by the charge, to, mass ratio of the molecules; size becomes the major factor when the ratio is approximately constant.
Separated bands after the run are stained/dyed (for instance ethidium bromide for DNA) and visualised under UV light in most experiments.
The principle is used for estimating fragment length by comparison with the standard “ladder” of known sizes and, this foundation, is widely employed in molecular biology works.
Parts of Gel Electrophoresis Apparatus
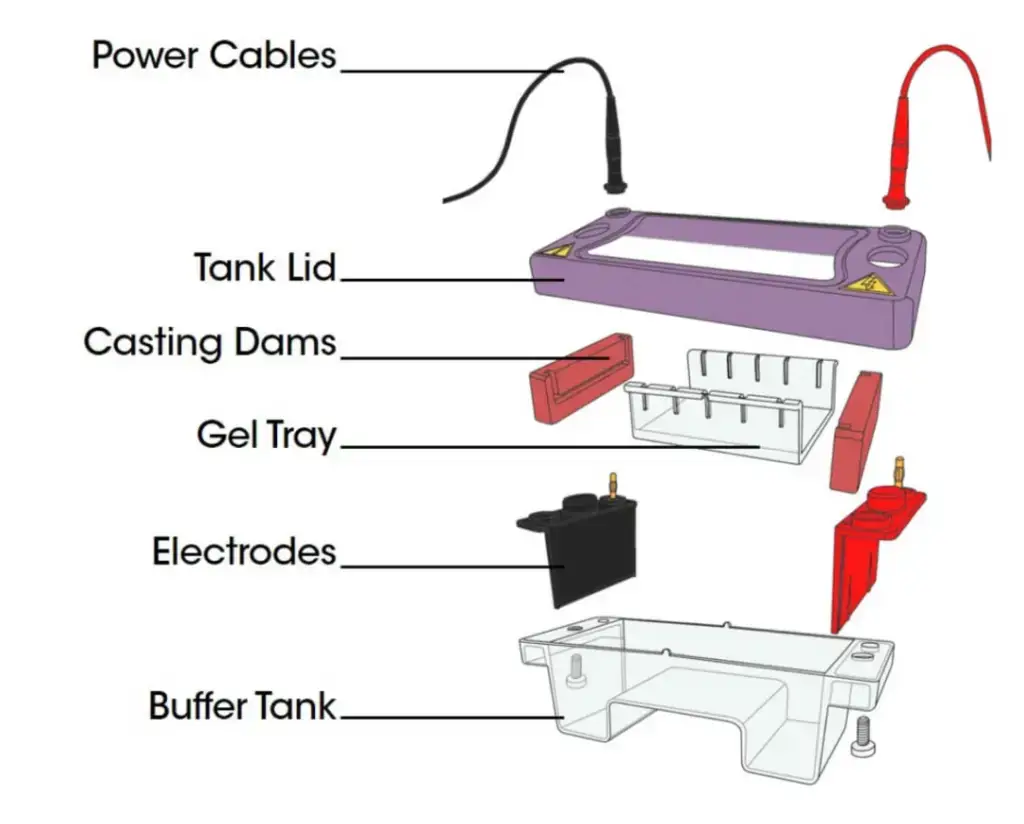
1. Power Supply – Electric current is supplied by it to gel box. The red wire (anode +) and black one (cathode -) is connected for running the current, sometimes the connection loosen if clips not tight. A constant voltage/current/power is maintained for uniform migration. If the current rise too high more heat is produce that can melt the gel, so the supply must be steady always.
2. Gel Casting Tray / Mold– It used for forming the gel slab before electrophoresis run. The liquid agarose or polyacrylamide is poured and after solidifying, the gel is lifted carefully. Sometimes bubbles form in gel which disturb migration, it must be avoid.
3. Comb – It placed in gel before it solidify to form wells (holes). These wells are used to load DNA / RNA / protein samples. After the gel become hard the comb is removed slowly otherwise wells get broken or uneven.
4. Gel Tank / Chamber – This tank hold the buffer solution (like TAE or TBE). The gel tray is placed inside horizontally. Electrodes are attached at both ends of tank, which allow electric current to pass by the gel. It’s usually made from clear acrylic plastic, so movement of dye can seen.
5. Buffer Solution – This acts as conductive medium for electricity between electrodes. The buffer maintain pH (around 8.0) and prevent sample damage. If buffer become too old or diluted, separation quality reduced.
6. Gel Matrix (like Agarose or Polyacrylamide)– Gel is used as a sieving medium where molecules move according their size/charge. The agarose concentration decide the pore size. Higher conc. make small pores, thus slow down big fragments.
7. Sample Loading Wells – The wells formed by comb, they hold DNA samples mixed with loading dye. These dyes help to visualize migration during run. Sometimes the sample float if loaded too fast.
8. Electrode Wires/ Leads – These are connected from power source to the chamber. Red lead connect to positive pole and black to negative. Molecules like DNA (which is negatively charged) move toward anode (+). Reversal of connection can completely ruin run, so polarity must be checked twice.
9. UV Transilluminator/ Gel Doc – After electrophoresis finish, the gel is placed on UV light box to see DNA bands stained by ethidium bromide or SYBR Safe. The UV exposure time must be short otherwise the DNA get damage.
10. Micropipette and Tips – They are used to load precise sample volume (2–20 µL etc.) into wells. Care is taken so that tip not pierce bottom of well. Wrong angle can cause sample mix with buffer.
11. Staining Tray– Used after run for staining and destaining the gel. Usually plastic trays used, but glass ones also sometimes used for better visibility.
12. Cooling System (optional) – Some systems used with cooling plate or circulating buffer to prevail overheating during long runs.
Types of Electrophoresis
There are several types of gel electrophoresis, namely:
1. Paper electrophoresis – It’s one of the oldest types where paper strip acts as supporting medium. Migration of molecules (proteins, amino acids etc.) occurs according to charge/size but resolution is not very high, still it used for simple separation.
2. Agarose gel electrophoresis – A gel of agarose is used as medium. It’s most widely used for DNA / RNA fragments separation. The pore size depends on agarose %; higher % slow down big fragments.
3. Polyacrylamide Gel Electrophoresis (PAGE) – The gel of polyacrylamide is used, mostly for proteins and small nucleic acids. It can run in native or denatured form, depending on whether structure is preserved.
4. SDS–PAGE – This is a modified PAGE where sodium dodecyl sulfate (SDS) detergent denatures protein and gives uniform negative charge, so separation happens only by molecular weight.
5. Pulse–Field Gel Electrophoresis (PFGE) – In this, the electric field direction is changed periodically; it’s used for separating very large DNA molecules (up to several Mb), commonly in genomic analysis.
6. Two–Dimensional Electrophoresis (2D–GE) – It combine two separations: first by isoelectric point (IEF), and second by size in PAGE. Used for protein profiling and comparison studies.
7. Immunoelectrophoresis (Rocket Electrophoresis) – It combines electrophoresis with antigen–antibody reaction. After separation, precipitation arcs (rockets) form showing antigen identity.
8. Difference Gel Electrophoresis (DIGE) – It’s an advanced fluorescent form of 2D–GE where multiple protein samples labeled with different dyes are separated in same gel to compare expression level.
9. Cellulose Acetate Electrophoresis – The medium is cellulose acetate film, useful for separation of serum proteins or hemoglobin variants. Gives faster run and clearer bands than paper.
10. Capillary Electrophoresis (CE) – It’s modern, done inside narrow fused–silica capillary under high voltage; separation happens very fast with small sample. Often used with detector for automation.
11. Isoelectric Focusing (IEF) – Separation of amphoteric molecules occurs in pH gradient where they move until net charge become zero. Highly precise for proteins.
12. Zymography – A PAGE method used for detecting enzyme activity. Substrate is mixed in gel, after electrophoresis bands of enzymatic activity appear as clear zones.
13. Isotachophoresis – Molecules move in discontinuous buffer system forming sharp zones (stacked form). It’s used for analytical and preparative purpose.
14. High Voltage Electrophoresis – Done under high voltage for fast separation in short time, but overheating must be prevented by cooling system.
15. Microchip Electrophoresis – Miniaturized form performed on microfluidic chip (lab-on-chip). It gives quick analysis with very low sample consumption.
Operating procedures of Electrophoresis
The operating procedures of electrophoresis, specifically focusing on DNA gel electrophoresis, can be summarized as follows:
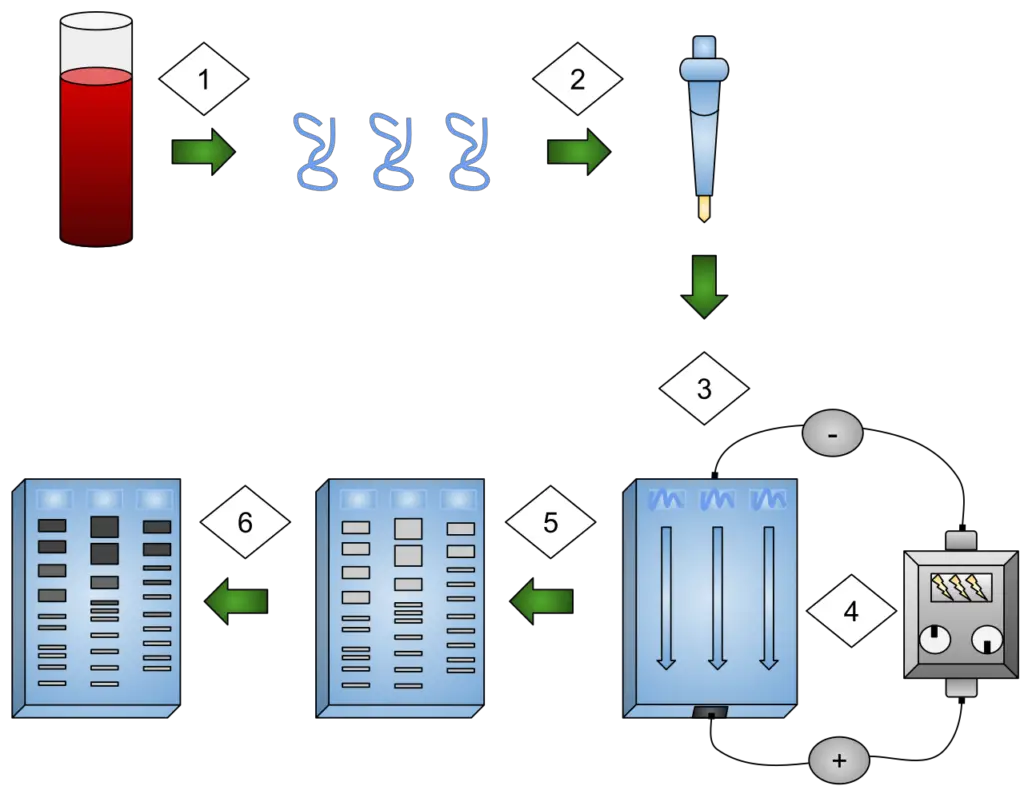
- The gel matrix is made by dissolving agarose (or polyacrylamide) in an appropriate buffer and the solution, while still hot, is poured into a casting tray and left to solidify.
- After the gel has been set the comb is removed and the casting tray is placed in the electrophoresis chamber in such a way that the wells are at the negative terminal side (the cathode) of the box.
- The running-buffer (for instance 1× TAE or TBE) is filled into the chamber until the gel is completely submerged and the buffer ions are free to conduct current
- The samples are made by mixing the DNA (or protein) with loading dye and in some cases the sample is heat-denatured or reduced (for proteins) so that the sample can easily enter the well and sinks down.
- One molecular‐weight ladder (marker) is inserted via a pipette into the first well, thereafter each sample is respectively loaded into its own well by careful pipetting thus avoiding the formation of bubbles and ensuring that the sample is “in the slot” and not spilling out.
- The chamber lid is set on and the electrodes connected to the power supply; the voltage is adjusted (for instance 80-150 V) and the operation is stopped when the dye front has migrated the desired distance.
- Due to the electric field, the negatively charged molecules (for instance DNA fragments) move towards the positive electrode (anode) with the small fragments leading the way as they are able to move faster through the gel matrix.
- Once separation is sufficient the power supply is turned off, electrodes removed, and the gel is taken out of the chamber carefully without tearing the gel.
- If the gel is not pre-stained, it is then stained with a proper dye (for DNA, ethidium bromide or some other, safer, dye; for proteins, Coomassie blue or silver stain) and the excess stain is removed by washing so as to reveal the clear bands.
- The bands, which are visualised by UV light or an imaging system, are photographed and the fragment sizes are calculated from the comparison to the ladder; the decisions made and data recorded.
- The equipment and waste are put in order, the buffer is either disposed of or recycled in a proper way and the safety regulations are followed (for example UV protection, proper disposal of stains etc.).
Applications of Electrophoresis
- In molecular biology research the separation of DNA or RNA fragments is performed by gel electrophoresis and this allows size-based sorting of nucleic acids which helps in gene mapping, PCR product checking, cloning workflows etc.
- Clinical diagnostics are supported when protein electrophoresis (for example immunoelectrophoresis) is used to detect abnormal proteins or antibodies in blood / urine, and often it is used for disease marker screening.
- Forensic science uses the separation of DNA by electrophoresis to build “fingerprints” (genetic profiles) from crime-scene samples, and thus identification of suspects is enabled by the method.
- In food / pharmaceutical quality control the technique is applied when the purity of antibiotics, vaccines or biopharmaceuticals is examined and the separated components are analysed for contaminants or integrity
- In genomics / proteomics large-scale studies are carried out when capillary electrophoresis / micro-chip electrophoresis is used for analysis of proteins, post-translational modifications, peptide mapping etc, advancing high-throughput science.
- In plant breeding / agricultural genetics the method is employed when molecular markers (for example SSRs, RFLPs) are subjected to electrophoresis so that genotyping/variant detection is achieved for crop improvement.
- Environmental / microbial ecology studies are aided as electrophoresis is applied to separation of nucleic acids from environmental samples and microbial communities are profiled by the resulting band-patterns.
Advantages of Electrophoresis
- High efficiency of separation is achieved when charged molecules (like DNA or RNA fragments) are resolved by size/charge in the matrix, which allows clear distinct bands to be formed.
- Only a small amount of sample is required for analysis, and thus precious/limited material can be used with minimal waste.
- The procedure is relatively simple and straightforward to carry out in many labs, hence it’s accessible for many researchers and technicians.
- Fast turn-around can occur, as analysis time is often shortened (for example runs may complete in minutes/hours) which means results are obtained quicker.
- The technique offers good resolution especially for biomolecules of moderate size, and variations (like capillary methods) push that even further.
- Economic benefits are present because equipment and consumables can be less expensive than many more complex instruments, so labs with limited budget can still use it.
- Versatility is afforded since electrophoresis is applied across many fields (molecular biology / clinical diagnostics / forensics) and many molecule-types can be analysed by one thing.
Limitations of Electrophoresis
- A limited throughput is encountered because only a modest number of samples can be processed at one time, and this slows down large-scale studies.
- The resolution is reduced for very small molecules (for example small ions / hormones) which may rush out of the gel or not separate properly.
- Substantial sample quantity is required for certain analyses, and when scarce material is available the method might not be feasible or cost-effective.
- The gel or support medium may undergo over-heating or melting under high voltage/long run, which distorts the separation and reduces accuracy.
- Electrophoresis is not highly quantitative by itself because band intensity/width may vary and precise concentration measurement is less reliable, so further steps often must be taken.
- The pore size / matrix uniformity may vary (for example in agarose gels) which leads to inconsistent migration-rates and sample bands that are smeared or diffused.
- Specialized equipment or conditions may be required for separating large or complex molecules (for example very large DNA fragments), which means that the standard setup cannot handle all molecule-types.
Precautions
- The equipment must be inspected carefully and thoroughly because cracks, buffer leaks or damaged cords could cause serious electrical shock.
- Gloves, lab-coat and goggles must be worn, and long pants & closed-toe shoes are required when the procedure is carried out.
- While preparing hot gels (for example agarose melted at 95°C or so) the container must be handled with insulated gloves and care, since boiled liquid may explode or scald.
- Only one hand at a time should be used to connect leads, and the power should be turned off before lid is opened or any cleaning is done.
- The chemical stains (like ethidium bromide or acrylamide solutions) must be handled in a fume-hood and disposed of as hazardous waste, because they are mutagenic / carcinogenic.
- Equipment must be placed away from sinks, metal plates or other unintended grounding points and the leads must not dangle or touch water / metal surfaces.
- Always follow manufacturer’s instructions, and never override safety interlocks – because unexpected surges or faults could happen and serious injury may occur.
FAQ
What is electrophoresis?
Electrophoresis is a technique used to separate and analyze molecules, such as proteins or nucleic acids, based on their size, charge, or other physical properties using an electric field.
What are the different types of electrophoresis?
There are several types of electrophoresis, including agarose gel electrophoresis, polyacrylamide gel electrophoresis (PAGE), SDS-PAGE, native PAGE, capillary electrophoresis, and isoelectric focusing (IEF).
What is the purpose of electrophoresis?
The main purpose of electrophoresis is to separate and characterize biological molecules based on their physical properties. It is used in various applications, such as DNA sequencing, protein analysis, genetic fingerprinting, and drug development.
How does electrophoresis work?
Electrophoresis works by applying an electric field to a gel or capillary containing the sample. The charged molecules migrate through the gel or capillary based on their charge and size, resulting in separation.
What is the role of a gel in electrophoresis?
The gel provides a matrix through which the molecules can migrate during electrophoresis. Agarose and polyacrylamide gels are commonly used, and their pore size or composition can be adjusted to suit different applications.
How are samples loaded onto an electrophoresis gel?
Samples are loaded into wells created in the gel using a pipette or a specialized sample-loading device. Care must be taken to ensure accurate and consistent loading of samples.
What is the purpose of staining in electrophoresis?
Staining is used to visualize the separated molecules on the gel. DNA or RNA can be stained with dyes like ethidium bromide, while proteins can be visualized using Coomassie Brilliant Blue or silver staining methods.
How is the separated material analyzed after electrophoresis?
After electrophoresis, the separated molecules can be further analyzed using techniques such as Western blotting, Southern blotting, or PCR to study specific molecules of interest.
What are the limitations of electrophoresis?
Some limitations of electrophoresis include the limited number of samples that can be analyzed simultaneously, difficulties in precise quantification, and challenges in separating very small or large molecules.
How do I troubleshoot common issues in electrophoresis?
Common issues in electrophoresis can include poor separation, smearing of bands, or unexpected results. Troubleshooting steps may involve optimizing gel composition, adjusting running conditions, or reviewing sample preparation techniques.