Ebola Virus
- Ebola, also known as Ebola virus disease (EVD) and Ebola hemorrhagic fever (EHF), is a hemorrhagic fever caused by ebolaviruses that affects humans and other primates.
- Typically, symptoms appear anywhere from two days to three weeks after infection. Typically, fever, sore throat, muscle discomfort, and headaches are the initial symptoms.Some individuals then begin to hemorrhage internally and externally.
- It kills between 25% and 90% of infected individuals, or roughly 50% on average. Death typically occurs between six and sixteen days after the onset of the first symptoms, and is frequently caused by fluid loss-induced shock.
- Early treatment of symptoms significantly increases the survival rate compared to delayed treatment.The US FDA authorized an Ebola vaccine in December of 2019.
- The virus is transmitted through direct contact with body fluids, such as blood from infected humans or animals, or through contact with objects recently contaminated with infected body fluids.
- There have been no documented cases of airborne transmission between humans and other primates, either in nature or under laboratory conditions.
- After recovering from Ebola, sperm and breast milk may harbor the virus for several weeks to months. Fruit bats are believed to be the natural carrier; they can transmit the virus without becoming infected.
- Ebola symptoms may resemble those of malaria, cholera, typhoid fever, meningitis, and other viral hemorrhagic fevers, among others.Blood samples are analyzed for the presence of viral RNA, viral antibodies, or the virus itself to confirm the diagnosis.
- Controlling outbreaks requires coordinated medical services and community participation, such as rapid detection, contact tracing of those exposed, fast access to laboratory services, care for those infected, and cremation or burial of the deceased.
- Preventative measures include donning protective clothing and washing hands when in close proximity to patients and while handling potentially infected bushmeat, and cooking bushmeat thoroughly.
- The US FDA authorized an Ebola vaccine in December 2019. While there is currently no approved treatment for Ebola, two treatments (atoltivimab/maftivimab/odesivimab and ansuvimab) are associated with improved outcomes.
- Additionally, supportive efforts enhance outcomes. These include oral rehydration therapy (drinking slightly sweetened and saline water) or intravenous fluid administration, as well as the treatment of symptoms.
- Atoltivimab/maftivimab/odesivimab (Inmazeb) was approved for medical use in the United States in October 2020 to treat Zaire ebolavirus disease.
Ebola Virus Classification
- Ebola virus is transmitted through direct contact with blood or other highly infectious bodily fluids, such as sperm, feces, or vomit, of infected individuals (or animals), including intimate contact with deceased EVD victims.
- Virus-contaminated objects, such as needles and syringes, as well as contaminated clothing and bedding, can also transmit infection.
- Most cases of Ebola virus transmission occur between family members or in health care settings with inadequate infection control, because individuals are most likely to come into contact with infected bodily fluids under these conditions.
- The virus penetrates the body of an unprotected individual through a skin break or through the eyes, nose, or mouth.
- In contrast to influenza and SARS, the Ebola virus does not travel through the air. It is neither transmitted by water nor by mosquitoes or other organisms.
- Ebola can only be transmitted from person to person while the infected individual is exhibiting symptoms, although it was recently discovered that the virus can persist for more than a year in the sperm of a small percentage of male survivors.
- As the disease progresses and the quantity of virus in the body rises, a person becomes more contagious. When a person is first infected, they typically do not produce high levels of the virus, so the risk of transmission is low. The average incubation period is between 8 and 10 days, ranging from 2 to 21 days.
Hosts of Ebola Virus
- There is no evidence that Ebola exists in a natural reservoir. But scientists think fruit bats are the most likely suspect. It has been hypothesized that three species of bats serve as a key natural reservoir for Ebola viruses due to their ability to retain the virus without showing any symptoms.
- However, only trace amounts of viral RNA fragments of Zaire Ebola virus have ever been described from fruit bats in and surrounding endemic locations. Potential reservoirs of this virus also include birds, plants, and insects.
- However, ELISA testing on blood collected from Zambia’s migrating fruit bats (Eidolon helvum) has revealed the presence of many filovirus strains.
- When the GP-EBOV interaction in humans was compared to that in African fruit bats, it was found that the GP exhibited very comparable biological features in the cells of both species.
- Therefore, evidence revealed that both interact similarly with EBOV-GP. The index case in Africa may have been exposed to the disease while playing near a colony of insectivorous free-tailed bats (Mops condylurus) because the disease survived experimental infections.
- Bats, shrews, tenrecs, rodents, and marsupials all have filovirus-like components incorporated into their genomes, as shown by phylogenetic and sequencing evidence. This demonstrates the antiquity of the gene-producing mammal-filovirus connection.
Structure of Ebola Virus
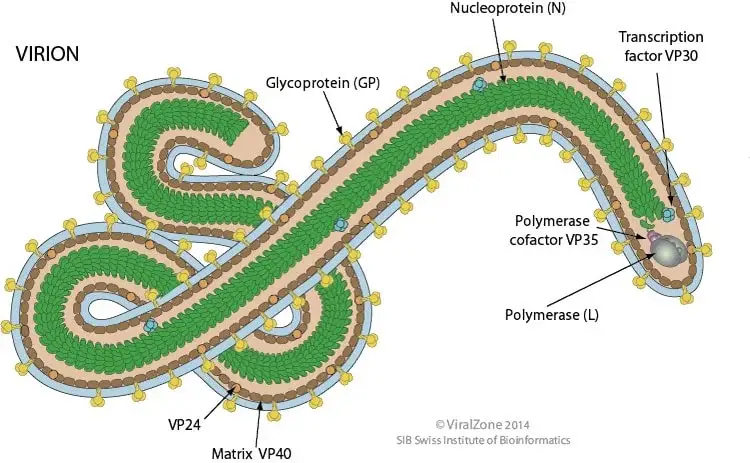
- The typical dimensions of an Ebola virus are 970 nm in length and 80 nm in width.
- They have a spherical or tubular shape and are made up of the viral envelope, matrix, and nucleocapsids.
- The virus typically takes the form of a long filament, though it can also be “U-shaped,” in the shape of a “6” (the “shepherd’s crook” appearance), or even round.
- They are characterized by spikes 7–10 nm in length that extend from the lipid bilayer surface and are composed of a glycoprotein (GP) encoded by a virus.
- Glycoproteins are proteins in which a chain of carbohydrate molecules (glycans) has been covalently linked to a polypeptide side chain.
- The Ebola virus relies only on its surface glycoprotein GP to connect to and enter new host cells.
- By inserting GP spikes into specific regions of the host cell membrane, production of the viral outer envelope begins.
- The structure of this virus, typical of the family Filoviruses, is reminiscent of a strand of thread.
Genome of Ebola Virus
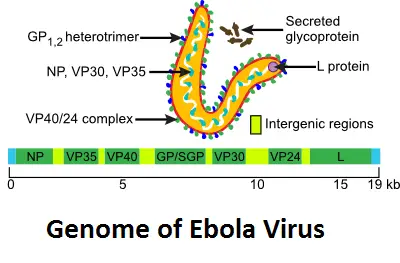
The genome is composed of a single strand of negative RNA and measures around 18–19 kilobases in length. There are seven proteins in all that are encoded by the genome. Neither the 3′ nor the 5′ end is polyadenylated or capped. About 19,000 base pairs are packed into it. Nucleoprotein (NP), polymerase cofactor (VP35), glycerol phosphate (GP), transcription activator (VP30), VP24, and RNA polymerase (L) are all encoded by this gene.
- Glycoprotein (GP) is a transmembrane glycoprotein that facilitates adhesion.
- Capsid assembly and packaging NP- nucleoprotein.
- VP24, a viral inhibitor, inhibits the synthesis of the antiviral protein interferon in the host cell.
- The generation of interferon or other antiviral responses to ds RNA is reduced by VP35-.
- The VP30 anti-terminator of transcription.
- The virion’s structural integrity is maintained by VP40, which is required for budding and capsid assembly.
- Viral polymerase L protein.
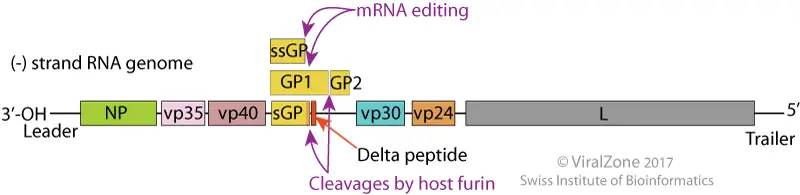
Epidemiology of Ebola Virus
- Ebola virus disease (EVD) is a severe and often fatal illness caused by the Ebola virus. The virus was first identified in 1976 during two simultaneous outbreaks in Sudan and the Democratic Republic of Congo (DRC), with the latter being the most severe. Since then, outbreaks of Ebola virus have occurred sporadically in several African countries.
- Ebola virus is primarily transmitted to humans through contact with bodily fluids of infected animals, such as fruit bats, monkeys, and apes. Once the virus is in human populations, it can spread through direct contact with bodily fluids of infected individuals, including blood, vomit, urine, and feces.
- The symptoms of Ebola virus disease can include fever, headache, muscle pain, weakness, diarrhea, vomiting, abdominal pain, and unexplained hemorrhage. These symptoms can appear 2-21 days after exposure to the virus, and the disease can be fatal in up to 90% of cases.
- Efforts to control and prevent Ebola outbreaks include identifying and isolating infected individuals, tracing their contacts, implementing infection control measures in healthcare facilities, and promoting safe burial practices. There is currently no licensed vaccine for Ebola virus disease, but several experimental vaccines are under development.
- The largest outbreak of Ebola virus to date occurred in West Africa from 2014-2016, with over 28,000 cases and 11,000 deaths reported. The outbreak highlighted the need for improved preparedness and response to emerging infectious diseases.
Ebola Entry Mechanism
- The process of EBOV entry into the host cell consists of three sequential stages, including attachment, co-receptor binding, and fusion.
- Viruses primarily utilize endocytic pathways for viral fusion, gaining access to the cytoplasm via macropinosomes, caveolae, and clathrin-coated vesicles.
- Different types of host cell-surface receptors are identified to play a role in EBOV entry into the host cell that are the β1 integrin receptors, galactose- and N-acetylgalactosamine-specific C-type lectin (hMGL), dendritic-cell-specific intercellular adhesion molecules DC-SIGN-related (DC-SIGNR) and (ICAM)-3-grabbing nonintegrin (DC-SIGN) factors. In order to enter host cells, the EBOV utilizes multiple molecules, including a variety of C-type lectins.
Transmission of Ebola Virus
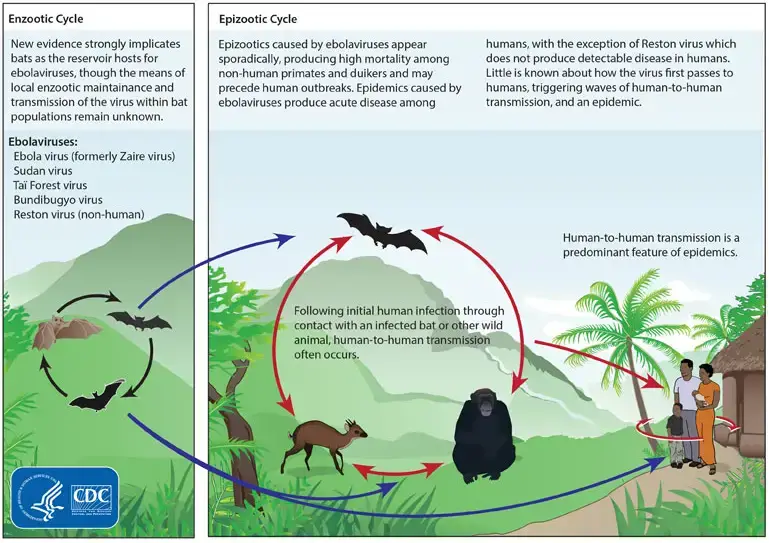
- Ebola virus is primarily transmitted to humans through contact with bodily fluids of infected animals, such as fruit bats, monkeys, and apes. The virus can be transmitted to humans through direct contact with the blood, vomit, urine, and feces of infected animals.
- Once the virus is in human populations, it can spread through direct contact with bodily fluids of infected individuals, including blood, vomit, urine, and feces. Transmission can also occur through contact with contaminated surfaces, such as needles and other medical equipment, that have been contaminated with the virus.
- Ebola virus is not spread through the air or by water, food, or mosquitoes. However, it is important to note that Ebola virus can survive in bodily fluids outside of the body for several days, making it important to practice good infection control measures when handling bodily fluids of infected individuals or contaminated surfaces.
- It is important to note that individuals who have recovered from Ebola virus disease can still transmit the virus through bodily fluids, including semen, for up to several months after recovery. Therefore, it is important for individuals who have recovered from Ebola to practice safe sex and use condoms or abstain from sex for at least three months after recovery.
Replication of Ebola Virus
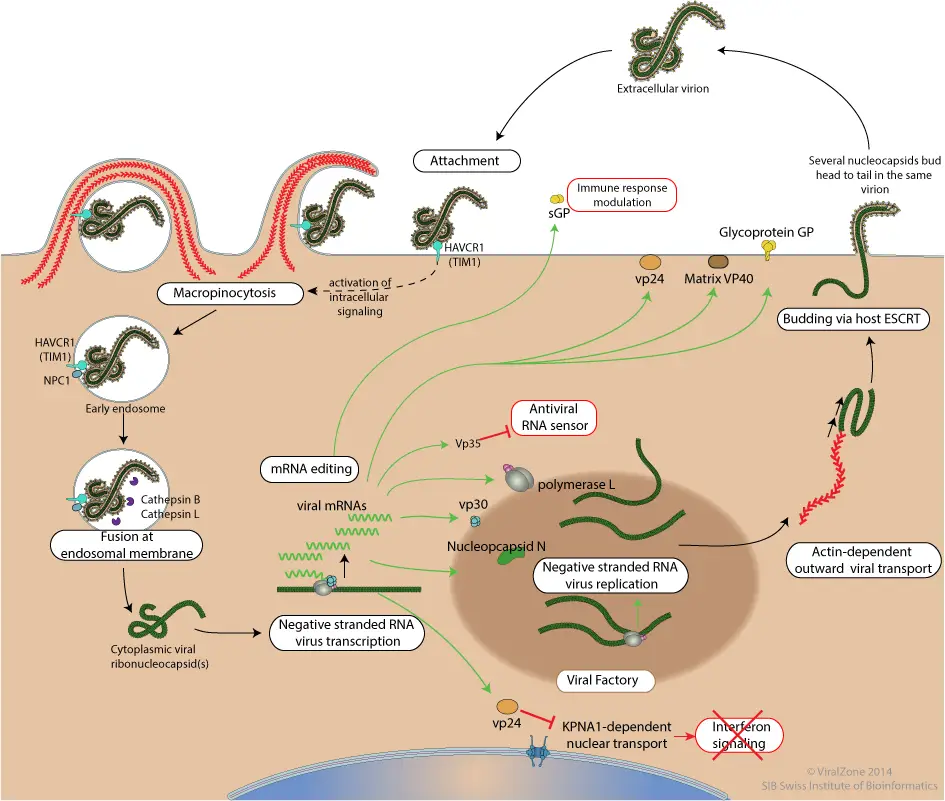
- Attachment to host receptors by means of GP glycoproteins, such as DC-SIGN and DC-SIGNR.
- A cellular receptor such as HAVCR1(TIM1) contacts phosphatidyl serine on the virion membrane, triggering the macropinocytosis program by transducing a signal into the cell.
- The virion penetrates the cell through the process of Macropinocytosis. In some culture cells, Cathepsin L and Cathepsin B can convert GP glycoprotein into 19kDa GP1 via processing by GP glycoprotein. However, this processing does not occur in every cell or for every ebolavirus.
- In the late macropinosome, GP1 interacts with the host NPC1 and promotes the fusion of the virus membrane with the vesicle membrane. Following this, the ribonucleocapsid is discharged into the cytoplasm.
- Viral mRNAs are capped and polyadenylated in the cytoplasm by polymerase instability during sequential transcription.
- Presumably, replication begins when sufficient nucleoprotein is present to encapsulate newly synthesized antigenomes and genomes.
- The virion is released by the interaction of the ribonucleocapsid with the matrix protein and the host ESCRT complexes with the plasma membrane.
Pathogenesis of Ebola Virus
The Ebola virus enters the body via the mucous membrane, cutaneous rupture, and parenteral route. Due to outbreak conditions, all data on the pathogenesis of the Ebola virus were obtained through laboratory tests on guinea pigs, mice, and other nonhuman primates.
- When Ebola enters the human body, the VP24 protein of the Ebola virus binds with the KPNA5 protein of the human host.
- KPNA5 is primarily responsible for cell-to-cell communication and signal transduction into and out of the nucleus.
- It functions just like a messenger. Then, KPNA5 transfers these signals to the nucleus of other cellular structures.
- These signals modulate numerous cellular functions, including immune response. According to Daisy Leung, when the Ebola protein VP24 binds to the messenger protein STAT1, crucial immune signal transmission is obstructed; STAT1 is the transporter inside the nucleus and activates the genes for antiviral responses.
- eVP-24 inhibits PY-STAT1 because it directly competes with NPI-1 subfamily KPNA for binding. Nucleus receives STAT-1 protein, which emits an alarm signal and releases interferons to combat virus, bacteria, or any other pathogen assaulting the cell.
Mode of Action
- The glycoprotein of Epstein-Barr virus (EBOV-GP) mediates viral entrance and promotes viral release from host cells. It has a molecular weight of around 140 kDa.
- Co-translational transfer of single-chain EBOV-GP precursors into the lumen of the endoplasmic reticulum yields trimers. Post-translational cleavage of the receptor-binding subunit of GP yields GP1 and GP2, which are required for entry and infection progression.
- Protease cleavage of GP is linked to virus entry, which is pH-dependent. Three cleavage fragments with molecular weights of 23, 19, and 4 kDa were generated by the cleavage process.
- The binding of GP to endosomal receptors and subsequent fusion-relevant conformational changes in GP are triggered by a 19-kDa core subunit. While the GP1 subunit is in charge of binding receptors, the GP2 subunit is in charge of securing the glycoprotein to the viral membrane.
- The GP1 subunit is very variable after the first 300 amino acids. The C-terminal variable region of GP1, also known as the mucin domain, has many O-linked glycosylation sites.
- X-ray crystallography solved the structure of GP2, which consists of a triple-stranded coiled coil in the center, followed by a disulfide-bonded loop. Initiating membrane fusion via bridging two membranes with GP2 is possible.
- Human neutralizing antibody KZ52 binds to the trimeric crystal structure of the surface glycoprotein (GP), which is necessary for attachment and fusion of viral and host membranes. Niemann-Pick C1 (NPC1), a human lysosomal cholesterol transporter, satisfies a defining feature of viral receptors by binding selectively to viral GP.
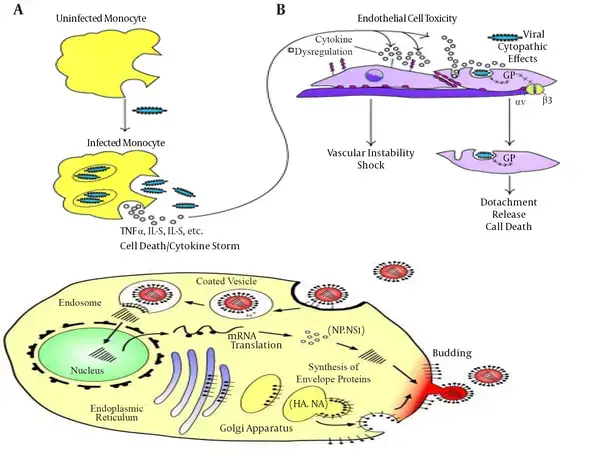
- Inducing cytotoxic effects in human endothelium cells, EBOV-GP is the primary viral determinant of Ebola virus pathogenicity. Explanted human or porcine blood arteries showed enhanced permeability after being exposed to GP, which has a mucin-like domain involved in substantial endothelial cell death within 24 hours.
- This effect was dependent on the mucin-like domain of GP, suggesting that this region was the viral determinant of Ebola’s pathogenicity. EBOV GP-dependent entrance also required a different component of AMPK in macropinocytic events.
- These filoviruses sacrifice effective entrance for the sake of glycoprotein glycosylation, which protects them from antibody neutralization and proteases. Proteolysis of the EBOV GP component GP1, which is carried out by enzymes like ascathepsin B (CatB) and cathepsin L (CatL), is crucial in reducing infectious ZEBOV replication.
Clinical manifestations of Ebola Virus
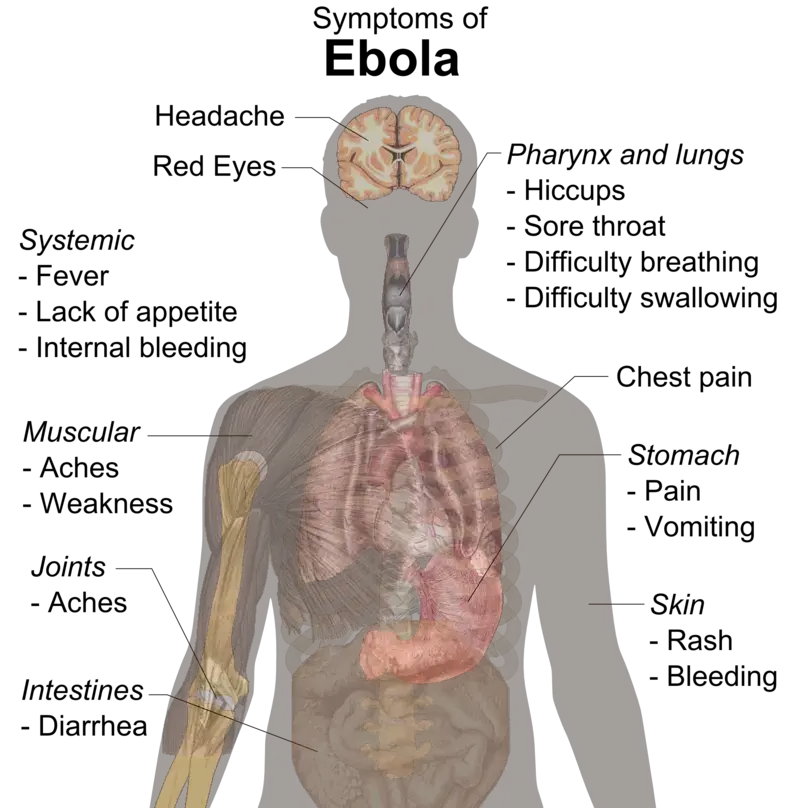
- During the incubation period of two to twenty-one days, patients infected with the Ebola virus develop flu-like symptoms, including fever, headache, decreased appetite, vomiting, abdominal pain, vascular dysfunction, diarrhea, and fatigue.
- During these conditions, fever levels persist above 38.3°C. Vomiting, abdominal pain, and occasionally chest pain and shortness of breath may follow these conditions. In this disease, severe and fatal stages are characterized by hemorrhage from small infections, hemorrhagic diathesis, reduced adaptive immune responses, shock, coagulation disorders, and eventual multiple organ failure.
- The fatal outcome is also associated with abnormal innate immune responses as a result of massive intravascular B- and T-lymphocyte apoptosis, which induces profound suppression of adaptive immunity in experimentally infected animals and humans.
- 7 to 14 days after the onset of the first symptom, recovery may begin. Due to extensive bleeding and fluid loss, decreased blood pressure is a common cause of death.
- Those who survive have a number of persistent health issues, including liver inflammation, muscle and joint pain, hearing loss, weight loss, and weakness. Patients who have recovered can no longer transmit the virus.
Lab Diagnosis of Ebola Virus
- The laboratory diagnosis of Ebola virus disease (EVD) is essential for identifying and managing outbreaks of the disease. The diagnosis of EVD is based on the detection of the Ebola virus in blood or other bodily fluids of infected individuals.
- Several laboratory tests can be used to diagnose EVD, including reverse transcription polymerase chain reaction (RT-PCR), antigen-capture enzyme-linked immunosorbent assay (ELISA), and virus isolation. These tests can be performed on blood, urine, saliva, semen, and other bodily fluids.
- In addition to laboratory testing, the diagnosis of EVD also requires a thorough clinical evaluation and consideration of the individual’s travel history and exposure to known cases of EVD.
- It is important for laboratory personnel to follow strict infection control measures when handling specimens from individuals suspected of having EVD. This includes wearing appropriate personal protective equipment and following biosafety protocols to prevent the spread of the virus.
- Laboratory testing for EVD is typically conducted at specialized laboratories with the appropriate equipment and expertise. Rapid diagnostic tests for EVD are also under development and may be useful for diagnosing the disease in resource-limited settings.
- Early diagnosis of EVD is important for initiating appropriate infection control measures, providing supportive care, and reducing the risk of transmission to others.
Treatment of Ebola Virus
- Currently, there is no specific treatment for Ebola virus disease (EVD). However, supportive care can improve the chances of survival for individuals with EVD. Supportive care includes treatment for symptoms such as fever, pain, and dehydration, as well as management of complications such as organ failure and bleeding.
- Intravenous fluids and electrolyte replacement can help prevent dehydration, and oxygen therapy may be necessary for individuals with breathing difficulties. Blood transfusions and medications to control bleeding may also be used in some cases.
- Experimental treatments for EVD are under development, including monoclonal antibodies, antiviral drugs, and convalescent plasma therapy. These treatments are not widely available and are still undergoing clinical trials to determine their safety and effectiveness.
- It is important for individuals with EVD to receive care in specialized treatment centers with trained healthcare providers and appropriate infection control measures to prevent the spread of the virus to others.
- Prevention measures, such as vaccination of healthcare workers and individuals at high risk of exposure, and strict infection control measures, are also essential for controlling and preventing outbreaks of EVD.
Prevention and control of Ebola Virus
Prevention and control of Ebola virus disease (EVD) requires a comprehensive approach that includes early detection, rapid response, and effective infection control measures. Here are some key strategies for preventing and controlling EVD:
- Surveillance and early detection: Monitoring and early detection of suspected cases of EVD is essential for controlling outbreaks. This includes active surveillance in high-risk areas and rapid reporting of suspected cases to public health authorities.
- Effective infection control measures: Strict infection control measures, including isolation of suspected and confirmed cases, use of personal protective equipment by healthcare workers, and appropriate disinfection of equipment and facilities, can help prevent the spread of the virus.
- Safe burial practices: Safe and dignified burial practices, such as avoiding direct contact with the body of the deceased, can help prevent transmission of the virus from infected corpses.
- Vaccination: Vaccination of healthcare workers and individuals at high risk of exposure can help prevent the spread of the virus and reduce the severity of the disease.
- Community engagement and education: Community engagement and education can help promote understanding of the disease and prevention measures, as well as reduce stigma and fear associated with the disease.
- Coordination and response planning: Effective response to outbreaks of EVD requires coordination and planning among public health authorities, healthcare providers, and other stakeholders.
- Research and development: Research and development of vaccines and treatments for EVD is essential for improving the prevention and control of the disease.
By implementing these strategies, it is possible to prevent and control outbreaks of EVD and reduce the impact of the disease on affected communities.
FAQ
What is Ebola virus disease (EVD)?
Ebola virus disease (EVD) is a severe and often fatal illness caused by the Ebola virus.
How is Ebola virus transmitted?
Ebola virus is transmitted through contact with blood, bodily fluids, or tissues of infected animals or humans.
What are the symptoms of Ebola virus disease?
The symptoms of EVD can include sudden onset of fever, weakness, muscle pain, headache, and sore throat, followed by vomiting, diarrhea, abdominal pain, and rash. More severe symptoms can include kidney and liver failure, bleeding, and shock.
Is there a cure for Ebola virus disease?
There is no specific cure for EVD, but supportive care can improve the chances of survival.
How is Ebola virus diagnosed?
The diagnosis of EVD is based on laboratory testing for the presence of the Ebola virus in blood or other bodily fluids of infected individuals.
How can Ebola virus be prevented?
Prevention of EVD includes surveillance and early detection, effective infection control measures, safe burial practices, vaccination, community engagement and education, and coordinated response planning.
Who is at risk for Ebola virus disease?
People who are in close contact with infected individuals or animals, or who live in or travel to areas where outbreaks of EVD occur, are at higher risk for contracting the disease.
Is there a vaccine for Ebola virus?
Yes, several vaccines have been developed for EVD and are being used in outbreak response efforts.
Can Ebola virus be spread through the air?
No, Ebola virus is not spread through the air. It is only transmitted through contact with infected bodily fluids or tissues.
What should I do if I think I have been exposed to Ebola virus?
If you think you have been exposed to Ebola virus, seek medical attention immediately. Early diagnosis and treatment can improve the chances of survival and prevent the spread of the virus to others.
References
- Laupland KB, Valiquette L. Ebola virus disease. Can J Infect Dis Med Microbiol. 2014 May;25(3):128-9. doi: 10.1155/2014/527378. PMID: 25285105; PMCID: PMC4173971.
- Qureshi, A. I. (2016). Ebola Virus. Ebola Virus Disease, 23–37. doi:10.1016/b978-0-12-804230-4.00003-0
- Keservani, Raj & Sharma, Anil Kumar & Jarouliya, Urmila & Singh, Amit. (2016). EBOLA VIRUS DISEASE AND ITS COMPLICATIONS. Universal Journal of Pharmaceutical Research. 1. 54-58. 10.22270/ujpr.v1i1.RW1.
- Qureshi, A. I. (2016). Ebola Virus. Ebola Virus Disease, 167–176. doi:10.1016/b978-0-12-804230-4.00012-1
- https://microbiologyinfo.com/structure-of-ebola-virus/
- https://www.nhs.uk/conditions/ebola/
- https://www.nhsinform.scot/illnesses-and-conditions/infections-and-poisoning/ebola-virus-disease
- https://www.cdc.gov/vhf/ebola/index.html#:~:text=Although%20Ebola%20disease%20is%20rare,Learn%20more%20about%20Ebola%20disease.
- https://www.who.int/news-room/fact-sheets/detail/ebola-virus-disease
- https://www.webmd.com/a-to-z-guides/ebola-fever-virus-infection
- https://www.bcm.edu/departments/molecular-virology-and-microbiology/emerging-infections-and-biodefense/specific-agents/ebola-virus
- https://brieflands.com/articles/ans-55883.html