What is a Cytotoxic T cell?
- A cytotoxic T cell (also known as TC, cytotoxic T lymphocyte, CTL, T-killer cell, cytolytic T cell, CD8+ T-cell or killer T cell) is a T lymphocyte (a type of white blood cell) that kills cancer cells, cells infected with intracellular pathogens (such as viruses or bacteria), and other damaged cells.
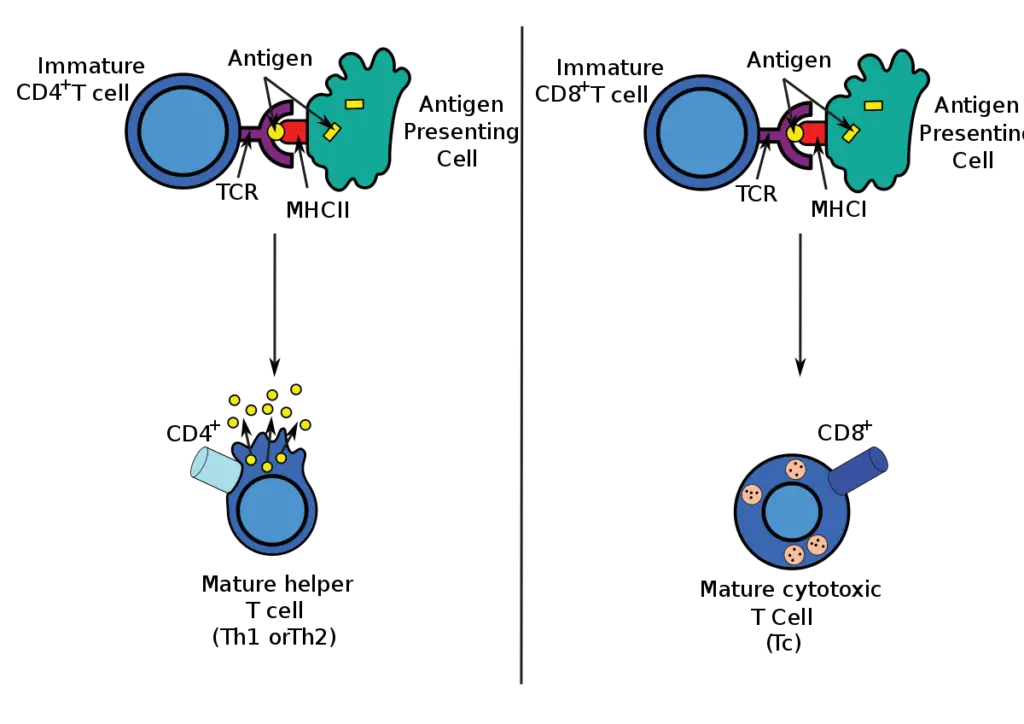
- The majority of cytotoxic T cells possess T-cell receptors (TCRs) that are capable of recognising a specific antigen. An antigen is a molecule that can stimulate the immune system and is frequently produced by cancer cells, viruses, bacteria, and intracellular signals.
- Antigens within a cell are attached to class I MHC molecules, which transport them to the cell’s surface where they can be identified by the T cell.
- If the TCR is specific for the antigen, it attaches to the complex of the class I MHC molecule and the antigen, destroying the cell.
- To bind to the class I MHC molecule, the TCR must be accompanied by the glycoprotein CD8, which binds to the constant region of the class I MHC molecule. Consequently, these T cells are known as CD8+ T cells.
- During antigen-specific activation, the affinity between CD8 and the MHC molecule keeps the TC cell and the target cell tightly attached.
- Once activated, CD8+ T cells are identified as TC cells and are typically classed as having a predetermined cytotoxic function within the immune system.
- However, CD8+ T cells also have the ability to produce antitumor and antimicrobial cytokines, such as TNF- and IFN-.
- The immunological response mediated by CTLs can be separated into two phases, each representing a distinct feature of the immune response. The initial step involves the activation and differentiation of naive TC cells into effector CTLs. In the second phase, effector CTLs detect antigen–class I MHC complexes on specific target cells, leading to their destruction.
Development of CD8+ T cells
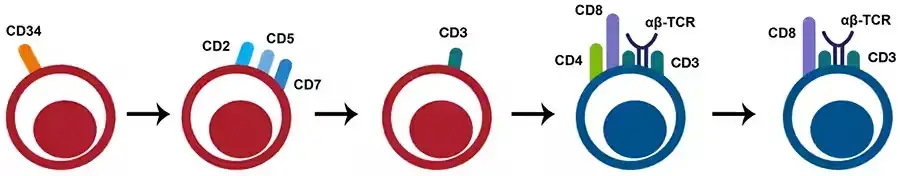
- The formation of CD8+ T- cells begins in the thymus with lymphoid progenitors that undergo a double-negative (DN) (CD8– CD4–) phase, followed by a double-positive (DP) (CD8+ CD4+) phase, before the thymocytes become single-positive (SP) CD8+ or CD4+ thymocytes.
- DP cells undergo positive selection in the thymic cortex by contact with peptide: MHC class I complexes, culminating in the development of CD8+ SP cells.
- The SP cells travel from the cortex to the medulla, where they undergo negative clonal selection to eliminate T cells with a strong affinity for self-antigens.
- Single-positive mature CD8+ T- cells are then discharged into circulation.
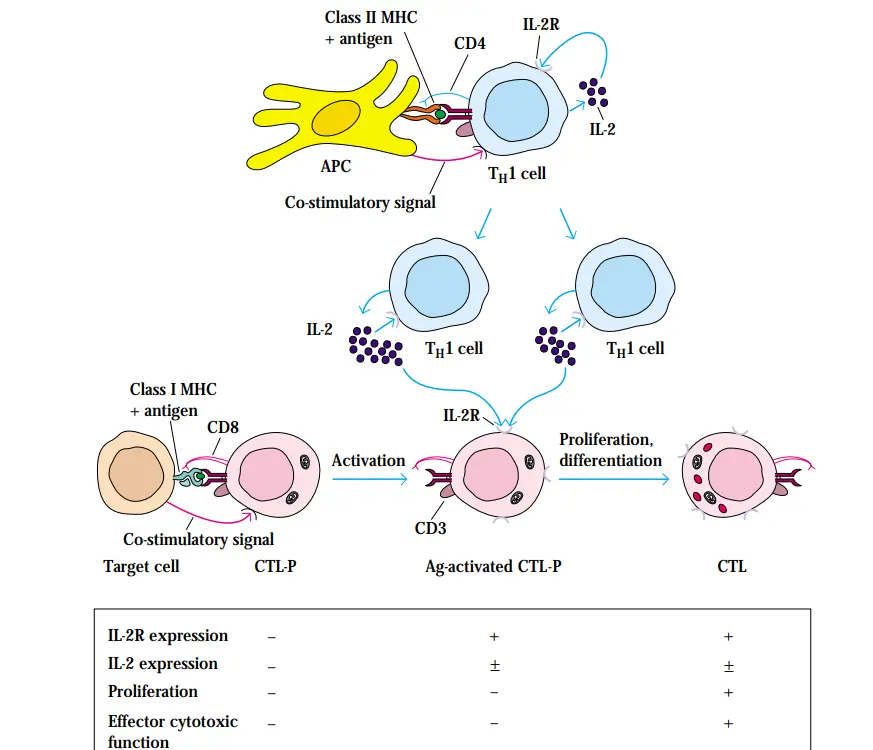
Effector CTLs Are Generated from CTL Precursors
- CTL precursors (CTL-Ps) are used to denote the immature condition of naive TC cells, which are incapable of killing target cells. After CTL-P activation, the cell will develop into a functional CTL with cytotoxic activity.
- It appears that the generation of CTLs from CTL-Ps requires at least three consecutive signals:
- Signal 1 transmitted by the TCR complex following identification of a peptide–class I MHC complex.
- A co-stimulatory signal transmitted by the CTL-P and antigen-presenting cell’s CD28-B7 interaction.
- Signal created by the interaction of IL-2 with the high-affinity IL-2 receptor, which leads to the proliferation and differentiation of antigen-activated CTL-P into effector CTLs.
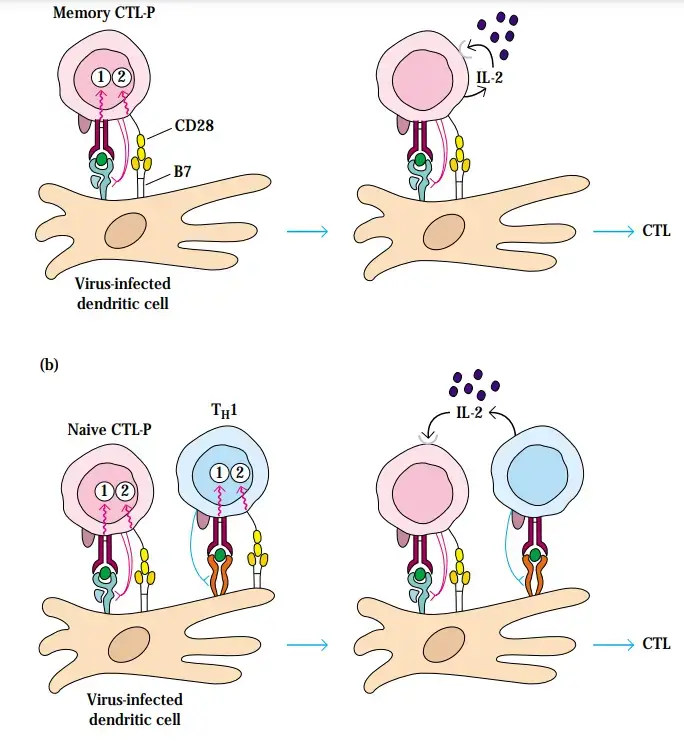
- CTL-Ps that have not been activated do not express IL-2 or IL-2 receptors, do not multiply, and lack cytotoxic function.
- Antigen stimulation stimulates CTL-P expression of the IL-2 receptor and, to a lesser extent, IL-2, the primary cytokine required for proliferation and differentiation of activated CTL-Ps into effector CTLs.
- This is especially true of memory CTL-Ps, which have lower activation needs than naive cells.
- Most activated CTL-Ps, however, require extra IL-2 produced by proliferating TH1 cells in order to proliferate and differentiate into effector CTLs. The fact that the IL-2 receptor is not produced until after a CTL-P has been activated by an antigen and a class I MHC molecule facilitates the clonal growth and acquisition of cytotoxicity by solely antigen-specific CTL-Ps.
- IL-2 is required for the proliferation and development of both antigen-activated TH1 cells and CTL-Ps.
- In IL-2-deficient animals, the absence of IL-2 eliminates CTL-mediated cytotoxicity. After antigen clearance, the level of IL-2 decreases, which promotes programmed cell death by apoptosis in TH1 cells and CTLs.
- Thus, the immune response is finished fast, reducing the chance of nonspecific tissue damage caused by the inflammatory response.
- It seems improbable that a TH1 cell and a CTL-P interact directly given the incomplete understanding of the role of TH1 cells in the production of CTLs from naive CTL-Ps.
- Nevertheless, IL-2 and costimulation are crucial for the transformation of naive CTL-Ps into effector cells, and TH1 cells can serve as mediators in the provision of these key requirements. As depicted in Figure, the interaction between helper cells and antigen-presenting cells can lead to the generation of IL-2 by TH1 cells. This cytokine’s paracrine effect on surrounding naive CTL-Ps with activated TCRs can induce their proliferation and differentiation into active CTLs.
- TH1 can also promote the upregulation of co-stimulatory molecules on the surface of antigen-presenting cells. In this way, TH1 cells facilitate the division and development of CTL-P by generating sufficient co-stimulation.
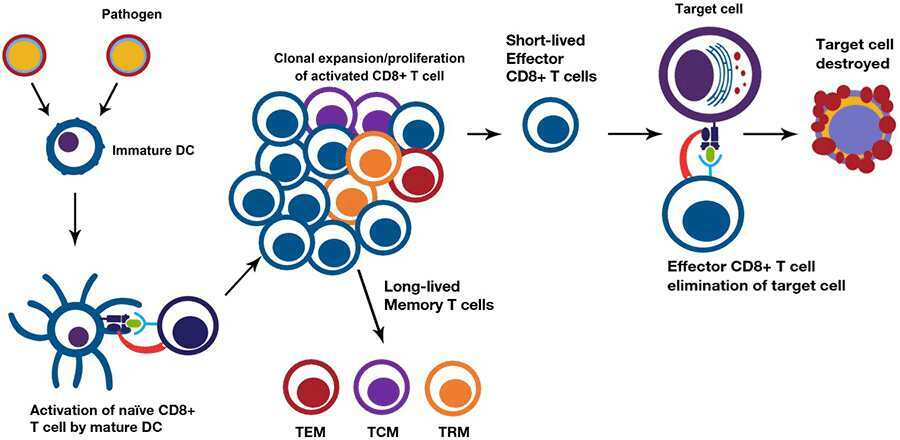
Activation and differentiation of CD8+ T cells
- When activated, naive CD8+ T cells identify antigens presented on MHC class I molecules (major histocompatibility complex) and differentiate into cytotoxic CD8+ T cells (CTLs).
- APCs, such as dendritic cells (DCs), typically deliver endogenous peptides in the context of MHC class I, which are recognised by the TCR and the CD8+ coreceptor on CD8+ T lymphocytes.
- Thus, DCs present altered peptides resulting from viral infection or tumour cells, which then activate antigen-specific CD8+ T lymphocytes via TCR interaction.
- CD8+ T cell activation requires additional costimulatory signals, including as CD80/86 signalling, as well as cytokine signalling from DCs and activated CD4+ T cells.
- The majority of CD8+ T cell activation requires CD4+ T cells to activate and upregulate the necessary costimulatory signals for effective stimulation.
- Certain infectious agents, such as viruses and bacteria, can also induce CD4-independent activation of DC by triggering Toll-like receptors or by promoting the release of IL-1 or type I interferons. CD8+ T cells, once activated, undergo clonal growth to generate phenotypically and functionally heterogeneous effector cells, whose primary job is to remove afflicted target cells.
- The majority of effector cells are short-lived and perish by apoptosis when infection or tumour cells are eliminated.
- A small proportion (5–10%) of T cells survive as long-lived memory cells with a variety of phenotypes, including tissue-resident memory T cells (Trm) that remain in the tissues that initiated the primary reaction, central memory T cells (Tcm) that circulate through secondary lymphoid tissue, and effector memory T cells (Tem) that circulate in non-lymphoid tissues.
Cytotoxicity Mediated by CD8+ T
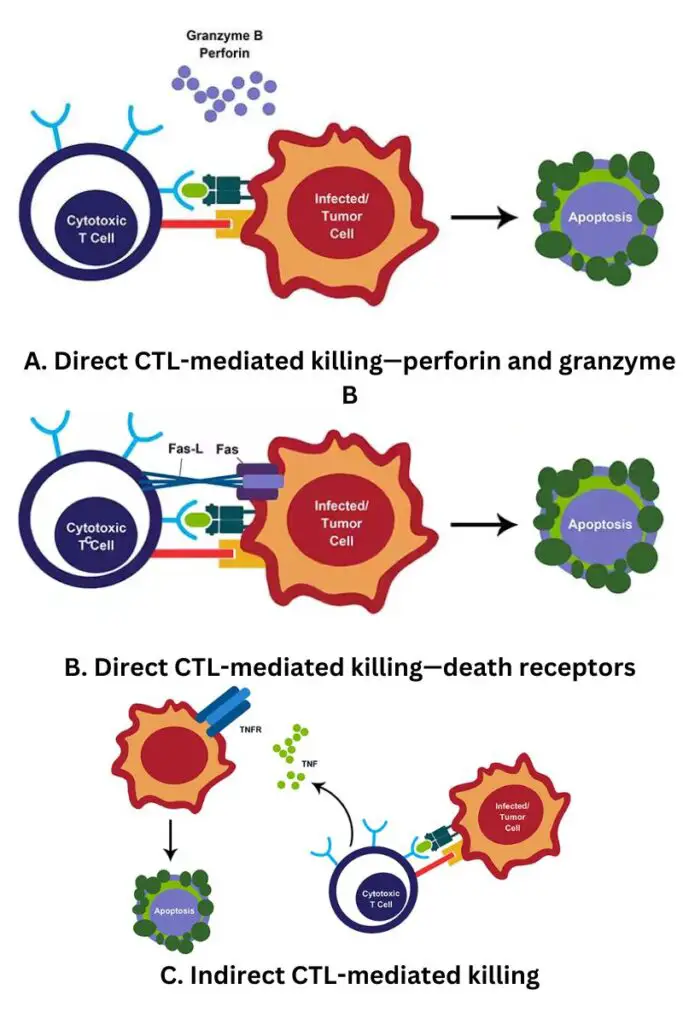
- Cytotoxicity, or the death of target cells by cytotoxic CD8+ T lymphocytes (CTLs), can occur via numerous pathways and involves a well organised chain of events that typically results in apoptosis and elimination of the target cell.
- Numerous CTLs begin the destruction of their targets by delivering pro-apoptotic chemicals and releasing cytotoxic granules.
- The synthesis of perforin and granzymes, which are stored in the cytosol, is triggered by the binding of the TCR to MHC class I. The granules are released at the point of contact, providing for targeted killing and minimal collateral damage.
- Perforin assembles on target membranes, allowing granzymes to enter the target cell. Granzymes are a class of serine proteases that induce cell death by activating caspases.
- Functionality also requires direct cell-to-cell interaction. By ligating the Fas receptor and Fas ligand (Fas L), which are expressed on lymphocytes and infected target cells, CD8+ T cells can trigger apoptosis.
- A number of cytokines, including IFNγ, TNFα, and lymphotoxin-, are produced by activated CD8+ T cells that contribute to host defence. IFNγ suppresses viral replication and increases MHC class I expression, hence increasing the likelihood that an infected cell will be detected.
Role in anti-viral immunity
- Effector cells develop from naive CD8+ T cells within a week of viral infection. Several pattern recognition receptors on innate immune cells identify viral antigens, which results in the creation of type I interferon, which in turn mediates the generation of CD8+ T cells effector response.
- The production of IL-12 by macrophages and DCs induces T-bet, which mediates the acquisition of antiviral cytotoxic capabilities.
- Other cytokines, including TNFα, IL-15, and IL-18, increase the CD8+ T cell response even further.
Role in anti-tumor immunity
- CD8+ T cells are regarded as significant antitumor immunity drivers. Tumor-infiltrating lymphocytes (TILs) that are CD8+ mediate tumour rejection by recognising tumour antigens and directly destroying altered cells.
- Effector CD8+ T cells in the tumour microenvironment release IL-2, IL-12, and IFNγ, which enhance the cytotoxic capacity of CD8+ T cells, hence facilitating the targeting and elimination of tumour cells.
- There is a correlation between elevated levels of cytotoxic CD8+ T lymphocytes in the tumour microenvironment and enhanced antitumor effects and prognosis in numerous forms of cancer.
What are Memory CD8+ T cells?
- It is possible to further classify T-αβ cell subsets depending on their effector memory differentiation state. These memory CD8+ T cells are self-renewing, dwell in lymphoid and nonlymphoid tissues, and are responsible for recall effector actions upon re-exposure to the same antigen. Memory CD8+ T cells have been divided into distinct subsets based on distinctions in effector functions, proliferative capability, and tissue-homing characteristics:
- Central memory cells (Tcm): These cells are primarily limited to lymphoid organs and express lymphoid homing markers CD62L and CCR7.
- Effector memory cells (Tem): These cells serve as the first line of defence against pathogens. They mediate immediate effector activities, are present in the circulation and nonlymphoid tissues, and have low levels of CD62L and CCR7 expression, which are lymphoid homing molecules.
- Tissue-resident memory cells (Trm): Tissue-resident memory cells (Trm): These cells are found at pathogen entrance sites.
All of these effector and memory subsets work together to generate the protective CD8+ T cell response. CD3, CD5, CD8, CD27, and CD28 are pan markers for mouse CD8+ T cells, while CD2, CD3, CD5, CD8, CD25++, CD27, and CD28 are pan markers for human CD8+ T cells.
Function of Cytotoxic T Cells
Cytotoxic T cells are also referred to as ‘killer’ T cells due to their ability to destroy infected cells, pathogens, and tumour cells. This is accomplished mostly by the transmission of cytotoxic granules to infected target cells, which kill both the cell and any infections it may contain.
Secretion of Cytotoxic Granules
- Cytotoxic T cells and natural killer (NK) cells destroy virus-infected cells and pathogens by an extremely similar method.
- Similarly to NK cells, cytotoxic T cells include cytotoxic granules containing the proteins perforin and granzymes, which destroy target cells.
- When cytotoxic T cells encounter an infected cell, their TCR receptors attach to the MHCI and release their cytotoxic granules.
- Perforin produces pores in the cell membrane of the target cell, through which granzymes can enter.
- Once within the cell, the granzymes induce apoptosis (programmed cell death), which kills the cell and any infections it carries.
- Virus-infected cells are often the target of cytotoxic T lymphocytes, therefore this mode of death is advantageous to the host because it contains and eliminates the viruses within the cell.
- If the cell were only lysed, the viruses would escape the cell and spread throughout the body.
- The primary distinction between NK cells and cytotoxic T cells is how they recognise their respective targets. The absence of MHCI, which is normally removed from the surface of tumour cells and pathogen-infected cells, stimulates natural killer cells to attack.
- However, cytotoxic T lymphocytes detect their targets based on the presence of particular antigens in the MCHI complex. Both cytotoxic T cells and natural killer (NK) cells are’serial killers’ and are capable of binding to and attacking many target cells.
Secretion of Cytokines
- Perforin and granzyme secretion is the cytotoxic T cell’s primary weapon, although it is not their only role. Cytotoxic T cells also release cytokines, which contribute in a variety of ways to the adaptive immune response.
- One of these cytokines (IFN-γ) suppresses viral replication directly, so slowing the progress of infection within the body.
- IFN-γ also activates macrophages and increases their migration to infection sites, where they serve as both effector cells (as they ingest and eliminate pathogens) and antigen-presenting cells.
Regulation of Cytotoxic T Cells
- Cytotoxic T cells are essential components of the adaptive immune response and are extremely efficient at eliminating infected cells from the body. However, cytotoxic T cells can contribute to an overactive immune response, causing damage to healthy host cells and tissues if left unchecked.
- In the majority of cases, this is prevented by the contraction phase, which occurs a few weeks after the initial infection. Typically, cytotoxic T cell activity peaks seven days after the first infection.
- In the days and weeks after the peak of cytotoxic T cell activity, the contraction phase commences and up to 95% of the cells die. The remaining 5-10% transform into memory T cells, which remain in the body for an extended period of time after the virus has been eliminated.
References
- Janeway CA Jr, Travers P, Walport M, et al. Immunobiology: The Immune System in Health and Disease. 5th edition. New York: Garland Science; 2001. T cell-mediated cytotoxicity. Available from: https://www.ncbi.nlm.nih.gov/books/NBK27101/
- Actor, J. K. (2012). T-Cell Immunity. Elsevier’s Integrated Review Immunology and Microbiology, 25–32. doi:10.1016/b978-0-323-07447-6.00004-1
- Groscurth, P., & Filgueira, L. (1998). Killing Mechanisms of Cytotoxic T Lymphocytes. Physiology, 13(1), 17–21. doi:10.1152/physiologyonline.1998
- https://www.celiackidsconnection.org/2018/05/06/what-are-the-different-types-of-t-cells/
- https://www.akadeum.com/blog/cytotoxic-t-cells/
- https://www.biologyonline.com/dictionary/cytotoxic-t-cell
- https://www.thermofisher.com/in/en/home/life-science/cell-analysis/cell-analysis-learning-center/immunology-at-work/cytotoxic-t-cell-overview.html
- https://biologydictionary.net/cytotoxic-t-cell/
- https://journals.physiology.org/doi/full/10.1152/physiologyonline.1998.13.1.17
- https://my.clevelandclinic.org/health/body/23547-cytotoxic-t-cells
- https://www.cancer.gov/publications/dictionaries/cancer-terms/def/cytotoxic-t-cell
- https://en.wikipedia.org/wiki/Cytotoxic_T_cell