One of those methods you’ve most likely heard of in passing, particularly if you have ever dipped a toe into chemistry or seen a crime thriller where they examine unidentified drugs, is chromatography. Fundamentally, it’s a means of separating difficult combinations into their component elements. Imagine you want to know what’s really in there and have a mix of elements, like chemicals in a medicine or ink from a marker. Chromatography then comes in really handy.
Leveraging two main phases—one that moves (like a gas or liquid) and one that remains place (like a specialty paper or gel—the method operates based on how various drugs interact. Every element moves at its own speed while the mobile phase propels the combination along. While some laggards behind the immobile phase, others fly forward. This variation in movement finally separates everything apart.
Without without knowing it, you have most certainly come across a basic form of this. Consider a coffee filter: the gritty particles are left behind when water goes through grinds extracts flavors and caffeine. Though much more precisely, chromatography works on a similar basis. Variations like gas chromatography for testing volatile substances or liquid chromatography for medicines abound in laboratories. The food business also utilizes it to look for pollutants or additives.
Its adaptability adds so much value. Chromatography is a go-to method for checking blood samples for drugs, making sure a new perfume smells just right, or even looking at environmental samples for toxins. It’s like having a molecular detective able to sort through anarchy to expose precisely what is lurking in the mix.
What is chromatography?
Chromatography is a scientific technique for separating the various components of a mixture. This approach dissolves the mixture in a fluid known as the mobile phase, which passes it across a system including a stationary phase—a substance kept fixed in situ. Factors including size, charge, and affinity affect how the components interact differently with the stationary phase while the mixture moves with the mobile phase. These distinct interactions drive the components to move at different rates, hence separating them.
Early in the 20th century Russian botanist Mikhail Tsvet invented the word “chromatography”. Tsvet refined this method to separate plant pigments including xanthophylls, carotenes, and chlorophyll between 1901 and 1905. Reflecting the method’s first application in distinguishing colored compounds, the term comes from the Greek words “chroma,” meaning color, and “graphein,” meaning to write.
With uses in chemistry, biology, and environmental science among other disciplines, chromatography has developed throughout time into a basic method in chemical analysis. Many chromatographic methods, each suited for certain kinds of studies and substances, have resulted from modern developments.
Chromatography Definition
Chromatography is a laboratory technique used to separate, identify, and purify the components of a mixture based on their differential affinities for a stationary phase and a mobile phase.
Principle of Chromatography – How does chromatography work
Chromatography is an analytical method that uses the differential mobility of components within a mixture to interact with two separate phases: stationary and mobile. Usually a solid substance or a liquid film deposited on a solid support, the sample is placed onto a stationary phase in this method and then subjected to a mobile phase to move the mixture either through or over the stationary medium. Chromatography is fundamentally based on the idea that, via adsorption and partitioning, the distinct components in the mixture interact differently with the stationary phase. Often affected by elements like molecule polarity, size, and weight, these different interactions lead certain components to stick to the stationary phase longer while others move more quickly with the mobile phase.
These different interactions define the efficiency of the separation. While those with a weaker attraction are rapidly swept away by the mobile phase, molecules with a great affinity for the stationary phase often move slowly and remain inside the system for a considerable duration. The fundamental premise of the chromatographic method is the effective separation of the components within the mixture resulting from this differential migration.
Chromatography has advanced recently to greatly raise the sensitivity and resolution of the separation process. Sophisticated detectors and digital processing methods included into modern systems allow the analysis of trace-level compounds in complicated matrices. New stationary phases, including surfaces reinforced by nanoparticles, which provide exceptional interaction characteristics and higher efficiency, have also come from material science innovations. Furthermore, the use of environmentally friendly mobile phases has been under increasing importance in order to match sustainable laboratory procedures with chromatographic methods.
Chromatography is essentially based on three fundamental elements: the separated analytes, the mobile phase, and the stationary phase. The stationary phase offers the surface for molecular interactions; the mobile phase serves as the sample carrier; and the different interactions among these components provide the effective separation of the mixture. From pharmaceutical research to environmental monitoring, the idea of chromatography is still fundamental in current analytical science given ongoing technical developments and an ever-growing spectrum of applications.
Types of Chromatography
Commonly used chromatography techniques are
- Column chromatography
- Ion-exchange chromatography
- Gel-permeation chromatography
- Affinity chromatography
- Paper chromatography
- Thin-layer chromatography
- Gas chromatography (GS)
- Dye-ligand chromatography
- Hydrophobic interaction chromatography
- Pseudoaffinity chromatography
- High-pressure liquid chromatography (HPLC)
1. Column chromatography
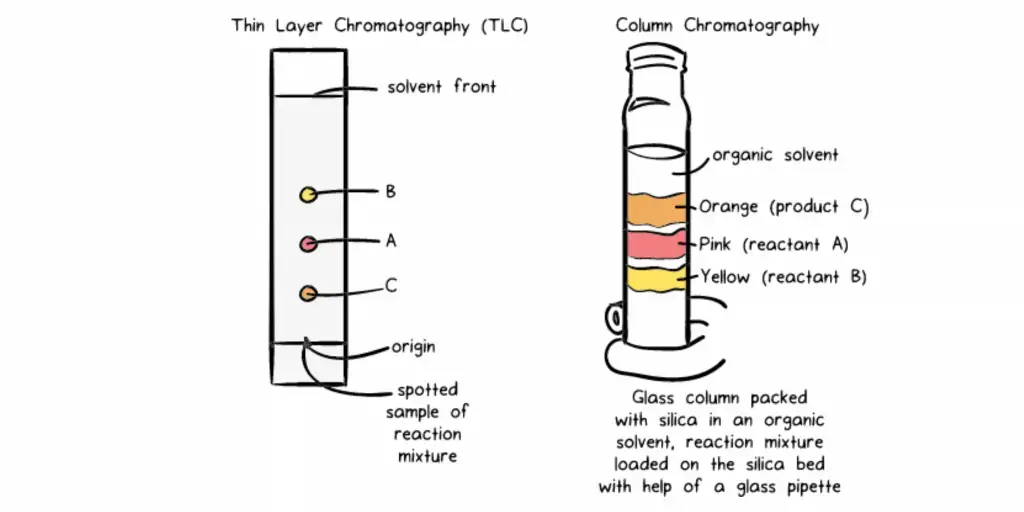
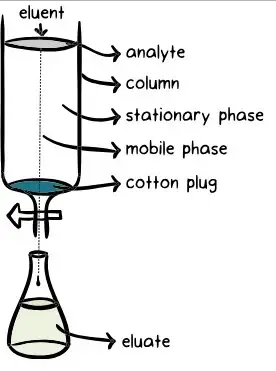
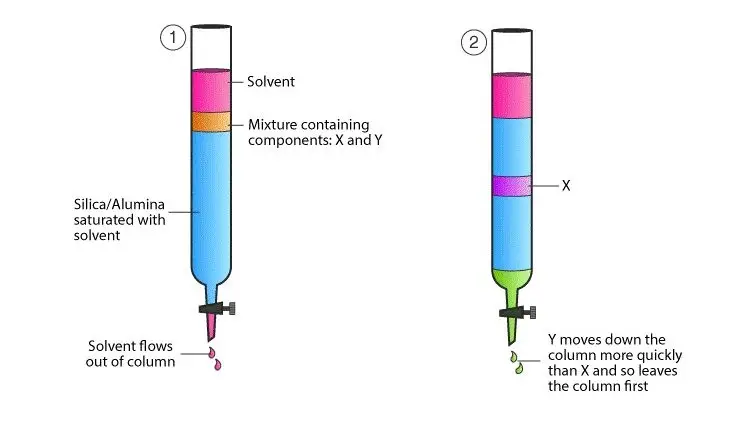
- One often used laboratory method for isolating and purifying specific components from complicated mixtures is column chromatography. This approach packs a vertical glass column with a solid stationary phase—often silica gel or alumina. Applied at the top of the column, the mixture to be separated passes via a liquid mobile phase sometimes referred to as the eluent. Based on their individual chemical characteristics—such as polarity and affinity—the components of the mixture interact differently with the stationary phase as it descends the column. The components separate as they elute from the column at various periods because of this differential interaction moving them at distinct speeds.
- Effective separation depends on a homogeneous stationary phase, hence the method starts with meticulous column packing to guarantee it. The top of the packed column is then the sample mixture placed onto. The components of the mixture accompany the mobile phase across the stationary phase. Components having more affinity for the stationary phase move more slowly; those with less affinity move quicker. Collecting the eluent in fractions at the column’s bottom allows particular components to be separated for additional study or use.
- Applications for column chromatography are many and include proteins, nucleic acids, and tiny chemical molecules among other compounds. Its uses in biochemistry, pharmacology, and environmental science include tasks like purifying reaction products, isolating natural substances, and evaluating complicated mixtures in each of which it finds use.
- Column chromatography’s developments have made it possible to create automated systems improving repeatability and efficiency. Pressurized gas is used in techniques like flash chromatography to drive the solvent through the column, hence drastically lowering separation time. Furthermore simplifying the purifying process is the real-time monitoring and automatic component collecting made possible by the combination of detectors and fraction collectors.
- All things considered, column chromatography is a basic method in analytical and preparative chemistry that is necessary for the component separation and purification in complicated mixtures. Its versatility and efficiency make it still a useful instrument in industrial uses and scientific study.
2. Partition chromatography
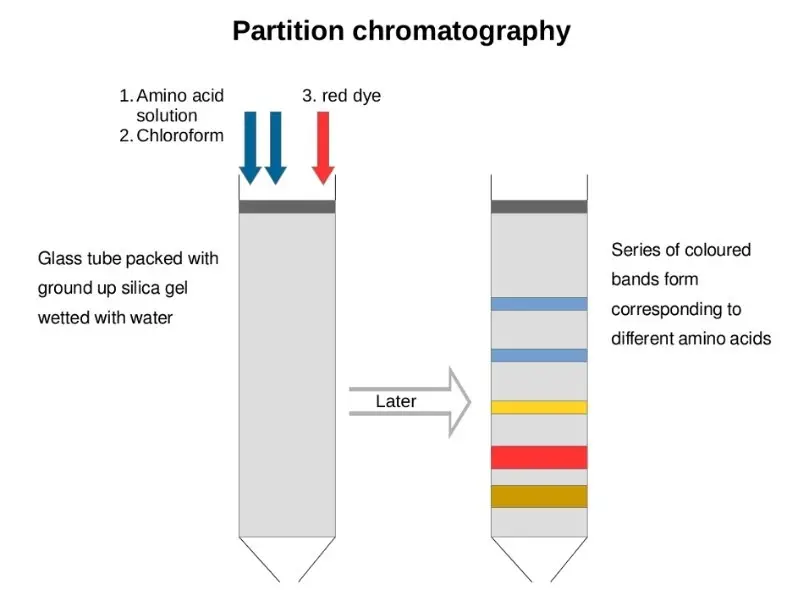
- Partition chromatography is a technique for separating mixture components based on their distribution in two immiscible liquid phases: a stationary phase and a mobile phase. Under this approach, another liquid flows through the system while the stationary phase is a liquid film sprayed onto a solid substrate. The separation results from distinct chemicals in the mixture having differing affinities for the two phases, which causes their varied partitioning.
- Partition chromatography’s foundation is the partition coefficient—that is, the ratio of a compound’s concentration in the stationary phase to its concentration in the mobile phase at equilibrium. Higher affinity compounds for the stationary phase will move more slowly throughout the system; those with higher affinity for the mobile phase will elute more quickly. This variation in movement lets the elements in the mixture separate.
- Usually, the process consists in creating a column with the stationary phase, bringing the mixture to be separated, and then running the mobile phase over the column. The components of the mixture travel in the mobile phase at various speeds, separating down the column. The separated chemicals are then found and counted using techniques of detection.
- Partition chromatography has extensive application in food analysis, environmental research, and medicines among other disciplines. Separating and evaluating similar-minded compounds—such as amino acids, nucleotides, and other tiny biomolecules—is very helpful using it. Its flexibility makes it a necessary instrument in industry as well as research.

3. Ion-exchange chromatography
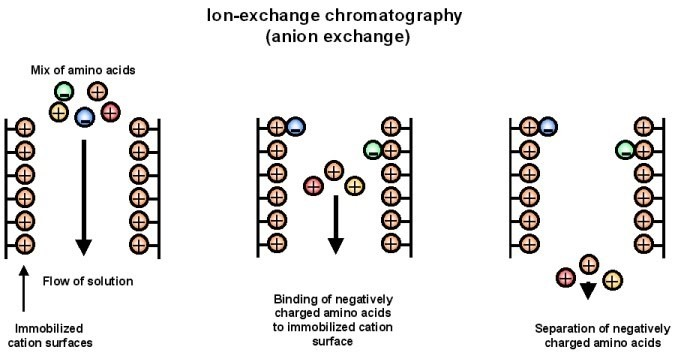
- Separating ions and polar molecules according to their affinity to ion exchangers is the basis of ion-exchange chromatography. The idea is the reversible ion exchange between ions bonded to an ion exchanger and target ions in the sample solution. Anionic exchangers, with their positively charged groups that draw negatively charged anions, and cationic exchangers, with their negatively charged groups that draw positively charged cations, are two primary varieties of ion exchangers.
- Usually, the process consists in equilibrating the ion exchange resin with a buffer solution, loading the sample onto the column, washing to eliminate unattached molecules, eluting the bound ions by adjusting the pH or ionic strength of the buffer, and so rejuvenating the column for next use.
- In many different disciplines, including water purification, protein and nucleic acid separation in biochemistry, and food and pharmaceutical analysis, ion-exchange chromatography finds application. It is a useful instrument for separating molecules depending on charge as it helps to purify and examine difficult combinations.
4. Gel- permeation (molecular sieve) chromatography
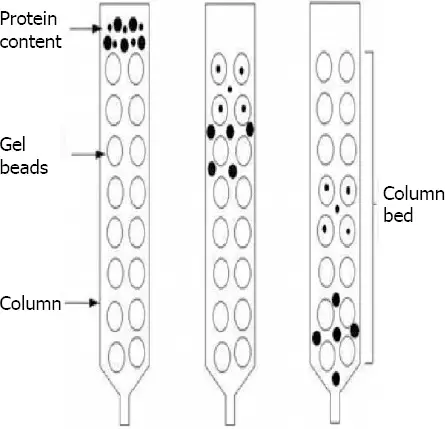
- Molecular sieve chromatography, often referred to as gel permeation chromatography, is a form of size-exclusion chromatography whereby molecules are separated in solution according to their size. This method uses a suitable solvent as the mobile phase while a porous gel substance packed in a column forms the stationary phase. Larger molecules cannot access the tiny pores of the gel when a mixture is added into the column, so elute from the column more rapidly. Smaller molecules elute later on because they pass through the pores and follow a longer trip. Analysis of molecular weight distribution inside a sample is made possible by this size-based separation.
- GPC’s basis is the differential exclusion of molecules by the porous stationary phase. Shorter retention durations follow from larger molecules’ less interacting passage in the column excluding from the pores. Smaller molecules pass through the column more broadly and enter the pores, which increases retention durations. Based on molecule size, this separation mechanism—which is essentially physical—does not entail chemical interactions between the analytes and the stationary phase.
- GPC starts by dissolving the sample in a suitable solvent and then injecting it into the chromatographic apparatus. The material goes across a column with a porous gel where molecular size causes separation. Refractive index or UV detectors among other detectors track component elution from the column to generate sample molecular weight distribution data.
- In polymer research, GPC is extensively applied to estimate the molecular weight distribution of polymers, therefore facilitating the knowledge of their physical properties and performance qualities. In biochemistry and biotechnology, it also finds use in the study of proteins and other macromolecules. Furthermore, GPC is a useful method for size-based component separation, so purifying difficult mixes.
5. Paper chromatography
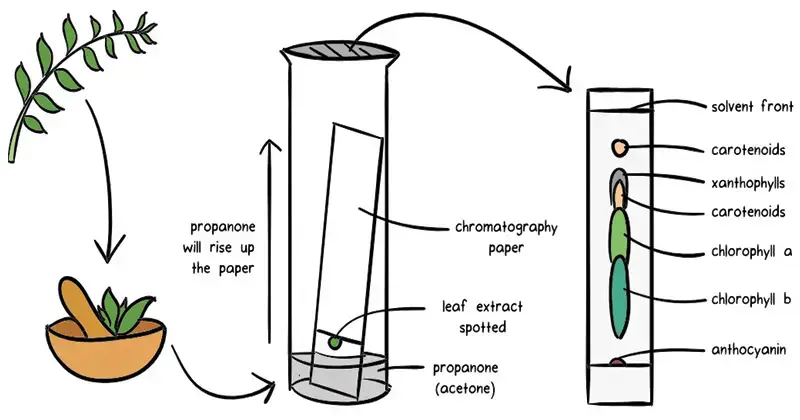
- A basic and reasonably priced analytical method for separating and identifying components within a mixture is paper chromatography. Under this technique, a solvent functions as the mobile phase and a specific paper acts as the stationary phase. Partition chromatography—where compounds spread themselves between the stationary phase (water molecules caught in the paper fibers) and the mobile phase—the solvent—forms the basis for the concept. various components travel at various speeds based on their solubility and affinity for the stationary phase when a sample is laid on the paper and the solvent travels through it by capillary action, thereby separating them.
- The technique consists on marking a tiny sample place close to one side of a filter paper strip. Then, within a sealed container, this edge is submerged in a solvent. Capillary action causes the solvent to climb the paper carrying the components of the mixture. Different solubility and contact with the paper cause the components to separate and create different heights’ worth of spots. Often utilizing staining or UV light, the paper is removed, dried, and the separated components are observed once the solvent front has progressed a sufficient distance.
- Among several disciplines, including chemistry, biology, and forensics, paper chromatography finds great application. It’s used to examine difficult combinations, pinpoint unidentified molecules, and track chemical processes’ development. It’s used, for example, to separate and identify amino acids, nucleotides, and sugars as well as to find toxins in food and drinks. Its low cost and simplicity make it a useful instrument for both pragmatic needs and teaching ones.
6. Thin-layer chromatography
- A basic, reasonably priced method for separating and examining mixtures is thin-layer chromatography (TLC). Under this approach, a tiny sample spot is placed close to the bottom of a plate covered in a thin layer of an adsorbent substance such as silica gel or alumina. The plate is then put in a closed container filled with a solvent that capillary action flows higher.
- Different chemicals in the mixture separate depending on their varied affinities for the stationary phase (the adsorbent) and the mobile phase (the solvent) as the solvent moves along the plate. Based on the partitioning principle, this procedure produces clearly visible patches usually seen under UV light or with a chemical stain. In laboratories, TLC is extensively used for preliminary separations in chemical and pharmaceutical research, compound purity checks, and reaction progress monitoring.
7. Affinity chromatography
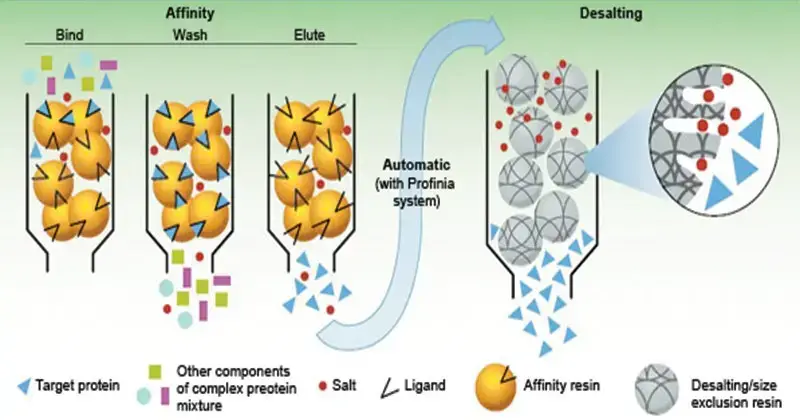
- Affinity chromatography is a technique for separating individual compounds from complicated mixtures by utilizing the target molecule’s unique interactions with a specific ligand. This approach is predicated on the idea that, like to a lock-and-key mechanism, some biomolecules naturally attract particular binding partners. Enzymes could, for instance, attach to substrates, antibodies to antigens, or receptors to ligands.
- Actually, the process entails packing a ligand onto a solid support—such as a resin or gel matrix—packed into a chromatography column immobilizing it. The mixture including the target molecule passes through the column therefore enabling the target to bind especially to the immobilized ligand. After washing away unbound contaminants, conditions—such as pH, ionic strength, or introducing a competing molecule—that disturb the interaction between the target and the ligand elute the target molecule.
- In biochemistry and molecular biology, affinity chromatography is extensively applied for protein, nucleic acid, and other biomolecule purification. Its great efficiency and sensitivity make it perfect for separating a specific molecule from a complicated mixture, therefore enabling research on the structure and behavior of the pure molecules.
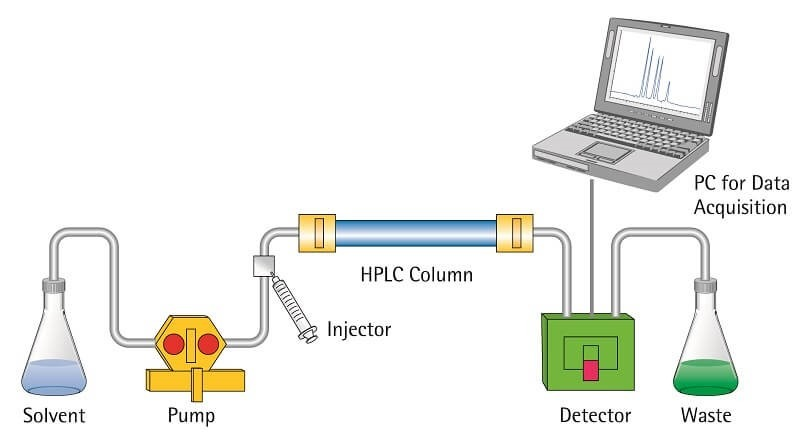
8. High-prssure liquid chromatography (HPLC)
- In analytical chemistry, high-performance liquid chromatography (HPLC) is a method for separating, identifying, and measuring components in a mixture. It works on the idea that various molecules in a sample would interact specifically with a stationary phase—a solid substance inside a column—and a mobile phase—a liquid solvent—running across it. These interactions separate each molecule by moving them through the column at distinct speeds.
- A liquid sample is actually introduced into the HPLC system and run over a column filled with the stationary phase under the mobile phase. Components of the sample segregate depending on their interactions with the stationary and mobile phases as it passes the column under high pressure. Detectors at the column’s exit track the separated substances to give information for identification and quantification.
- Because of its accuracy and efficiency in assessing complicated mixtures, HPLC is extensively applied in many disciplines including medicines, environmental monitoring, and food safety.
9. Hydrophobic interaction chromatography (HIC)
- Separating and purifying proteins depending on their hydrophobic character is accomplished using hydrophobic interaction chromatography (HIC). The idea underlying HIC is the interaction of hydrophobic areas of proteins with hydrophobic groups bonded to a stationary phase in a chromatography column.
- These hydrophobic interactions are strengthened under high-salt circumstances, which helps proteins to attach to the column material. HIC uses a buffer with a high salt content to apply a sample to the column thereby encouraging the binding of proteins with exposed hydrophobic regions to the hydrophobic ligands on the stationary phase.
- Proteues elute from the column in increasing hydrophobicity as the salt concentration is progressively lowered. Because this approach runs under somewhat moderate circumstances compared to other chromatographic methods, it is especially helpful for purifying proteins while preserving their biological function.
10. Dye- ligand chromatography
- Dye-ligand chromatography is a chromatographic method based on the binding affinity between some proteins or enzymes and particular dye molecules. The realization that several enzymes may bind purine nucleotides, including Cibacron Blue F3GA dye, motivated the creation of this method.
- Planar ring structure with negatively charged groups in Cibacron Blue F3GA dye mimics the arrangement of NAD (nicotinamide adenine dinucleotide). Acting as an analog of ADP-ribose, it has been observed that the dye may attach to the adenine and ribose binding sites of NAD. About 10–20 times stronger than other adsorbents used in affinity chromatography, this binding capacity of dye-ligand adsorbents is rather remarkable.
- Typically a solid support, the stationary phase in dye-ligand chromatography is an adsorbent material functionalized with the particular dye ligand. The dye ligand binds and retains the target enzyme or protein specifically on the adsorbent material within the chromatography column.
- High-ionic strength solutions elute the bound proteins from the column under suitable pH conditions. This makes use of the adsorbent material’s ion-exchange characteristics. The binding relationships between the dye ligand and the target proteins can be upset by changing the pH and ionic strength, therefore releasing them from the column.
- Protein purification benefits from various aspects provided by dye-ligand chromatography. Effective capture and purification are made possible by the great binding affinity between the target proteins and the dye ligand. While other toxins are eliminated, the selectivity of the dye ligand guarantees the particular separation of the intended proteins. Furthermore a flexible method in many biochemical and biotechnological uses is dye-ligand chromatography, which may be used on a broad spectrum of proteins, enzymes, and nucleic acids.
- Based on their particular binding interactions with dye ligands, dye-ligand chromatography offers generally a strong and efficient way for the purification of proteins and other macromolecules. This method finds uses in many scientific and industrial environments and has greatly helped the area of protein purification.
11. Pseudoaffinity chromatography
- Using chemicals with high affinity for particular enzymes or proteins, pseudoaffinity chromatographic methods selectively capture and purify target molecules. Often employed in protein purification, immobilized metal affinity chromatography (IMAC) is one kind of pseudoaffinity chromatography.
- In pseudoaffinity chromatography, certain ligands are immobilized onto a solid support—such as beads or resin—and show affinity for particular enzymes or proteins. Usually employed as the immobilized ligands in IMAC are metal ions including nickel, cobalt, or zinc.
- Specific interactions with the target enzymes or proteins define the affinity of the ligands in pseudoaffinity chromatography. For instance, azo-dyes and anthraquinone dyes are known to be affine for enzymes including reductases, kinases, transferases, and dehydrogenases.
- The sample with the combination of molecules is run on the pseudoaffinity column during the chromatographic procedure. While non-target molecules pass through the column, the target enzymes or proteins having affinity for the immobilized ligands bind to the ligands only.
- We use certain elution conditions to eliminate the bound target molecules. These disorders can affect pH, ionic strength, or add competing ligands to upset the affinity between the target molecules and the immobilized ligands.
- For the separation and purification of highly selective and specific enzymes or proteins, pseudoaffinity chromatography offers a strong tool. Based on their affinity to certain ligands immobilized on the solid support, it enables the isolation of target molecules from complicated mixtures.
- Using metal ions as the immobilized ligands, the immobilized metal affinity chromatography (IMAC) variation of pseudoaffinity chromatography has become somewhat well-known in protein purification. Many proteins include histidine, a residue of certain amino acids that metal ions as nickel, cobalt, or zinc have great affinity for. Commonly utilized in recombinant protein production and purification systems, this helps the histidine-tagged proteins to be selectively bound and purified.
- Pseudoaffinity chromatography—including methods like immobilized metal affinity chromatography (IMAC)—uses particular ligands with great affinity for target enzymes or proteins to attain selective capture and purification in general. Pseudoaffinity chromatography is a useful instrument for protein purification and separation in many research and biotechnological uses by using the particular interactions between the ligands and the target molecules.
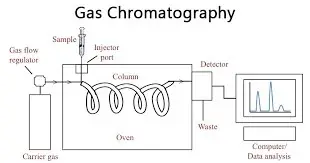
12. Gas chromatography
- Operating on the “gas-liquid” chromatographic concept, gas chromatography is a quite extensively used chromatographic method. This technique uses a column as the stationary phase, which is placed into the instrument and features deposited liquid stationary phase onto the surface of an inert solid support. Comprising gases like helium (He) or nitrogen (N2), the mobile phase—often referred to as the carrier gas—is
- Gas chromatography is the technique whereby the mobile phase—an inert gas—is passed under high pressure over the column. First evaporated, then injected into the gaseous mobile phase is the material to be examined. On the solid support, the elements of the sample spread out within the column between the mobile phase and the stationary phase.
- Among the various benefits of gas chromatography are simplicity, adaptability, great sensitivity, and rapid analysis times. Even in trace levels, it is very appropriate for the separation and study of tiny compounds. From chemistry to pharmaceuticals to environmental analysis to forensic sciences, the method is extensively used in many different scientific disciplines.
- Excellent separation capacity of gas chromatography makes it possible to detect and count individual components in complicated combinations. Differentiation in the affinity of the sample components for the stationary phase and their volatility helps to achieve the separation. Whereas those with weaker contacts elute faster, components with stronger interactions with the stationary phase will spend more time in the column and elute later.
- Depending on the particular analytical needs, the separated components are found by a detector—thermal conductivity detector (TCD), flame ionization detector (FID), or another kind of detector. The detector generates a recorded and examined signal that creates a chromatogram illustrating the component distribution in the sample.
- One very effective method that enables exact quantitative analysis and component identification inside a mixture is gas chromatography. Many different businesses utilize it routinely for quality control, research, and regular analysis. In analytical chemistry, gas chromatography is a useful instrument due of its adaptability and sensitivity.
Commonly employed chromatography techniques include
| Technique | Stationary phase | Mobile phase | The basis of separation | Notes |
|---|---|---|---|---|
| *Paper Chromatography | solid (cellulose) | liquid | Polarity of molecules | compound directly on cellulose paper |
| *Thin Layer Chromatography (TLC) | Solid (silica or Alumina) | liquid | Polarity of molecules | glass is coated with a thin layer of silica. On it is seen the chemical |
| *Liquid column chromatography | Solid (silica or Alumina) | liquid | Polarity of molecules | Glass column is filled with silica-slurry |
| Size exclusion chromatography | Solid (microporous pieces of silica) | liquid | the size of molecules | small molecules get stuck in tiny pores within the stationary phase while larger molecules move through the spaces between the beads and have very short retention time. Thus, larger molecules emerge first. In this form of chromatography there isn’t any interplay whether chemical or physical, between the analyte and stationary phase. |
| Ion-exchange chromatography | (cationic or anionic resin) (cationic or anionic resin) | liquid | The charge of the ions in molecules | molecules with the same charge to resins will be able to bind strongly to the resin. molecules with similar charges to the resin will move through the column, and then elute out first. |
| Affinity Chromatography | Solid (agarose as well as porous glass beads which immobilized molecules are like antibodies and enzymes) | liquid | the binding affinity of the chemical analyte to the molecule that is immobilized on the stationary phase | If the molecule acts as an enzyme’s substrate that it binds to the enzyme, and the non-bound analytes will move through the mobile phase and then elute out of the column leaving the substrate attached to the enzyme. It is then removed of the stationary part and then eluted from the column using the appropriate solvent. |
| Gas chromatography | Solid or liquid support | gas (inert gas such as Helium or argon) | the boiling point of molecules | The samples are dissolved and the sample with the lowest boiling point is taken out first. The molecule that has the highest boiling point exits the column last. |
Applications of Chromatography
- Medicinal Industry: Drug purification, or the separation of intended molecules from contaminants, Quality control—that is, trace contamination detection and quantification—in
- Environmental study: Pollutant detection—that is, chemical and toxic identification of samples
Monitoring air and water quality—that is, environmental sample analysis—hereby - Food Industry: Additive and contaminant analysis—that is, search for undesired compounds—by means of Toxin detection—identification of toxic chemicals such as aflatoxins—involutions
- Molecular Biology: Purification of proteins and enzymes with HPLC-based methods
Research on nucleic acids, or DNA, RNA, and associated molecules: - Chemical Industry: Purity evaluation, or determining the chemical products’ degree of purity,
Process monitoring—ensuring ideal manufacturing conditions. - Forensics Science: Drug, explosive, and other compound identification—analysis
Ink analysis—that is, study of document ink compositions. - In biotechnology: Bioprocessing, or product purification including monoclonal antibodies and vaccines, Metabolic profile analysis, the study of metabolic profiles in biological specimens,
Advantages of Chromatography
- Elevated Separation Efficiency: Capable of isolating intricate combinations into distinct constituents.
- Versatility: Compatible with many substances (gases, liquids, solids).
- Elevated Sensitivity: Identifies and measures even minimal quantities of substances.
- Reproducibility: Ensures consistent and dependable outcomes.
- Adaptability: Capable of being customized for various analytical and preparative requirements.
- Coupling Capability: Seamlessly integrable with additional detectors (e.g., mass spectrometry) for improved analysis.
- Scalability: Appropriate for both small-scale analytical and large-scale preparative separations.
Limitations of Chromatography
- High Cost: Requires expensive instruments, maintenance, and consumables.
- Time-Consuming: Method development and analysis can take a long time.
- Complex Sample Preparation: Often needs careful and sometimes complicated preparation steps.
- Limited Resolution: May struggle to separate compounds with very similar properties.
- Solvent Use: Uses chemicals that can be hazardous and require proper disposal.
- Equipment Sensitivity: Instruments require careful handling, calibration, and regular maintenance.
- Skilled Operation: Requires trained personnel to set up and interpret results.
FAQ
What is chromatography?
Chromatography is a technique used for separating and analyzing complex mixtures into their individual components. It involves the separation of substances based on their different affinities to a stationary phase and a mobile phase.
What are the different types of chromatography?
There are several types of chromatography, including gas chromatography (GC), high-performance liquid chromatography (HPLC), thin-layer chromatography (TLC), ion-exchange chromatography, affinity chromatography, and more.
What is the principle behind chromatography?
Chromatography relies on the differential interactions of the components in a mixture with the stationary phase and the mobile phase. Components that have stronger interactions with the stationary phase will elute more slowly, resulting in separation.
What are the applications of chromatography?
Chromatography has a wide range of applications, including pharmaceutical analysis, environmental monitoring, food analysis, forensic investigations, biochemistry research, quality control in various industries, and more.
What is the difference between HPLC and GC?
HPLC is a liquid chromatography technique that uses a liquid mobile phase, while GC is a gas chromatography technique that uses a gas mobile phase. HPLC is typically used for analyzing compounds that are soluble in liquid, while GC is used for volatile and gaseous compounds.
How does chromatography separate components in a mixture?
Chromatography separates components based on their distribution between a stationary phase (solid or liquid) and a mobile phase (liquid or gas). The components that interact more with the stationary phase will have a slower migration and elute later, resulting in separation.
What is the role of the stationary phase in chromatography?
The stationary phase provides a surface for the components in the mixture to interact with. It can be a solid material (such as silica gel or a resin) or a liquid immobilized on a solid support.
What is the role of the mobile phase in chromatography?
The mobile phase carries the sample through the stationary phase. It can be a liquid or a gas and is chosen based on the type of chromatography and the properties of the components being separated.
How is the separated mixture detected in chromatography?
Various detection techniques are used in chromatography, including UV-Vis spectroscopy, fluorescence, mass spectrometry, refractive index measurement, and electrochemical detection. The choice of detection method depends on the nature of the analytes and the specific chromatographic technique.
How can I optimize chromatography separations?
Optimizing chromatography separations involves adjusting parameters such as the choice of stationary and mobile phases, column dimensions, flow rates, temperature, and detection conditions. Method development and optimization can be achieved through experimental exploration and systematic adjustments to achieve the desired separation goals.
- https://thesciencenotes.com/chromatography-definition-principle-types-application/
- https://chem.libretexts.org/Bookshelves/Analytical_Chemistry/Instrumental_Analysis_%28LibreTexts%29/26%3A_Introduction_to_Chromatographic_Separations/26.06%3A_Applications_of_Chromatography
- https://www.geeksforgeeks.org/applications-of-chromatography/
- https://byjus.com/chemistry/applications-of-chromatography/
- https://www.peakscientific.com/discover/news/5-everyday-uses-for-chromatography/
- https://studyrocket.co.uk/revision/level-3-applied-science-btec/practical-scientific-procedures-and-techniques/application-of-chromatography
- https://www.vedantu.com/chemistry/applications-of-chromatography
- https://www.geeksforgeeks.org/chromatography/
- https://chromtech.com/applications-of-chromatography/
- https://scienceinfo.com/chromatography-principles-types-applications/
- https://www.bio-rad.com/en-us/applications-technologies/introduction-hydrophobic-interaction-chromatography
- https://www.bio-works.com/blog/hydrophobic-interaction-chromatography-hic-principles
- https://thesciencenotes.com/high-performance-liquid-chromatography-principle-instruments-applications/
- https://microbeonline.com/hplc-high-performance-liquid-chromatography/
- https://laboratoryinfo.com/hplc/
- https://www.pharmaacademias.com/affinity-chromatography-introduction-theory-types-instrumentation-and-applications/
- https://acmeresearchlabs.in/2024/02/12/paper-chromatography-procedure-principle-applications/
- https://www.pharmaacademias.com/ion-exchange-chromatography-iec-introduction-principle-classification-mechanism-factor-affecting-and-applications/
- https://www.universallab.org/blog/blog/understanding_gel_permeation_chromatography/
- https://www.studyread.com/gel-permeation-chromatography/
- https://www.chemistrylearner.com/chromatography/partition-chromatography
- https://lab-training.com/partition-chromatography/
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.