What are epimers?
- Epimers are a type of stereoisomers found in chemistry. They are pairs of molecules that share the same molecular formula and connectivity but differ in the arrangement of atoms in three-dimensional space. Epimers are characterized by having opposite configurations at only one stereogenic center, while all other stereogenic centers in the molecules remain the same.
- To illustrate this concept further, let’s consider the example of twins. Imagine there are two identical twin brothers named Ajay and Rahul. While they may look almost identical, there is one distinguishing feature: Ajay has a mole on the left side of his face, while Rahul has it on the right side. Despite their striking resemblance, the presence of the mole on different sides sets them apart as individuals. In a similar manner, epimers exhibit distinct differences despite sharing similarities in their overall structure.
- A classic example of epimers in the field of chemistry can be found in the case of D-glucose and D-galactose. Both of these molecules are monosaccharides and belong to the group of carbohydrates. The primary distinction between them lies in the placement of the hydroxyl (-OH) group at a specific position. In D-glucose, the hydroxyl group is located at the first carbon (C1), whereas in D-galactose, it is found at the fourth carbon (C4). This difference in the position of the hydroxyl group results in the molecules being classified as epimers.
- It is worth noting that epimers not only differ in their physical characteristics but also exhibit variations in their chemical properties. These dissimilarities arise due to the different spatial arrangement of atoms, which can influence their interactions with other molecules and biological systems.
- In practical applications, epimers find use in various fields, including pharmaceuticals. For instance, doxorubicin and epirubicin are two epimers that are commonly employed as drugs. These substances possess similar structures except for their configuration at a specific stereogenic center, which can impact their therapeutic properties and effects.
- In summary, epimers are a type of stereoisomers that share the same molecular formula and connectivity but differ in the arrangement of atoms in three-dimensional space. They have opposite configurations at one stereogenic center, while all other stereogenic centers remain the same. Epimers, such as D-glucose and D-galactose, showcase the concept of distinct molecules that are related but differ in specific characteristics, highlighting the fascinating world of stereochemistry.
Epimer Definition
Epimers are a type of stereoisomers that differ in configuration at only one stereogenic center while having the same configuration at all other stereogenic centers.
Epimerization
- Epimerization refers to a chemical process in which an epimer, a specific type of stereoisomer, undergoes a transformation into its diastereomeric counterpart. This process can occur spontaneously, although it is typically a slow reaction, or it can be catalyzed by enzymes or other factors.
- One example of epimerization is observed in the depolymerization reactions of condensed tannins. During these reactions, the epimerization process can lead to the conversion of one epimeric form of a compound to its diastereomer. Enzymes, such as renin-binding protein, can catalyze epimerization reactions, facilitating the conversion between specific epimers. An instance of enzymatic epimerization occurs between the sugars N-acetylglucosamine and N-acetylmannosamine.
- In the field of pharmaceuticals, epimerization plays a significant role. For instance, the synthesis of epibatidine, a potent alkaloid, includes an epimerization step known as the penultimate step in Zhang & Trudell’s classic epibatidine synthesis. This process involves the conversion of an epimer to its diastereomer, leading to the formation of the desired compound.
- In the realm of drug development, epimerization can also impact the pharmacological properties of compounds. For example, the erythro isomers of methylphenidate can undergo epimerization to the threo isomers. The threo isomers are typically the pharmacologically preferred forms due to their lower energy states. This transformation can affect the efficacy and physiological effects of the drug.
- However, epimerization is not always desirable in pharmaceuticals. Undesired in vivo epimerization can occur, leading to the conversion of a drug to its diastereomer, which may possess different pharmacological properties. An example of this is observed in tesofensine, which can undergo in vivo epimerization to brasofensine, potentially altering its intended therapeutic effects.
- In summary, epimerization is a chemical process in which an epimer is converted into its diastereomeric counterpart. It can occur spontaneously or be catalyzed by enzymes. Examples of epimerization can be found in various fields, such as the depolymerization of condensed tannins, drug synthesis, and pharmaceutical applications. Understanding and controlling epimerization processes are essential in drug development and other areas of chemical research.
Main Characteristics of Epimers
The main characteristics of epimers can be summarized as follows:
- Configuration at One Chiral Carbon: Epimers differ from each other in the configuration at one specific chiral carbon in a molecule. This means that out of the several chiral carbons present in a molecule, only one isomerism difference exists between the epimers.
- Variation in Few Chiral Carbons: While epimers share the same configuration at most chiral carbons, they differ in the configuration at only a few specific chiral carbons. This means that the majority of chiral carbons in the molecules remain the same in both epimers.
- Non-Mirror Images: Epimers are not mirror images of each other. Unlike enantiomers, which are non-superimposable mirror images, epimers have distinct three-dimensional structures that are not identical or related through simple reflection.
- Different Physical and Chemical Properties: Epimers exhibit differences in both physical and chemical properties. Due to their distinct three-dimensional structures resulting from the differing configurations at specific chiral carbons, epimers may have varying reactivity, stability, solubility, and other properties. These differences can influence their interactions with other molecules and their behavior in chemical reactions.
How Do Epimers and Anomers Differ?
Epimers and anomers are both types of stereoisomers found in carbohydrate (sugar) molecules. While they share some similarities, there are distinct differences between epimers and anomers:
- Structural Difference: Epimers differ from each other in the configuration at one or more specific chiral carbons, which are typically not the anomeric carbon. On the other hand, anomers specifically differ in their three-dimensional structure at the anomeric carbon, which is the hemiacetal or hemiketal carbon in the cyclic carbon chain of a sugar molecule.
- Location of Difference: Epimers can have differences in configuration at multiple chiral carbons, whereas anomers only differ at the anomeric carbon. Anomers have distinct forms based on the orientation of the hydroxyl (-OH) group at the anomeric carbon: alpha (α) and beta (β). The alpha form has the -OH group pointing down, while the beta form has the -OH group pointing up.
- Naming Convention: Epimers are usually named based on the specific chiral carbon(s) where the configuration differs. Anomers, on the other hand, are named based on the orientation of the -OH group at the anomeric carbon. The alpha designation is given when the -OH group points down, and the beta designation is given when the -OH group points up.
- Chiral Center Configuration: In epimers, the difference in configuration can occur at any chiral carbon(s) in the molecule, while anomers specifically differ in the configuration at the anomeric carbon. The configuration at other chiral centers in the molecule remains the same in both anomers.
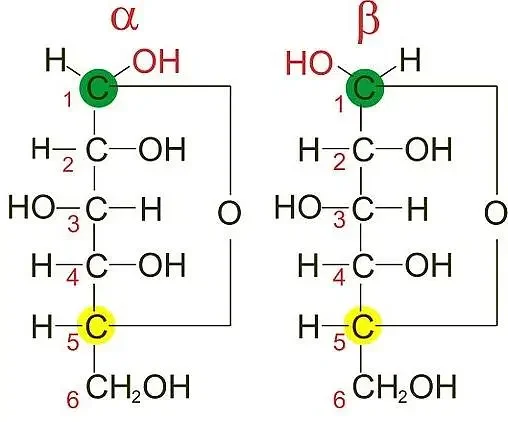
To illustrate this distinction, consider the example of glucose. The alpha anomer of glucose has the -OH group pointing down at the anomeric carbon (C1), while the beta anomer has the -OH group pointing up at the anomeric carbon. The difference in configuration occurs only at the anomeric carbon, and the configuration at other chiral centers remains the same.
In summary, while both epimers and anomers are types of stereoisomers, epimers can differ at multiple chiral carbons, while anomers specifically differ at the anomeric carbon. Anomers are named based on the orientation of the -OH group at the anomeric carbon, and their difference in configuration is limited to the anomeric carbon, whereas epimers can have differences at other chiral centers as well.
Example of Epimers
Here are some examples of epimers:
- β-D-glucopyranose and β-D-mannopyranose: They differ in the stereochemistry at the C-2 position of glucose.
- α-D-glucopyranose and β-D-glucopyranose: They differ in the orientation of the -OH group at the anomeric carbon (C-1) of glucose.
- Dextrotartaric acid and levotartaric acid: They are diastereomers and epimers that differ in configuration at one stereogenic center.
- Epirubicin and doxorubicin: They are epimers used as drugs.
- Epi-inositol and inositol: They are epimers that exhibit different stereochemistry.
- Lipoxin and epilipoxin: They are epimers found in specific compounds.
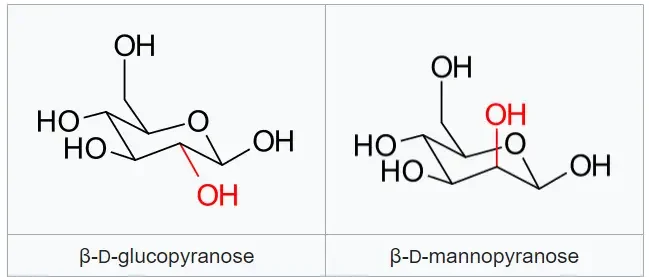
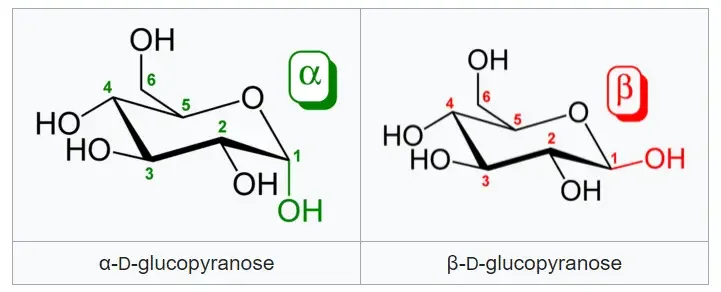
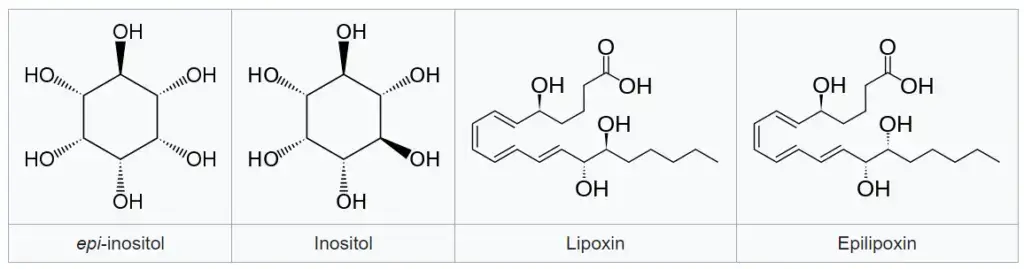
These examples illustrate the concept of epimers, where molecules have similar structures but differ in the arrangement of atoms at specific stereogenic centers, leading to distinct chemical and biological properties.
Epimers vs Anomers
Anomers and epimers are two different types of stereoisomers that have distinct characteristics and differences. Here are some key points differentiating anomers and epimers:
Definition:
- Anomers: Anomers are stereoisomers that result from the difference in configuration at their anomeric carbon. The anomeric carbon is the carbon involved in the formation of the cyclic structure of carbohydrates.
- Epimers: Epimers, on the other hand, are a type of stereoisomers that differ from each other only at one specific chiral carbon.
Carbon where Isomerism occurs:
- Anomers: Isomerism occurs specifically at the anomeric carbon of the anomers. This carbon is the hemiacetal or hemiketal carbon in the cyclic carbon chain of a sugar molecule.
- Epimers: Isomerism occurs at the epimeric carbon of the epimers. This carbon can be any chiral carbon in the molecule, excluding the anomeric carbon.
Structure of Sugar Molecule:
- Anomers: Anomers are typically cyclic molecules. They exist in a ring form due to the formation of hemiacetal or hemiketal bonds.
- Epimers: Epimers can be either acyclic or cyclic molecules. They may or may not have a ring structure and can exhibit different arrangements and configurations at specific chiral carbons.
Focus of Difference:
- Anomers: The main focus of difference in anomers is the orientation of the hydroxyl (-OH) group at the anomeric carbon. Anomers are categorized as alpha (α) or beta (β) based on the orientation of the -OH group.
- Epimers: Epimers, on the other hand, primarily differ in the configuration at one specific chiral carbon. They can have various arrangements and orientations at this particular carbon, leading to different stereoisomeric forms.
While anomers and epimers both involve differences in configuration, their specific points of difference and the nature of the isomerism set them apart. Anomers are specifically related to the cyclic structure of sugar molecules and involve the anomeric carbon, while epimers can encompass any chiral carbon in the molecule and may or may not be cyclic.
| Aspect | Epimers | Anomers |
|---|---|---|
| Definition | Differ from each other at one specific chiral carbon | Differ in configuration at the anomeric carbon |
| Carbon where Isomerism occurs | Epimeric carbon | Anomeric carbon |
| Structure of Sugar Molecule | Can be acyclic or cyclic | Typically cyclic |
| Focus of Difference | Configuration at one specific chiral carbon | Orientation of hydroxyl (-OH) group at the anomeric carbon |
FAQ
What are epimers?
Epimers are a type of stereoisomers that differ in the configuration at one or more specific chiral carbons while having the same configuration at all other chiral carbons.
How do epimers differ from enantiomers?
Epimers differ from enantiomers in that they have the same connectivity and arrangement of atoms but differ in the configuration at specific chiral carbons, while enantiomers are non-superimposable mirror images of each other.
What is the significance of epimers?
Epimers play a crucial role in stereochemistry as they contribute to the diversity of stereoisomers in organic compounds. Their differing configurations can lead to distinct physical and chemical properties, influencing their interactions and behaviors.
How can epimers be identified?
Epimers can be identified by comparing the configurations of chiral carbons in two related compounds. If the configuration differs at one or more specific chiral carbons while remaining the same at others, the compounds are epimers.
Can epimers have different biological activities?
Yes, epimers can exhibit different biological activities due to their varying three-dimensional structures. Even a slight change in configuration at a specific chiral carbon can impact how an epimer interacts with biological receptors and enzymes, leading to different effects.
How do epimers relate to carbohydrates?
Epimers are commonly found in carbohydrate molecules. One particular type of epimers in carbohydrates is called anomers, which differ in configuration at the anomeric carbon, the carbon involved in the formation of the cyclic structure of carbohydrates.
Can epimers interconvert?
Yes, epimers can interconvert through a process called epimerization. Epimerization involves the conversion of one epimer to another, typically through chemical reactions or enzymatic catalysis.
Can epimers have different physical properties?
Yes, epimers can have different physical properties such as melting point, boiling point, solubility, and optical rotation. These variations arise from the differences in their three-dimensional structures and interactions with other molecules.
Can epimers be separated or purified?
Yes, epimers can be separated and purified using various techniques such as chromatography, crystallization, and distillation. These methods exploit the differences in physical properties between epimers to achieve separation.
Are epimers commonly found in pharmaceuticals?
Epimers can be found in pharmaceutical compounds. The presence of epimers in drugs can have implications for their potency, bioavailability, and therapeutic effects. Understanding and controlling the configuration of chiral carbons is important in drug development and manufacturing processes.
Are epimers diastereomers?
Yes, epimers are a type of diastereomers. Diastereomers are stereoisomers that have different configurations at one or more chiral centers while retaining the same configuration at some other chiral centers. Since epimers differ from each other in configuration at one or more specific chiral carbons while having the same configuration at other chiral carbons, they fall under the category of diastereomers.