What is Flow cytometry?
- Flow cytometry is an indispensable instrument for the contemporary immunologist.
- It was created by the Herzenbergs (Leonore and Leonard) and their colleagues, and among its initial applications was the analysis of blood cells, namely lymphocyte subpopulations.
- Flow cytometry is an analytical method that counts the frequencies of cells attaching to fluorescent antibodies and scattering light in a distinctive manner.
- When a flow cytometer is modified to sort cell subpopulations based on fluorescence and light scattering, it is known as a fluorescence-activated cell sorter (FACS) (FACS).
- Approximately at the same time, monoclonal antibody and FACS technologies were invented, and the two technological advances proved complementary: the more antibodies accessible for cell type and sorting, the more informative flow cytometric tests became.
- The applications of fluorescent microscopy described thus far are extremely valuable qualitative tools, but they do not provide quantitative data regarding the frequencies of cells within a population that are stained with specific antibodies and therefore belong to experimentally defined subpopulations.
- The advent of the flow cytometer, which was meant to automate the examination and separation of cells labelled with different fluorescent antibodies, overcame this deficiency.
Flow cytometry Instrumentation
- Laser Light: To generate a laser beam.
- Photodiode: Picks up light scattered in the forward direction.
- Sensitive photomultiplier tubes: side-scattered light is measured by sensitive photomultiplier tubes.
- Photomultiplier tubes: To record the emitted light.
- Computer: To store the data.
- Software: To analyze the data.
Flow cytometry Principle
- The reported fluorescent antibody techniques are incredibly useful qualitative tools, but they do not provide quantitative data. This deficiency was overcome with the invention of the flow cytometer, which was designed to automate the analysis and separation of fluorescent antibody-stained cells.
- Using a laser beam and a light detector, the flow cytometer counts single intact cells in suspension.
- Each time a cell passes through the laser beam, light is deflected from the detector, and this disruption in the laser signal is recorded.
- Those cells with a fluorescently labelled antibody linked to their cell surface antigens are activated by the laser and release light, which is detected by a second detection device positioned at a right angle to the laser beam.
- A computer provides charts of the number of cells as the ordinate and their fluorescence intensity as the abscissa. The instrument’s basic form counts each cell as it passes through the laser beam and records the level of fluorescence it emits.
- The equipment is capable of sorting populations of cells into distinct containers based on their fluorescence signature in more advanced models.
- Analysis is the use of the instrument to determine whether and how many individuals of a cell population bind fluorescently tagged antibodies; cell sorting is the use of the instrument to separate cells based on their reactivity patterns.
Information Obtained from Flow cytometry
The flow cytometer has various clinical and scientific applications. The determination of the type and amount of white blood cells in blood samples is a typical clinical application. By treating properly processed blood samples with a fluorescently tagged antibody and doing flow cytometric analysis, the following information can be obtained:
- How many cells express the target antigen as an absolute number and as a proportion of cells passing through the beam? Using a fluorescent antibody specific for an antigen present on all T cells, for instance, it would be feasible to measure the proportion of T cells in the entire population of white blood cells. Using the flow cytometer’s cell-sorting capabilities, it would then be able to separate the T-cell component of the leukocyte population.
- The distribution of cells within a population sample based on antigen density as assessed by fluorescence intensity. Thus, it is possible to derive a measure of the antigen density distribution within the population of cells containing the antigen. This is a potent characteristic of the instrument, as the same type of cell may express varying quantities of antigen depending on its developmental or physiological condition.
- The size of individual cells. This information is acquired from an evaluation of the light-scattering properties of the examined cell population. Flow cytometry also permits the examination of cell populations that have been fluorescently labelled with two or even three distinct antibodies. For instance, if a blood sample is reacted with a fluorescein-tagged antibody specific for T cells and a phycoerythrin-tagged antibody specific for B cells, the percentages of B and T cells can be calculated concurrently using a single analysis. Numerous variants of these “two-color” studies and “three-color” tests are performed daily. With the aid of the proper software, highly advanced versions of the flow cytometer can even do “five-color” analysis.
Flow cytometry is now an integral part of immunology and cell biology, as well as a vital clinical tool. In numerous medical facilities, the flow cytometer is a crucial instrument for the detection and classification of leukemias (see the Clinical Focus). The choice of treatment for leukaemia is highly dependent on the cell types involved; therefore, accurate identification of the neoplastic cells is crucial for clinical practise. Similarly, flowcytometric analysis is commonly used for the fast detection of T-cell subpopulations, a crucial indication of prognosis in AIDS. Labeled monoclonal antibodies against the major T-cell subtypes bearing CD4 and CD8 antigens are utilised to determine their ratios in the patient’s blood during this process. When the patient’s CD4 T cell count falls below a certain threshold, opportunistic infections are quite likely.
Flow cytometry Process
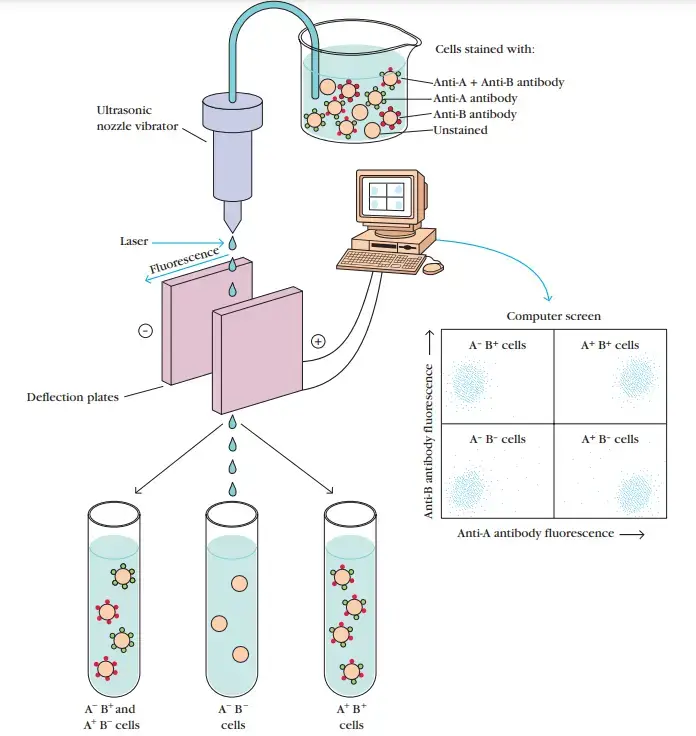
- Flow cytometer separation of fluorochrome-labeled cells. In the illustration, a population of mixed cells is stained with two antibodies, one specific for surface antigen A and the other specific for surface antigen B.
- Fluorescein (green) is used to label anti-A antibodies, while rhodamine is used to label anti-B antibodies (red).
- The labelled cells are placed into the cytometer’s sample chamber. One cell at a time is released from a small vibrating nozzle that produces microdroplets containing no more than one cell.
- Each droplet receives a little electrical charge as it exits the nozzle, and the computer that runs the flow cytometer can precisely determine when a droplet formed by the nozzle passes across the laser light beam that activates the fluorochrome.
- A computer screen displays the intensity of the fluorescence generated by each droplet containing a cell, as measured by a detector.
- Because the computer tracks the location of each droplet, it is feasible to predict when a certain droplet will reach the deflection plates.
- By providing a transient charge to the deflection plates when a droplet passes between them, it is possible to redirect a droplet’s route into one or more collecting vessels.
- This permits the separation of a cell population into subpopulations with distinct profiles of surface markers.
- Each dot on the computer monitor symbolises a cell.
- Antibody anti-A or anti-B did not react with the cells in the lower left panel, which exhibit background levels of fluorescence.
- Those in the upper left panel reacted with anti-B but not with anti-A, while those in the lower right panel reacted with anti-A but not with anti-B.
- The upper right panel comprises cells that exhibit anti-A and anti-B reactivity. In this illustration, the A-B- and A+B+ subpopulations have been separated into different tubes.
- Anti-A and anti-B fluorescent antibodies permit the differentiation of four subpopulations: A-B-, A+B+, A+B+, and A+B-.
Flow Cytometry and Leukemia Typing
- Leukemia is the uncontrolled multiplication of a clone of defective hematopoietic cells. Leukemic cells typically respond poorly or improperly to regulatory cues, exhibit abnormal differentiation patterns, or fail to differentiate.
- In addition, they occasionally inhibit the growth of normal lymphoid and myeloid cells. Leukemia can develop at any stage of maturation in any of the hematopoietic lineages.
- Lymphocytic leukemias have many traits with cells of the lymphoid lineage, whereas myelogenous leukemias share characteristics with myeloid lineage cells.
- In addition to lineage, many leukemias can be categorised as either acute or chronic. Acute lymphocytic leukaemia (ALL), the most prevalent form of childhood leukaemia, acute myelogenous leukaemia (AML), which is more prevalent in adults than in children, and chronic lymphocytic leukaemia (CLL), which is uncommon in children but the most prevalent form of adult leukaemia in the Western world, are some examples.
- A fourth kind, chronic myelogenous leukaemia (CML), affects older adults more frequently than youngsters.
- The diagnosis of leukaemia is based on two observations. One is the identification of aberrant blood cells, and the other is the observation of abnormal bone marrow cells.
- Clinical experience has demonstrated that in order to establish the most effective treatment for a patient, it is necessary to identify the type of leukaemia present. In this regard, two crucial concerns are: (1) What is the ancestry of the aberrant cells, and (2) What is their stage of maturation? Various techniques, such as cytologic evaluation of cell morphology and staining features, immunophenotyping, and, in some instances, gene rearrangement studies, can be utilised to answer these concerns.
- Immunophenotyping, the determination of the profile of specific cell-surface markers expressed by leukemic cells, is one of the most effective of these methods.
- Although leukemia-specific antigens have not yet been identified, profiles of expressed surface antigens can frequently establish cell lineage and aid in identifying the maturational stages of leukemic cell populations.
- For instance, an aberrant cell that expresses immunoglobulin on its surface would be allocated to the B-cell lineage, and its maturational stage would correspond to that of a mature B cell. At contrast, a cell with cytoplasmic heavy chains but without surface immuno-globulin would be a B-lineage leukemic cell in the pre-B cell maturational stage.
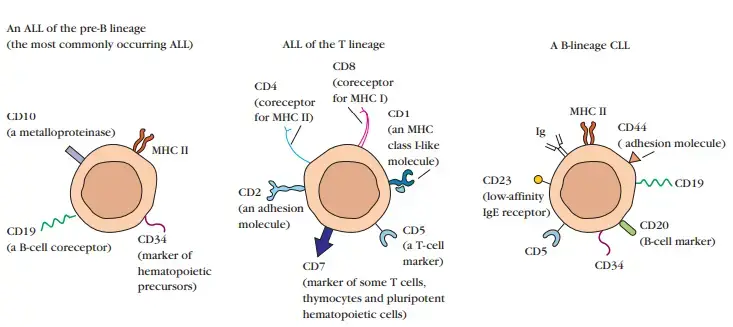
- Flow cytometry and monoclonal antibodies are the most efficient and accurate technologies for immunophenotyping.
- The availability of monoclonal antibodies specific for each of the dozens of antigens found on various types and subtypes of hematopoietic cells has made it possible to identify antigen expression patterns that are characteristic of cell lineages, maturational stages, and a number of types of leukaemia.
- The vast majority of cancer centres are outfitted with flow cytometers that are capable of performing and interpreting the multiparameter analyses required to provide relevant profiles of surface markers on tumour cell populations.
- Flow cytometric immunophenotype determination permits:
- Validation of the diagnosis.
- Diagnosis when morphological or cytochemical staining patterns are unable to provide a definitive answer.
- Identification of abnormal antigen profiles that can aid in the detection of leukemia’s reappearance during remission.
- Improved prognosis of the disease’s progression
Use of Flow cytometry
Flow cytometry is utilised in a variety of research applications, including:
- Cell measurement.
- Cell sorting.
- identifying cell function
- Determining the properties of cells.
- Microorganism detection, including bacteria, fungi, and yeast.
- Finding biomarkers (characteristics that indicate normal function).
- The diagnosis and therapeutic treatment of malignancies of the blood and bone marrow.
References
- McKinnon KM. Flow Cytometry: An Overview. Curr Protoc Immunol. 2018 Feb 21;120:5.1.1-5.1.11. doi: 10.1002/cpim.40. PMID: 29512141; PMCID: PMC5939936.
- Brehm-Stecher, B. F. (2014). Flow Cytometry. Encyclopedia of Food Microbiology, 943–953. doi:10.1016/b978-0-12-384730-0.00127-0
- Chantzoura, E., & Kaji, K. (2017). Flow Cytometry. Basic Science Methods for Clinical Researchers, 173–189. doi:10.1016/b978-0-12-803077-6.00010-2
- Berny-Lang, M. A., Frelinger, A. L., Barnard, M. R., & Michelson, A. D. (2013). Flow Cytometry. Platelets, 581–602. doi:10.1016/b978-0-12-387837-3.00029-8
- Roederer, M., Parks, D. R., Herzenberg, L. A., & Herzenberg, L. A. (1998). Flow Cytometry. Encyclopedia of Immunology, 932–943. doi:10.1006/rwei.1999.0243
- https://www.bu.edu/flow-cytometry/files/2010/10/BD-Flow-Cytom-Learning-Guide.pdf
- https://www.slideshare.net/tashagarwal/flow-cytometry-46618943
- https://www.abcam.com/protocols/introduction-to-flow-cytometry
- https://www.bosterbio.com/protocol-and-troubleshooting/flow-cytometry-principle
- https://www.technologynetworks.com/cell-science/articles/what-is-flow-cytometry-343977
- https://www.thermofisher.com/in/en/home/life-science/cell-analysis/cell-analysis-learning-center/molecular-probes-school-of-fluorescence/flow-cytometry-basics/flow-cytometry-fundamentals/how-flow-cytometer-works.html
- https://nanocellect.com/blog/how-does-flow-cytometry-work/
- https://en.wikipedia.org/wiki/Flow_cytometry
- https://my.clevelandclinic.org/health/diagnostics/22086-flow-cytometry