What is Citric Acid?
- Citric acid, having the chemical formula C6H8O7, is a weak organic acid. Aspergillus species produce a huge range of metabolites, including citric acid.
- Citric acid is a naturally occurring acid. It is present in a number of fruits and vegetables. The citrus fruit with the highest concentration of citric acid is the lemon.
- Carl Wilhelm Scheele initially extracted citric acid from lemon juice in 1822 and defined its chemical composition.
- Citric acid is utilised in the food and beverage industries for numerous consumer goods. In soft drinks and syrups, as an acidulant, it stimulates a natural fruit flavour and imparts the necessary tartness.
- Citric acid is an intermediate organic component in the tricarboxylic acid (TCA) cycle and is naturally present in citrus fruits, pineapples, pears, and calcium citrate crystals. It is created mostly through fermentation.
- Citric acid generates several metallic salts, such as complexes with copper, iron, manganese, magnesium, and calcium.
- It is utilised as a sequestering agent in industrial processes and as an anticoagulant blood preservative due to the presence of these salts. In fats and oils, it decreases metal-catalyzed oxidation by chelating trace amounts of metals such as iron.
- Its function as a flavouring includes two components: first, its acidity, which leaves little aftertaste, and second, its capacity to accentuate other flavours.
- Citric acid is used as a scrubber to remove sulphur dioxide from flue gases, making a complex ion that then combines with H2S to produce elemental sulphur and regenerate citrate.
- This may become more significant as environmental forces increase.
- The triethyl, butyl, and acetyl tributyl esters of citric acid are employed as plasticizers in plastic films, and monostearyl citrate is utilised instead of citric acid as an antioxidant in oils and fats.
Properties of Citric Acid – C6H8O7
| C6H8O7 | Citric Acid |
| Molecular Weight/ Molar Mass | 192.124 g/mol |
| Density | 1.66 g/cm³ |
| Boiling Point | 310 °C |
| Melting Point | 153 °C |
Citric Acid structure – C6H8O7
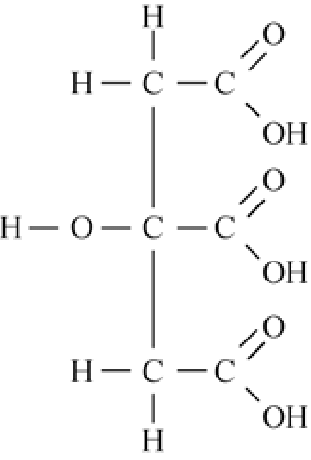
Which Microorganism is used for Citric Acid Production?
Numerous microorganisms are utilised in the production of citric acid. There are bacteria, fungi, and yeasts among these. However, A.Niger and saccharomycopsis sp. are utilised for commercial production due to its numerous benefits.
Advantages of using Aspergillus niger
Aspergillus niger is utilised in the majority of processes due to the following factors:
- Can be cultivated with ease.
- Process biochemical properties to be uniform.
- Under controlled conditions, only a small amount of oxalic acid is produced.
- Produce an abundant amount of citric acid.
Other Microorganisms Used For Citric Acid Production
Bacteria
- Bacillus licheniformis
- Arthrobacter paraffinens
- Corynebacterium species
Yeasts
- Candida tropicalis
- C.oleophila
- C.guilliermondii
- C.Citroformans
- Hansenula anamola
- Yarrowia lipolytica
Fungi
- Aspergillus nagger
- A.aculeatus
- A.awamori
- A. carbonarius
Production of Citric Acid / Fermentation of Citric Acid
The industrial production of citric acid can be conducted in three distinct ways:
- Surface fermentation
- Submerged fermentation
- Koji or Solid-state fermentation
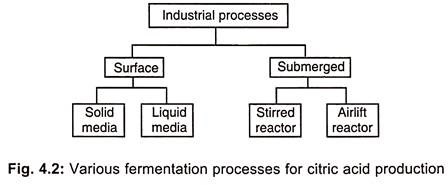
1. Surface Culture Process for Production of Citric Acid
- This process is also known as “liquid surface fermentation.” In 1919, surface culture fermentation was the first technique introduced for the synthesis of citric acid.
- In liquid surface fermentation, the culture medium (5-6 pH) is put to shallow, 5-20 cm-deep aluminium trays.
- In the fermentation chamber, which ensures uniform air circulation and maintains relative temperature and humidity, the process is carried out.
- First, A.niger spores are blown onto the surface of the culture medium for approximately 5 to 6 days, followed by the passage of dry air.
- The pH of the medium is then adjusted between 1.5 and 2 pH.
- After 24 hours, the spores begin to germinate and white mycelium begins to form on the surface of the culture media.
- After the moulds have utilised the sugar content, the residual liquid is removed from the mycelial mat.
- A. niger produces a tiny quantity of citric acid as a main metabolite during the surface culture phase.
Process of Surface Culture Method
This process consists of four phases:
- Inoculum production,
- Preparation of medium,
- Fermentation process and
- Harvest and recovery.
1. Inoculum Production
- Suspension of spores is employed as an inoculum in the synthesis of citric acid. From a stock culture, a suitable and high-yielding strain of A. niger is selected.
- Glass vials containing sporulating media are infected with the stock culture. At 25°C, the bottles are incubated for 10-14 days.
- Trace elements, such as manganese, zinc, and iron salts, must be appropriately maintained in the sporulating medium; otherwise, they will impact the output of citric acid during actual fermentation.
- Suspension of spores is achieved by suspending mature spores in an appropriate diluent, such as water containing the wetting ingredient sodium lauryl sulphate. In addition to the quantity, the viability of the spore crop is crucial.
2. Preparation of Medium
- The medium used to produce citric acid must have a source of carbohydrates and inorganic salts.
- As a carbon source, numerous substances can be utilised. However, sucrose and beet molasses are typically employed as carbon sources.
- Sucrose is the best carbon source among the evaluated organic compounds. A medium containing less than 15% sucrose is reported to have a high citric acid production.
- When sucrose is partially replaced by fructose or glucose, citric acid output is diminished.
- Commercially, beet molasses is widely employed as a carbon source in the manufacture of citric acid by A. niger.
- In addition to sugars, beet molasses contains an abundance of inorganic salts. Before it is utilised in the creation of the medium, it is treated with ferrocyanide or ferricyanide to eliminate these excessive inorganic salts.
- The inorganic salts can also be extracted by passing the beet molasses through a cation exchange resin.
- In addition to carbon, elements such as nitrogen, potassium, phosphorus, and magnesium are required in the media.
- In minimal amounts, ammonium nitrate, potassium dihydrogen phosphate or potassium monohydrogen phosphate, and magnesium sulphate are added to the medium.
- Higher concentrations of these components decrease the yield of citric acid and increase the output of oxalic acid.
- Adjusting the pH of the medium to 3.4-3.5 with hydrochloric acid is required. According to reports, a medium with a low pH facilitates less contamination, the synthesis of more citric acid, the suppression of the formation of oxalic acid, and simple sterilisation.
- In one litre of deionized water, salts and carbohydrates are dissolved. The medium must be sterilised at 55-103 to 69-103 Nm-2 per square inch of steam pressure for 30 minutes.
3. Fermentation Process
- The production media is placed in shallow pans in such a way that a 1 to 2.5 cm thick layer of medium is generated.
- The inoculum spores are introduced to the medium to maintain their floating state. This is accomplished through modulating devices.
- Incubation occurs in 30-40°C incubation chambers. Figure 4.3 depicts the standard fermentation setup.
- During fermentation, the temperature is held constant at 30 degrees Celsius. Air current ventilation is also essential for gas exchange, as the rate of citric acid generation decreases when CO2 levels in the environment reach 10%.
- The germinating spores develop a thin layer of mycelium on the surface of the nutrient solution 24 hours after inoculation. Due to the incorporation of ammonium ions, the pH of the culture medium falls to between 1.5 and 2.0.
- If the iron concentration is high after 30 hours of fermentation, oxalic acid and yellow pigment are produced, which inhibits the recovery process.
- The mature mycelium floats as a thick, whitish, convoluted layer atop the liquid media. After 8-14 days, the fermentation ceases.
- The rate of sugar bioconversion to citric acid is proportional to the ratio of surface area to medium volume.
- If the ratio is reduced, the yield of citric acid will increase. In this shallow pan approach, the ratio of surface area to medium volume is reduced, which exposes a vast surface area of mycelial mat to a thin layer of medium.
- Under these conditions, sugar is increasingly transformed into citric acid. This is the reason why this method is considered superior to the submerged culture method. This method yields between 1.2 and 1.5 kilogramme of citric acid monohydrate per square metre of fermentation surface per hour.
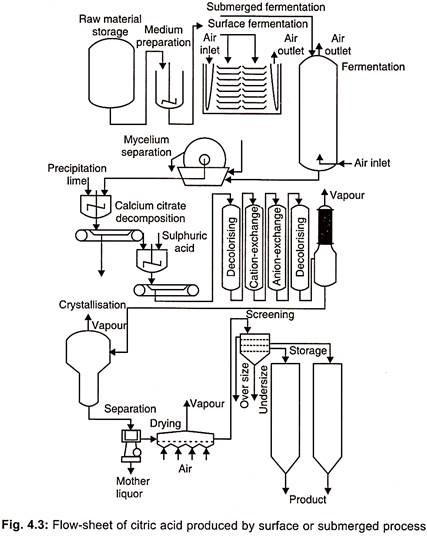
4. Harvest and Recovery
- Separating the mycelium from the fermentation broth. By pressing the mycelium, any intracellular citric acid present in the mycelium can be extracted.
- Calcium hydroxide is used to treat the filtered broth. There is filtration and washing. The substance is then treated with an equivalent volume of sulphuric acid to produce citric acid. In this procedure, a precipitate of calcium sulphate is generated.
- Filtration is used to isolate the precipitate. By decolorizing and demineralizing an impure citric acid solution with activated carbon, an impure citric acid solution is created.
- Through evaporation, pure citric acid crystals are created. It is also extracted using a counter-current extraction technique.
2. Submerged Culture Process for Production of Citric Acid
- This process is also known as “Submerged culture fermentation.” Approximately 80% of citric acid generation is accomplished through submerged fermentation.
- Submerged fermentation utilises black Aspergillus, also known as A. japonicus. It is conducted in a stainless steel bioreactor equipped with aeration, cooling system, impellers, etc.
- Substrates such as beet molasses, maize starch, etc. are used as carbon sources. As a source of nitrogen, ammonia is utilised.
- This approach requires pretreatment of the substrate, such as nutrition addition, sanitation, etc.
- The culture medium is injected with A. japonicus and maintained at 30 degrees Celsius.
- Typically, submerged fermentation is conducted in a batch bioreactor that can produce 1500 kg of citric acid and 500 kg of biomass from 2500 kg of glucose and 860 kg of oxygen.
- Important for production in the submerged culture process are three elements. They are the qualities of the metal used to make fermenters, mycelium structures, and oxygen delivery systems.
- Candida lipolytica, an alkane-utilizing fungus, can also be used in the continuous fermentation generation of citric acid. It yields 45% more citric acid than standard production.
Process of Submerged culture fermentation
1. Inoculum Production
- In this method, mycelial mats known as pellets are employed as inoculum for fermentation. From a stock culture, suitable and high-yielding strains of A. niger are selected.
- A seed fermenter induces germination of the spores. In this seed fermenter, a nutrition solution containing 15% sugar from molasses is employed.
- Cyanide ions are introduced to the medium to stimulate the production of mycelial pellets.
- The production of pellets is significantly influenced by the concentration of cyanide ions in the media. If cyanide ions are in lower concentration, citric acid production is diminished.
- Lower concentrations of cyanide ions encourage the production of regular mycelium rather than pellets.
- The spores germinate at 32 degrees Celsius and form 0.2 to 0.5 mm pellets within 24 hours. Throughout this time, the pH lowers to 4.3. These pellets are then employed as starter cultures in production fermenters.
2. Preparation of Medium
- The same medium used for surface culture is also used for this method.
3. Fermentation Process
- The majority of fermenters used for citric acid manufacturing have a capacity between 10 and 220 klt. They must be made from stainless steel to prevent heavy metal leaching.
- Normal steel, if used in the building of fermenters, may limit the synthesis of citric acid at pH levels between 1-2.
- Due to their huge surface-to-volume ratio, small stainless steel fermenters with a capacity of up to 1000 litres should be lined with plastic. Nevertheless, huge stainless steel fermenters do not require such a plastic lining.
- The mycelial pellets grown in the seed tank are transferred aseptically to the fermenters and incubated at a constant temperature of 30 degrees Celsius.
- The structure of the mycelium that forms in the fermenter is essential to the success of the manufacturing process. If the mycelium is loose and filamentous with few branches and no chlamydospores, little citric acid is generated.
- The optimal amount of citric acid is produced when the mycelium is in pellet form. The iron-to-copper ratio in the media determines the type of the mycelium. In certain instances, production fermenters are directly inoculated with spores.
- Although A. niger has a low oxygen requirement, it is vulnerable to oxygen shortage. The oxygen concentration must be between 20 and 25 percent of the saturation value during the fermentation process.
- Short interruptions in the oxygen supply permanently halt manufacturing. During the acid generation phase, the aeration rate should be between 0.2-1 volume per minute.
- Low viscosity makes stirring unnecessary. Consequently, while some factories utilise stirred fermenters, airlift reactors can also be used.
- Foaming is an issue during submerged culture. However, it can be regulated by often adding antifoaming substances such as lard oil.
- Both airlift and stirred bioreactors require a foam chamber one-third the size of the fermenter volume.
- There are additional mechanical antifoaming devices available. By calculating the fermentation’s sugar and citric acid concentrations on a regular basis, the fermentation’s progress is regularly checked.
3. Solid State Fermentation
- It is known as the “koji procedure.” Japan is where the koji method was originally introduced. It relates to the utilisation of agro-industrial leftovers in the synthesis of citric acid.
- In the Koji process, common raw ingredients include apple pomace, sugar cane, and beet molasses, among others. Aspergillus niger makes use of raw resources.
- The pH and moisture content of the raw material are adjusted to 4-5 and 70%, respectively. Then, chill the raw material to temperatures between 30 and 60 degrees Celsius.
- Then, inoculate with A. niger. After inoculation, the medium is moved to big trays with a depth of 3-5 cm and incubated for 3-7 days at 25-30 degrees Celsius.
- The citric acid is finally collected from the fermenting vessel. The starch content of the raw material is converted to citric acid by the amylase enzyme of Aspergillus niger.
- Because trace elements have little effect on citric acid synthesis, the koji method does not require a pretreatment of the substrate.
- The solid substrate is saturated with water to a water content of 65-70 percent. Following the removal of superfluous water, the mass is subjected to a steaming procedure.
- The surface of sterile starch paste is infected with Aspergillus niger conidia in the form of an aerosol or liquid conidia solution.
- The pH of the substrate is approximately 5-5.5, and the incubation temperature is 28-30 degrees Celsius.
- By adding Alpha-amylase, growth can be enhanced. Although the fungus can hydrolyze starch with its own alpha-amylase, it cannot do so efficiently. During citric acid synthesis, pH values went below 2
- Five to eight days are required for the solid-state surface procedure, after which the complete is removed with hot water. In some instances, citric acid is extracted from the cells using mechanical passes.
- All of these procedures occurred during solid-state fermentation to produce citric acid.
Recovery of Citric Acid
- The product of fermentation is a liquor that appears cloudy due to antifoaming chemicals, mycelia, etc.
- To separate these substances, a slurry of calcium hydroxide, i.e. Ca (OH)2, is used to generate a calcium citrate precipitate.
- The calcium citrate precipitate is filtered and rinsed. After filtration, use sulphuric acid to precipitate calcium from a filtrate as “Calcium sulphate” (CaSO4).
- After passing successively through the ion exchange bed, calcium sulphate is next treated with activated carbon, which demineralizes it.
- The resulting solution is then exposed to circulating crystallizers. The crystals that form as a result of crystallisation are subsequently removed by centrifugation.
- Following these procedures, the residual solvent is dried, sieved, and finally packaged. The remaining mother liquor is extracted using the same method.
Factors Affecting the Citric-Acid Production
1. Fungi
- It is crucial to select a good fungal strain for the optimal production of citric acid.
- It has been claimed that numerous fungus may make citric acid. For instance, Aspergillus niger, A. clavatus, Penicillium luteum, P. citrinum, Paecilomyces divaricatum, Mucor piriformis, Ustulina vulgaris, and other Mucor species have been used to manufacture citric acid in the laboratory or commercially.
- Among these fungal strains, however, only A. niger strains are utilised for the following reasons:
- These are productive (high-yielding) strains.
- They have relatively similar biological characteristics.
- They create zero or minor amounts of oxalic acid if fermentation conditions (pH and salts) are optimised to promote the synthesis of citric acid.
- They are simple to cultivate.
2. Preparation of inoculum
- The spores of Aspergillus niger strain necessary for inoculating shallow pans are produced by cultivating the fungus from a stock culture on a suitable solid sporulation medium at 25 degrees Celsius for four to fourteen days.
- In the composition formula of inoculum medium, trace levels of manganese salts, if not balanced by adequate amounts of zinc or iron salts, may reduce citric acid yields in the actual fermentation of spore-inoculated media.
- The spores are suspended by suspending them in a suitable diluent, such as water containing a wetting agent.
- Since this is a surface-culture method, inoculum spores are introduced to the production medium to keep them floating on the surface. This can be achieved using a specialised inoculation equipment.
3. Source of carbon
- Sucrose, according to laboratory tests, is the best carbon source among a variety of investigated organic compounds, especially sugars, for creating high yields of citric acid.
- It was also stated that sucrose concentrations beyond 15% should not be employed since the extra sugar (less than 3%) was not converted into citric acid.
- When sucrose was partially replaced with fructose or glucose, citric acid production were lower than when sucrose was used alone as a control.
- Therefore, care must be exercised when utilising sucrose to prevent incomplete hydrolysis. Molasses from sugar beets is widely utilised as a carbon substrate in the industrial synthesis of citric acid by fungi.
- Molasses from sugar beets requires pretreatment because it contains excessive levels of trace metals. Before sterilisation, then, ferrocyanide or ferricyanide may be added to the production medium.
- As a result of forming a combination with the additional chemical agent, the metals in the beet molasses are precipitated out.
- The clarified molasses can also be run through a cation-exchange resin. Under laboratory circumstances, the best yields of citric acid can be achieved by passing sucrose over an ion-exchange resin.
4. Inorganic salts
- In addition to the carbon, hydrogen, and oxygen supplied by the additional carbohydrate, the fermentation medium used to produce citric acid requires the trace metals nitrogen, potassium, phosphorus, sulphur, and magnesium.
- Note: Salts and sugars are dissolved in distilled water and formed up to 1 litre in volume. The pH of the medium is adjusted with N/1 HCI to a range of 2.20 to 1.60. The medium is sterilised at 8 to 10 pounds of steam pressure per square inch for 30 minutes.
- It is of the utmost importance to add NH4NO3, KH2PO4 or K2HPO4, and MgSO4 7H2O in the smallest amounts possible, as the presence of these metallic salts in excess adversely affects the fermentation.
- More than 2.50 grammes of NH4NO3, 1.50 grammes of potassium monohydrogen phosphate, and 0.30 grammes of MgSO4 7H2O, for instance, enhance the yield of oxalic acid while decreasing the output of citric acid.
- It is highly desired for mycelial mats to be thin and sporulation to be light or nearly absent in order to obtain large citric acid yields.
- Numerous researchers have investigated the qualitative and quantitative effects of various metallic ions. In conclusion, the type and amount of additional metallic ions (such as iron and zinc) required for citric acid—Aspergillus niger fermentation are highly dependent on the fungal strain to be employed in the process.
- For instance, Aspergillus niger 62 required 0.1 mg of iron per litre to generate optimal results in an experiment done by Perlman, Dorrell, and Johnson (1946) using highly pure media.
- A. niger 59, on the other hand, required 10 mg of iron per litre for optimal growth. Moreover, the effects of metallic ions are interconnected. Consequently, the optimal concentration of one metal may depend on the concentration of other metals in the medium.
- As a result of advancements in analysis techniques, it is now possible to add the precise amount of trace metals (e.g., a fraction of a part per million) to the medium.
5. pH
- According to Currie, HCI should be used to modify the pH of the medium to 3.4 to 3.5. In addition, Doelger and Prescott discovered that low pH levels (1.60 to 2.20) are the most favourable.
- These researchers also recommended for the use of HCI, which has been found to be superior to nitric, sulphuric, and acetic acids for increasing citric acid output.
- In general, calcium carbonate is not added to the production medium to neutralise generated citric acid because its presence promotes contamination.
- In addition, its absence favours higher citric acid production and a shorter fermentation period. For the following reasons, low pH values in Aspergillus niger – citric acid fermentation are desired:
- Medium sterilisation is more easily accomplished.
- Citric acid formation is favoured.
- Oxalic acid formation is inhibited.
- The risk of contamination is reduced.
- Last but not least, stationary pans or trays must be corrosion-resistant or made of stainless steel or aluminium due to the use of production media with low pH values and the sensitivity of the fermentation process to excessive iron.
6. Temperature
- The exact temperature necessary for incubation is contingent upon the fungus strain and fermentation conditions. Doelger and Prescott regard a temperature range of 26 to 28 degrees Celsius to be acceptable.
7. Ratio of surface area to volume
- This is a crucial bioparameter in this process, as the rate of sugar bioconversion to citric acid is dependent on the ratio of surface area to medium volume.
- The citric acid output increases as the volume to surface area ratio decreases. A significant surface area of the mycelial mat is exposed to a relatively thin layer of production media in this shallow-pan approach.
- Under those conditions, significant sugar bioconversions occur. This method is preferable to the submerged-culture method in this regard.
- Doelger and Prescott investigated the effect of altering the volume-to-surface area ratio of the medium.
- Typically, the production media is placed in shallow pans in such a way that a 1 to 2.5 cm (but occasionally up to 8 cm) layer of medium is generated.
8. Aeration
- Air must be provided to the surface of the seeded media. According to laboratory research, aeration rates that are either higher or lower than the optimal rate result in reduced citric acid outputs.
- Therefore, it is vital to estimate the air supply rate required for each newly installed piece of equipment.
9. Time
- The shallow-pan approach (i.e. surface-culture process) requires between 7 and 10 days for the progression and completion of the fermentation process.
10. Yields
- It has been claimed that citric acid yields can range from 60 to 80 g of anhydrous citric acid per 100 g of integrated sugar, or even more.
- Wells, Moyer, and May achieved a maximum output of 90.7% citric acid from glucose based on the amount of sugar consumed.
11. Nitrogen Source Concentration
- Ammonium salts such as urea, ammonium sulphate, etc. result in a reduction in pH, which is essential for the formation of citric acid.
- The nitrogen concentration must fall within the range of 0.1 to 0.4 N/L.
- A high nitrogen source will enhance microbial growth, which will increase sugar consumption and decrease citric acid output.
12. Phosphorous Source Concentration
- Potassium dihydrogen phosphate is regarded as the optimal phosphorous source for optimal fungal production and development.
- For optimal yield, the phosphorus concentration must range between 0.5 and 5.0 g/l.
- In the presence of excessive phosphorus, sugar acids are produced, which inhibit carbon dioxide fixing and increase fungal growth.
13. Presence of Trace Elements
- During fermentation, divalent metals such as iron, zinc, manganese, etc. are produced. The addition of KH2PO4 to zinc will increase production, however trace elements such as manganese, iron, and a high zinc concentration may reduce yield.
14. Lower Alcohols Concentration
- Reduced alcohols such as ethanol, methanol, etc. promote citric acid production. The lower alcohol percentage must be between 1-3%.
- Ethanol stimulates Citrate synthetase enzyme activity, which doubles the time by decreasing Aconitase enzyme activity by 75%.
- Coconut oil (about 3%) also influences the synthesis of citric acid. Lower alcohols not only enhance the formation of citric acid, but also the sporulation of bacteria.
15. Miscellaneous Compounds
- Calcium fluoride, sodium fluoride, and other chemicals promote the generation of citric acid, but potassium ferrocyanide reduces the output.
Applications of Citric Acid
1. Food Additive
- Citric acid is used as a flavouring ingredient and preservative in food.
- It is utilised in processed foods such as beverages and soft drinks.
- Due to its sour flavour, it is utilised in the production of sour candies.
- Sometimes the sour candy is topped with a citric acid-based white powder.
- Several ice cream manufacturers utilise it as an emulsifier to keep fat globules at bay.
2. Cleaning Agent
- The acid citric is among the chelating agents.
- With the use of citric acid, limescale is eliminated from evaporators and boilers.
- The acid is utilised in soaps and laundry detergents because it softens water.
- In addition to citric acid, kitchen and bathroom cleansers also include citric acid.
- In addition to being a cleaning, it is also utilised as a deodorizer.
3. Cosmetics
- As citric acid aids in the elimination of dead skin, it is utilised in homemade masks.
- Reduces the appearance of wrinkles, acne scars, and other blemishes by enhancing the texture and development of the skin.
- In order to balance the pH levels, citric acid is a typical component in cosmetics.
- It is present in hand soap, body wash, nail polish, facial cleansers, shampoos, and other cosmetics.
4. Water Softener
- Citric acid is utilised in detergents as a water softener due to its organic acid, chelating, and buffering capabilities.
- Citric acid’s chemical features as a weak organic acid make it an effective water softener.
- It functions by breaking down the trace amounts of metal found in water, making it an ideal all-natural remedy for hard water.
5. Industrial Uses
- Citric acid is used in the production of detergents, electroplating, and leather tanning.
- Additionally, citric acid is employed as a blood preservative, buffer, and antioxidant in the pharmaceutical and cosmetics sectors.
- Several acids produced from fungi have substantial commercial value and find widespread use in the food, feed, pharmaceutical, and polymer sectors.
References
- https://www.hansrajcollege.ac.in/hCPanel/uploads/elearning/elearning_document/IEM_Unit_3_merged_citric_acid,_penicilin_and_ethanol.pdf
- https://www.vogelbusch-biocommodities.com/technology/organic-acid-process-plants/citric-acid-technology/
- https://jjbs.hu.edu.jo/files/v8n3/Paper%20Number%208m.pdf
- https://www.biotechnologynotes.com/industrial-biotechnology/citric-acid/citric-acid-structure-fermentation-process-and-uses-in-food-industries-biotechnology/13744
- https://biologyreader.com/production-of-citric-acid.html
- https://www.vaisala.com/en/industries-applications/cane-and-beet-sugar-milling-and-refining/citric-acid-production
- https://webstor.srmist.edu.in/web_assets/srm_mainsite/files/files/citric.pdf
- https://www.narajolerajcollege.ac.in/document/sub_page/20200418_101850.pdf
- https://thebiologynotes.com/citric-acid-production/
- https://www.biologydiscussion.com/acids/citric-acid/citric-acid-discovery-fermentation-and-recovery-microbiology/66045
- https://ccelms.ap.gov.in/adminassets/docs/10112020063211-Citric_acid_production_ppt_pdf.pdf