Continuous Culture Definition
Continuous Culture is an ‘open’-culture system for the cultivation of microorganisms or cells in which fresh sterilized medium is introduced at a steady flow rate and from which the culture fluid emerges at the same rate.
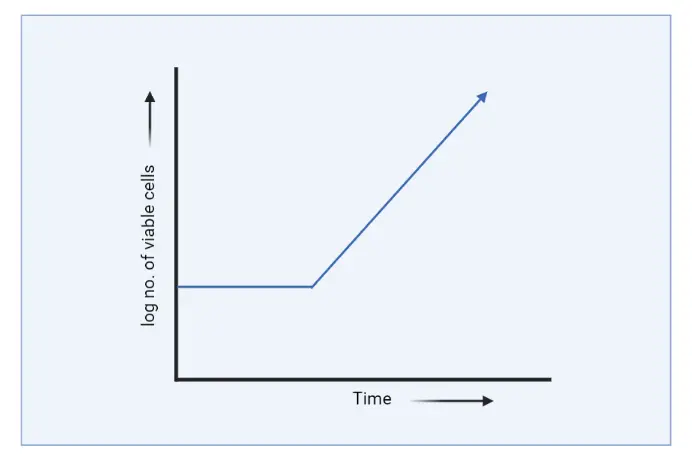
- In both experimental research and industrial processes, it is often necessary to maintain a bacterial population growing at a particular rate in the log phase. This condition is known as steady-state growth or continuous growth.
- In the case of continuous culture, there is no stationary phase and decline phase due to the addition of fresh medium continuously. So, here we can obtain only lag phase and log phase.
- For the following reasons the lag phase in continuous culture will be long; if the inoculum is taken from old culture, if the inoculum is synthetic, if the culture is incubated at unfavorable temperature.
- There are available different types of continuous culture methods, among them the chemostat method was popular.
- Chemostats support steady-state concentrations of growth-limiting substrates to be kept at a set level in the culture fluid, which ends in extremely reproducible ‘steady-state’ growth situations in which variations in cell density, physiological state, and medium composition of the culture are untraceable.
- All the environmental parameters such as pH, oxygen tension, population density, and concentration of excretion products can be easily controlled within the continuous cultures.
Continuous Culture Advantages
- We may obtain all the physiological and biochemical activities of the cell.
- We can study its genetics and cell biology.
- Give an idea about all the toxic substances that kill the same species.
- We can determine the cell’s growth pattern and metabolic activity.
- Reduce the labor costs and increasing product yields
System applied for Continuous Culture
There are two systems used for Continuous Culture such as;
- Chemostat
- Turbidostat
Chemostat
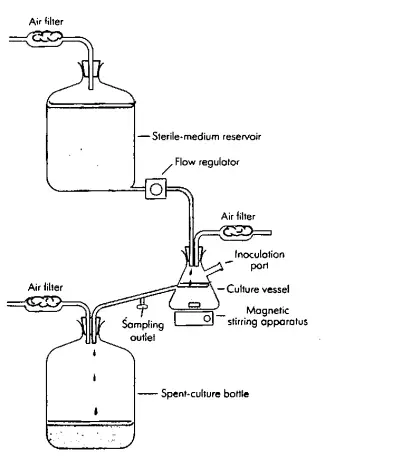
- This system is used to culture the organism continuously. It is an open system/open fermenter where nutrients added and product removed at a steady rate throughout.
- It is constructed so that a sterile medium is fed into the culture vessel at the same rate as the medium containing microorganisms is removed.
- The culture medium for the chemostat process and essential nutrient in limiting quantities, because of the presence of limiting nutrients.
- The growth rate is determined by the rate at which the new medium is fed into the growth chamber.
- The concentration of substrate within the culture vessel is in turn controlled by the dilution rate (the rate at which fresh medium is being added to the culture divided by the volume of the culture vessel). Therefore in chemostat by adjusting the dilution rate we can control the growth rate.
- The cell reaches a high density because they are leaving the culture vessel at a very slow rate: moreover, they have time to use the substrate completely. Therefore, the substrate concentration is maintained at low levels within the vessel. This low substrate concentration permits the cell to grow at only a slow rate.
- On the other hand, if the dilution rate is high, the cell density is low because the cells are leaving the vessel at a high rate; moreover they have little time to utilize the substrate that is entering the vessel, and therefore the substrate concentration is maintained at a high level within the vessel.
- This system depends on the fact that the concentration of essential nutrients within the culture vessel will control the growth rate of the cell.
- The addition rate of the fresh medium within the chemostate system determines the rate of growth because the fresh medium always contains a limiting nutrient.
- In a chemostat the bacterial culture can be grown and maintained at relatively constant conditions, depending on the flow rate of the nutrients.
- The main disadvantage of Continuous Culture is, there is a higher risk of contamination because of the constant adjustments.
- It is feasible only when the inoculated cells are genetically stable.
Turbidostat
- This system is used for culturing the organism continuously.
- This is the second type of continuous culture apparatus, turbid state. It has a photocell that measures the absorbance or turbidity of the culture in the growth vessel.
- The photoelectric cell continuously monitors the cell density and controls the dilution rate to maintain cell density at a constant rate.
- If the cell density is too high, the dilution rate is increased, if the cell density is low the solution rate becomes decreased.
Perfusion
- Perfusion is another system of Continuous Culture, in this method, the cells are retained within the bioreactor or recycling the cells back to the bioreactor. After that, the fresh medium is added and cell-free supernatant is removed from the bioreactors at the same rate.
- The cell density is constantly incrasing depending on the chosen media perfusion rate, also called the dilution rate factor D.
- The cell growth is limited to the nutrient or oxygen constraints or by waste product inhibition, leading to a quasi-steady-state of cells, metabolites and product concentration
Application of Continuous Culture
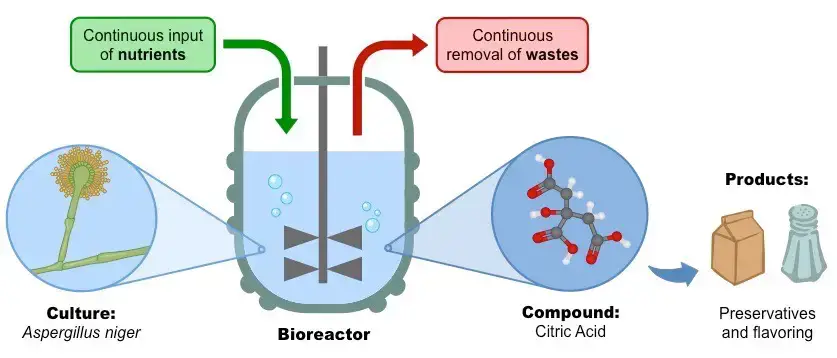
- The continuous Culture method is used for the production of citric acid in industries.
- Used for the Continuous Manufacturing of Recombinant Proteins.
- Continuous cultures have been used to optimize many other process parameters such as Plasmid stability, Promoter strength characterization, Metabolic flux distribution in the pyruvate node, etc.
- Chemostat cultures allow tight control over all growth conditions and the option to modify μ of host cells by limiting the feeding rate of an essential growth substrate. For this reason, chemostats have been widely used to determine the product formation kinetics—the relationship between µ and qp—in many different organisms.
- Continuous cultures have been applied to basic physiology studies of wild-type cells.
- Chemostats are frequently used in the industrial manufacturing of ethanol.
- Used in the biotechnological industry as an experimental model.
Citric acid Production in Continuous Fermentation
- During the citric acid production via the Continuous culture method, the Carbohydrates are continuously added into the fermenter.
- To reduce the further conversion of citric acid within the Krebs cycle, the Iron (Fe2+ ions) is eliminated from the mixture.
- As citric acid stores, it is obtained as part of the medium that is being continuously removed from the fermenter.
References
- J.G. Kuenen, O.J. Johnson, Continuous Cultures (Chemostats), Editor(s): Moselio Schaechter, Encyclopedia of Microbiology (Third Edition), Academic Press, 2009, Pages 130-147, ISBN 9780123739445, https://doi.org/10.1016/B978-012373944-5.00112-7.
- Peebo, K., & Neubauer, P. (2018). Application of Continuous Culture Methods to Recombinant Protein Production in Microorganisms. Microorganisms, 6(3), 56. https://doi.org/10.3390/microorganisms6030056
- https://www.infors-ht.com/en/blog/continuous-culture-for-beginners/
- https://ib.bioninja.com.au/options/untitled/b1-microbiology-organisms/batch-versus-continuous.html
- https://en.wikipedia.org/wiki/Chemostat