What is Centrifuge?
- A centrifuge is a device spinning materials at high speeds using centrifugal force to separate them depending on variations in their densities.
- It works by generating a force pushing heavier particles away that lets lighter particles stay nearer the center.
- Labs, businesses, and therapeutic settings all employ centrifuges to separate elements like cells from liquids or solids from mixes.
- Laboratory centrifuges, gas centrifuges, and ultracentrifuges—each intended for particular uses—are among the several forms of centrifuges available.
- In biological study, they are crucial for separating organelles, proteins, DNA, and other cellular constituents.
- Centrifuges are applied in both industrial and clinical environments for operations like dairy processing, blood separation, wastewater treatment, and even uranium enrichment.
- Usually consisting of a motor-driven rotor holding sample containers, the device generates a force several times greater than gravity.
- Preventing mechanical breakdowns and guaranteeing safe operation during high-speed rotation depend critically on proper balance and safety precautions.
Definition of Centrifuge
A centrifuge is a laboratory instrument that uses spinning and centrifugal force to separate mixtures based on their density.
Who first invented the Centrifuge?
- Early 1700s English military engineer Benjamin Robins created a whirling arm device using centrifugal force, therefore establishing the conceptual basis for centrifugation.
- Antonin Prandtl first put the centrifuge in use practically in 1864 when he suggested a dairy centrifuge to extract cream from milk.
- Later on, Antonin’s brother Alexander Prandtl perfected this design, raising its practical value and efficiency.
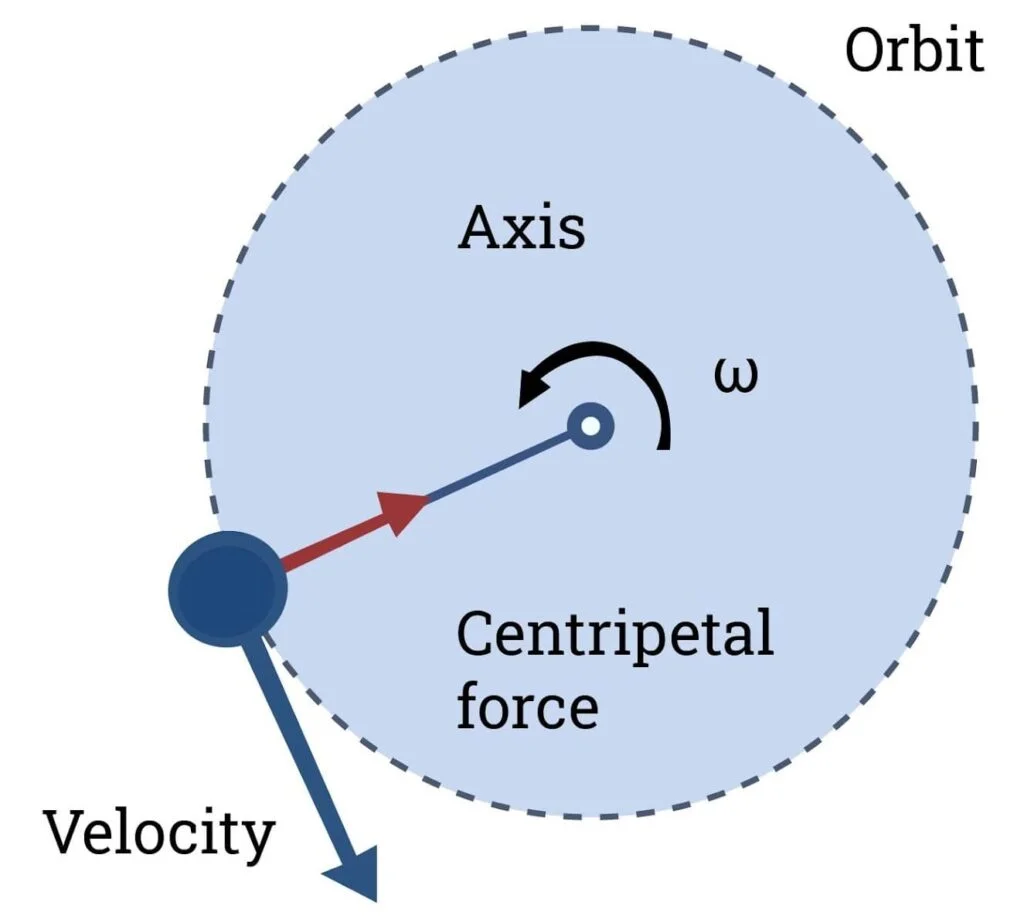
Principle of a Centrifuge
- A centrifuge generates centrifugal force from fast spinning a sample that pushes particles away from the center of rotation.
- While smaller components remain closer to the center as the supernatant, the heavier or denser particles suffer a larger force and go toward the outer edge to form a pellet.
- This method speeds up the natural sedimentation under gravity so that components may be effectively separated depending on density, size, and form.
- Factors include the speed of the rotor (RPM), its radius, and variations in density and viscosity of the components in the sample affect the separation efficiency.
- Relative centrifugal force (RCF), which measures how many times more the force is compared to Earth’s gravitational attraction, frequently defines the efficiency of the centrifuge.
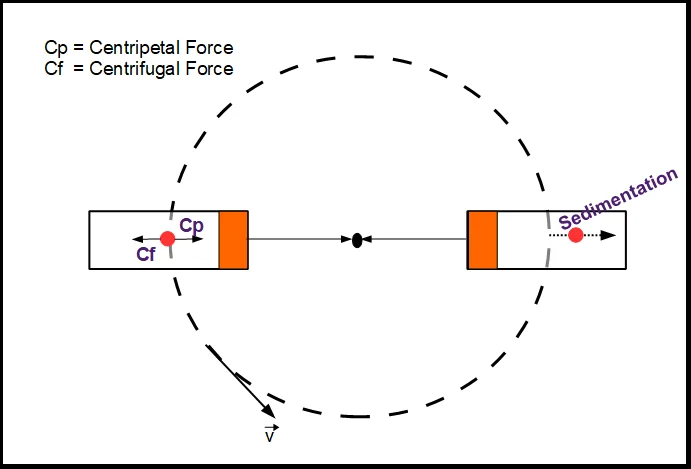
Sedimentation coefficient
- When a particle experiences an external force like gravity or centrifugal force, the sedimentation coefficient gauges how quickly it settles in a fluid.
- It is stated as the particle’s sedimentation speed relative to the applied acceleration.
- Its units are Svedberg units, whereby one Svedberg corresponds to 10^-13 seconds.
- Particle mass, shape, and density as well as fluid viscosity determine the value of the sedimentation coefficient.
- In biochemistry, macromolecules including proteins and ribosomes are often described using it.
Factors Affecting Sedimentation coefficient
- Mass – A major determinant is the mass of the particle; larger particles often sink quicker, increasing the sedimentation coefficient.
- Shape – Particle frictional resistance is affected by its shape and conformation; so, compact or spherical particles single more quickly than elongated or irregular ones.
- Viscosity – The viscosity of the surrounding fluid is quite important; increased viscosity reduces the sedimentation coefficient and slows down sedimentation.
- Density– The effective mass causing sedimentation is found by the density differential between the particle and the fluid—that is, the buoyancy factor.
- Temperature– Temperature changes the viscosity of the fluid, therefore indirectly influencing sedimentation.
- Volume– Reflecting their internal hydration and density, the partial specific volume of the particle influences its effective sedimentation behavior.
- Concentration– Under non-ideal circumstances, concentration effects and particle aggregation can change the computed sedimentation coefficient.
Parts of a Centrifuge
The main parts of a centrifuge include:
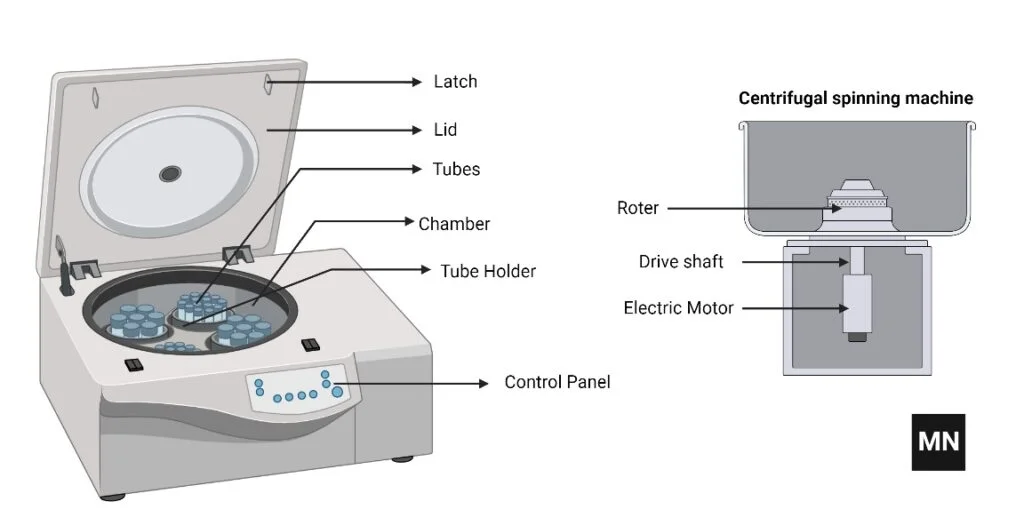
A centrifuge is a tool used in laboratories to spin components of a mixture at high speeds, therefore separating them according to density. A centrifuge consists mostly in:
- Rotor: The rotor whirls the sample tubes around a central axis. Each of the several rotors—fixed-angle and swing-out rotors—is intended for a particular use.
- Motor: The motor powers the rotation of the rotor, therefore supplying the required speed to produce centrifugal force.
- Chamber: The contained area the rotor revolves in. Sensitive samples are protected in refrigerated centrifuges by temperature control of the chamber.
- Lid: Usually including a locking mechanism to prevent opening while the rotor is rotating, the lid covers the chamber to provide safety during operation.
- Control Panel: Users of a control panel can select settings like temperature, time, and speed (RPM). Advanced variants could have programmable settings and digital displays.
- Spindle: Connects the rotor to the motor thereby transferring the rotational force required for centrifugation.
- Imbalance Detection System: Monitors the weight distribution within the rotor to prevent unequal loads, therefore preventing damage or dangerous operating circumstances.
- Safety Features: Modern centrifuges have several safety elements like reinforced enclosures to guard users, automated rotor identification, and emergency stop systems.

Centrifuge Safety Features
There are several safety dangers associated with these gadgets, however they are typically fitted with safety mechanisms to protect people and the environment. Several characteristics include the following:
- Electric Lid Lock : This function prevents the lid from accidently opening while the centrifuge is in operation.
- Imbalance Sensor : The Imbalance Sensor detects when the centrifuge is not properly balanced and puts the centrifuge on pause if the vibration level increases.
- Sealed Rotors : These prevent biohazardous chemicals or substances from escaping or leaking throughout the process.
- Rotor Recognition Technology : The Rotor Recognition Technology determines which rotor is mounted and prevents the rotor from exceeding the limit operating speed.
Types of Centrifuge Rotors
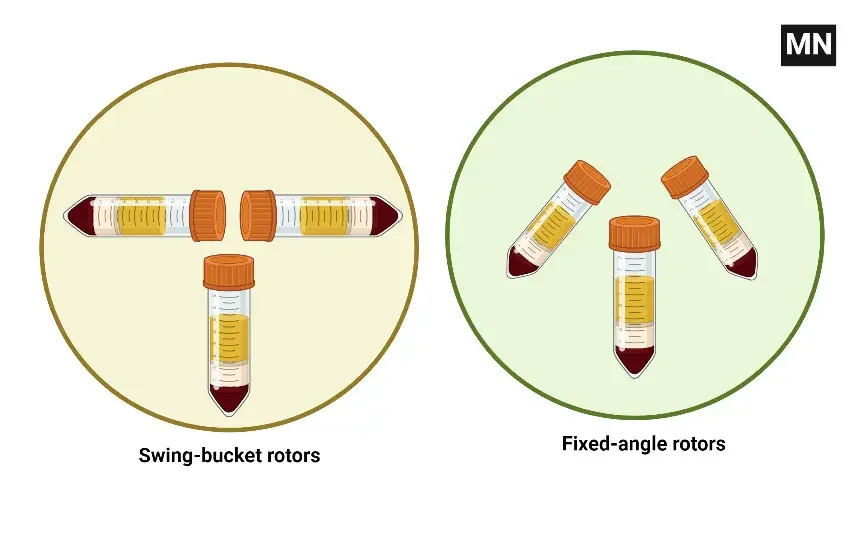
Essential parts that hold and spin samples at high speeds to separate molecules depending on density are centrifuges rotors. The particular application and desired separation result determine the type of rotor to be used. Centrifuges rotors mostly come in two forms:
- Fixed-Angle Rotors: Usually ranging between 25° and 45° relative to the vertical axis, fixed-angle rotors retain sample tubes at a constant angle. Because particles move a limited distance before pelleting against the side and bottom of the tube, they are perfect for pelleting particles from a suspension, say cells or subcellular components.
- Swinging-Bucket Rotors: Known also as horizontal rotors, swinging-bucket rotors feature buckets that start in a vertical position and swing out to a horizontal position during centrifugation. Applications like density gradient separations and isopycnic separations find this design appropriate as it lets samples encounter a homogenous centrifugal force.
- Vertical Rotors: Tubes maintained parallel to the axis of rotation in vertical rotors. Mostly employed for isopycnic separations—where particles are segregated according to buoyant density—they are Though they might not be appropriate for pelleting uses, the small pathlength in these rotors enables very fast separations.
- Continuous Flow Rotors: Designed for large-scale separations, constant flow rotors let samples be continuously added and removed during centrifugation. They are frequently utilized in industrial settings or while processing vast numbers of cell cultures.
Types of centrifugation techniques
Centrifugation is a technique used to separate substances based on their density. There are several different types of centrifugation techniques, including:
1. Sedimentation centrifugation
- Sedimentation centrifugation is a method of separation whereby the natural settling (sedimentation) of particles in a liquid is accelerated using centrifugal force.
- While lighter components remain in the supernatant, heavier and denser particles are quickly driven forth swiftly to create a pellet at the bottom of the tube when the sample is spun at high speeds.
- The particle size, shape, density, viscosity of the medium, and rotor speed—or relative centrifugal force—all affect the effectiveness of the separation.
- From cell fractionation, protein purification, and food processing to other scientific studies and commercial activities, this approach is extensively applied to rapidly and successfully separate components from complicated mixtures.
2. Density gradient centrifugation
- A density gradient centrifugation is a method of particle separation based on buoyant density by use of a continuous gradient of rising density.
- Usually formed using liquids like sucrose or cesium chloride, which build layers with increasingly greater density within the centrifuge tube, the gradient is generated.
- Particles migrate until they reach an area where the density of the surrounding medium equals their own, sometimes known as the isopycnic point, when the sample is carefully stacked on top of the gradient and subjected to high-speed centrifugation.
- Particles create separate bands at this equilibrium point that may be separated and enable the individual component separation from complicated mixes.
- In molecular biology, biochemistry, and cell biology this approach is extensively applied for very precise nucleic acid, protein, organelles, and virus purification.
- Two basic techniques exist: rate-zonal centrifugation, in which separation is based on the sedimentation rate prior to equilibrium; isopycnic centrifugation, in which separation is based just on density.
3. Rate-zonal centrifugation
- A prepared density gradient is used in rate-zonal centrifugation to segregate particles according to sedimentation speed.
- This technique centers the sample gently on top of the gradient—often derived from sucrose or Ficoll—then centrifuged for a specified, restricted duration.
- Particles move over the gradient at rates dictated by their size, shape, and mass while centrifugation runs, producing separate bands or zones.
- The process is stopped before the particles find their equilibrium (isopycnic) density, therefore they separate just based on their sedimentation rate.
- In molecular biology and biochemistry, this method is widely used to separate and purify organelles, viruses, and macromolecules with perhaps identical buoyant densities but different mass or size.
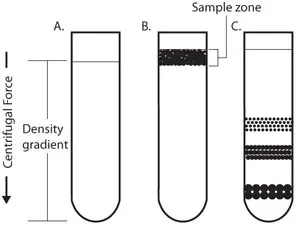
4. Continuous flow centrifugation
- Unlike discrete batch processing, continuous flow centrifugation is a technique whereby the sample is continually supplied into the centrifuge and treated as it passes around the rotor.
- Compared to conventional batch centrifugation techniques, this technology greatly lowers handling time and boosts throughput by allowing vast quantities of material to be handled fast.
- Usually by guiding the denser particles to one outlet (forming a pellet) and the lighter material to another exit (the supernatant), the rotor of a continuous flow system is made to constantly separate components.
- Applications like blood in apheresis, cell component separation in bioprocessing, and high volume industrial or biological material purification make constant flow centrifugation extensively employed in many fields.
- For high-throughput operations, the main benefits include scalability, effective handling of big sample quantities, and less downtime as the rotor does not have to stop and resume often.
5. Precipitation centrifugation
- Precipitation centrifugation is a method for rapidly separating an insoluble solid (precipitate) from its liquid surroundings by use of centrifugation.
- This approach uses a chemical process to lower the solubility of a substance by varying parameters such pH, ionic strength, or temperature, therefore precipitating out of the solution.
- The liquid is centrifuged once the precipitate occurs, where the great centrifugal force quickly settles the solid particles into a compact pellet at the bottom of the tube.
- Carefully decanted or discarded, the residual liquid—known as the supernatant—leaves behind the pure precipitate for additional processing or study.
- Laboratories and businesses extensively apply this method to improve the speed and efficiency of chemical separation and purification following a precipitation reaction.
6. Isopycnic centrifugation
- Separating particles just based on their density is done by isopycnic centrifugation, often referred to as buoyant density or equilibrium density-gradient centrifugation.
- Usually utilizing cesium chloride, sucrose, or Percoll, this method spins a sample in a media that creates a continuous density gradient and each particle migrates to the point in the gradient where its density matches that of the surrounding medium (its isopycnetic point).
- Isopycnic centrifugation lets particles be separated with great resolution, even if they are comparable in size, as long as they vary in density, unlike other centrifugation techniques depending on variations in size or form.
- This method is extensively applied in molecular biology for purification of nucleic acids, proteins, and organelles; it was especially used in the Meselson-Stahl experiment to show the semi-conservative DNA replication.
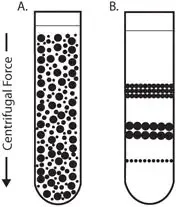
What is Differential centrifugation?
- Differential centrifugation is a technique for separating components in a homogenized sample based on size and density variations by exposing the sample to a series of centrifugation stages with varying speeds.
- While smaller components stay suspended in the supernatant, in the first spin the largest and most dense particles—such as nuclei—sink rapidly to form a pellet.
- After that, the supernatant is pipled to a fresh tube and centrifuged faster to pellet smaller organelles like lysosomes, mitochondria, and other subcellular particles.
- By use of this progressive separation, scientists can fractionate cells into separate components for additional biochemical or molecular investigation.
Types of Centrifuges
Essential tools for separating mixture components depending on size, density, and rotor speed are centrifuges in laboratories. Different kinds of centrifuges are made to fulfill particular uses and criteria:
- Low-Speed Centrifuges – Appropriate for separating complete cells or big organelles, low-speed centrifuges run at speeds ranging from 5,000 RPM.
- High-Speed Centrifuges – Perfect for pelleting cellular trash, bacteria, or yeast cells, high-speed centrifuges may run up to 25,000 RPM.
- Ultracentrifuges – Separating tiny particles like ribosomes, proteins, and viruses, ultracentrifuges allow speeds of 100,000 RPM.
- Microcentrifuges – Commonly found in molecular biology labs for DNA or RNA precipitation, microcentrifuges are made for tiny sample quantities.
- Refrigerated Centrifuges – Refrigerated Centrifuges: Designed with cooling systems to preserve low temperatures—necessary for temperature-sensitive materials.
- Benchtop Centrifuges – Compact and flexible, benchtop centrifuges fit for ordinary laboratory work with modest sample quantities.
- Continuous Flow Centrifuges – Perfect for large-scale separations in industrial use, continuous flow centrifuges let samples be added and removed constantly.
- Gas Centrifuges – Particularly utilized for the separation of isotopes of gases, like as uranium enrichment procedures are gas centrifuges.
- Hematocrit Centrifuges – Designed specifically to find the volume proportion of red blood cells in blood samples, hemocrit centrifuges
- Vacuum Centrifuges/Concentrators – Use vacuum to evaporate solvents, therefore concentrating samples without heat damage.
| Type of Centrifuge | Description | Common Applications | Advantages | Limitations |
|---|---|---|---|---|
| Benchtop Centrifuges | Compact, portable units designed for small-scale separations, typically used on laboratory benches. | Routine lab separations, cell and particle pelleting, DNA/RNA extractions. | Compact and cost-effective, suitable for small samples. | Limited capacity and speed, less versatile for larger or more complex separations. |
| Floor-Standing Centrifuges | Larger units placed on the floor, suitable for higher-throughput applications and larger samples. | Blood component separations, protein purification, industrial processing. | Higher capacity and speed, more rotor options. | Larger footprint, higher cost, and more complex operation. |
| High-Speed Centrifuges | Designed for very high speeds, facilitating efficient separations of denser samples. | Subcellular fractionation, protein isolation, high-resolution separations. | High separation efficiency, capable of handling dense samples. | High cost, more complex maintenance, and specific safety precautions required. |
| Refrigerated Centrifuges | Equipped with cooling systems to maintain temperature during operation, ideal for temperature-sensitive samples. | Enzyme and cell separations, sedimentation of fine particles, molecular biology applications. | Temperature control preserves sample integrity, increased separation efficiency for certain samples. | Higher cost due to refrigeration system, larger size, and energy consumption. |
| Microcentrifuges | Small, high-speed units for microvolume samples, commonly used in molecular biology and biochemistry labs. | DNA/RNA isolation, pelleting of small particles, enzyme assays. | Compact and fast, designed for small volumes. | Limited capacity, not suitable for large-scale separations. |
| Industrial Centrifuges | Heavy-duty centrifuges designed for large-scale and continuous processing in industrial settings. | Wastewater treatment, oil and gas, food and beverage production. | High throughput, robust design for demanding applications. | High initial and operating costs, requires skilled operators, large footprint. |
| Haematocrit Centrifuges | Specialized for measuring the ratio of the volume of red blood cells to the total volume of blood. | Blood analysis, medical diagnostics, research on blood disorders. | Precise measurement of haematocrit levels. | Limited to blood sample analysis, requires careful handling of capillary tubes. |
| Low-Speed Centrifuges | Operate at lower speeds, suitable for gentle separations where high centrifugal force might damage the sample. | Cell culture, sedimentation of bulky particles, some bioseparations. | Gentle on samples, reducing potential for damage. | Limited separation capabilities for particles with small size or density differences. |
| Continuous Flow Centrifuges | Allow continuous introduction and separation of material without stopping the machine, ideal for large volumes. | Industrial processing, chemical manufacturing, high-throughput bioseparations. | High processing capacity, efficient for large-scale operations. | Complex setup and operation, higher maintenance requirements, may not be suitable for all types of samples. |
| Ultracentrifuges | Ultra-high-speed centrifuges for separating molecules of very small size and high density. | Molecular biology research, virology, protein structure analysis. | Very high resolution and separation efficiency. | Expensive, requires specialized training to operate, potential risk with ultra-high speeds. |
| Basket Centrifuges | Utilize a spinning basket to separate solid from liquids, often used in the pharmaceutical and chemical industries. | Solid-liquid separations, sludge dewatering, crystal harvesting. | Effective for solid-liquid separations, can handle a range of particle sizes. | Periodic cleaning required, not continuous flow, may require manual unloading. |
| Decanter Centrifuges | Feature a rotating drum and are used for the continuous separation of solids from liquids in suspension. | Wastewater treatment, sludge processing, food industry separations. | Continuous operation, suitable for a variety of slurry types. | Complex machinery, high capital and maintenance costs, requires skilled operation. |
| Other Types | Including specialized centrifuges like inverting filter, vertical solid bowl, pusher, peeler, and oil centrifuges, each designed for specific applications and separation challenges. | Diverse applications across industries depending on the centrifuge type. | Tailored to specific separation needs, offering solutions for challenging separations. | Each has its own set of limitations, often related to the specific design and intended application, such as maintenance requirements or operational complexity. |
Operating Procedure of Centrifuge
- Before beginning to guarantee there is no obvious damage and that the machine is clean and free from dust or any residues that would impede its functioning, carefully inspect the centrifuge.
- Check that the centrifuge is securely fastened and on a level, sturdy platform to stop any movement or vibration during high speed running.
- During centrifugation, don the proper personal protective gear—a lab coat, gloves, and safety goggles—to guard yourself from any spills, aerosols, or other risks.
- Before turning on the instrument, make sure all control knobs, switches, and indicator lights are in their manufacturer’s recommended normal, default positions.
- Carefully open the centrifuge lid using the appropriate lock or release mechanism to guarantee that the lid is completely open and that any interlock devices are correctly disengaged for safe loading.
- Considering the type of samples, the necessary speed, and the rotor capacity, choose the right rotor for your experiment; confirm that the rotor is suited for operation with the centrifuge model in use.
- Look for any flaws in the rotor or the centrifuge tubes or containers; destroy any broken parts right away to prevent imbalance or possible mechanical failure.
- Load the centrifuge tubes into the rotor, being sure to balance them by weight instead of volume; If needed, utilize a precision balance to guarantee that equally spaced groups or matched samples have matching mass.
- Use a swinging bucket or fixed-angle rotor to make sure all positions are occupied as needed; if utilizing a swinging bucket rotor, load all buckets even if some are empty by arranging suitably weighted counterbalances.
- Particularly when processing hazardous or volatile materials, securely cap every centrifuge tube to stop leaks and reduce the danger of aerosol production.
- To engage the safety interlock system—which is meant to stop the machine from running should the lid be improperly closed—tightly close the centrifuge lid.
- Starting the centrifuge power, progressively change the speed control knob to make sure you slowly raise the rpm to the required amount without running beyond the manufacturer’s maximum safe speed.
- Use the relevant push button or dial for timer settings to establish the run duration, thereby verifying that the selected time interval and speed settings fall within the safe operating limitations stated by the equipment handbook.
- Till maximum operational speed is attained, constantly monitor the centrifuge during the acceleration period; Look for any odd vibrations that might point to a mechanical problem or imbalance. Listen for any such sounds.
- While the centrifuge is running, never leave it alone; should you find any anomalies or indications of instability, be ready to stop the machine right away.
- Allow the rotor to naturally come to a complete stop following the centrifugation cycle; Wait to try opening the lid until all of the moving components have completely slowed down.
- Wait an extra period—usually around 10 minutes—after the rotor stops for operations involving hazardous chemicals, biological materials, or radioactive substances to let any aerosols settle safely before lifting the lid.
- Only open the cover gently until you are certain the rotor has stopped; then, carefully discharge the centrifuge tubes to prevent spillage or contamination.
- To guarantee appropriate documentation and traceability, record all operational data including the run time, speed settings, and any noted equipment log or laboratory record abnormalities.
- Using a non-abrasive cleaning agent, clean and disinfect the rotor, centrifuge chamber, and tubes following each usage; this helps to avoid the accumulation of residues that can cause corrosion or imbalance in next runs.
- Follow any further manufacturer instructions or laboratory-specific guidelines for routine preventative maintenance including frequent inspections, lubrication, and component centering of centrifuges.
- Immediately stop the centrifuge, unplug it from the power source, and call a certified service professional for a thorough inspection and required repairs if any unexpected sounds, too strong vibration, or operational problems are seen when running.
- Before every usage, always consult the user handbook and particular laboratory standard operating procedures; make sure all staff members have received sufficient instruction on the safe running and maintenance of the centrifuge.
- When handling more sensitive or dangerous products, use extra safety precautions include safety cups, sealed rotors, or operating under a fume hood to further reduce risk.
- Share with your lab mates the operating state of the centrifuge and any accidents; follow accepted emergency procedures right away should an accident or exposure happen during spinning.
- When not in use, keep the rotor and other detachable parts in a clean, regulated environment. Following planned maintenance and inspection schedules will help to increase the lifetime of the centrifuge and guarantee continuous safe operation.
Centrifuge Balancing – How to balance a centrifuge?
- Starting any operation, first make sure the centrifuge and its rotor are clean, undamaged, and correctly seated.
- Check any tubes or containers for any flaws or cracks; next, make sure they fit the rotor being used.
- Load each tube with your sample such that the liquid level is constant in every tube so that variations in volume do not cause weight imbalances.
- Using a precision balance, weigh every tube with its contents—including the cap—to confirm that all tubes have the exact same mass as even minute variations might lead to appreciable imbalance at high speeds.
- Set the tubes in the rotor in symmetrical pairs, orienting them exactly opposite one another to distribute mass equally throughout the rotor.
- If you have an odd number of tubes, add a dummy tube filled with a liquid whose mass matches that of your sample to preserve symmetry and equilibrium.
- For rotors with more than two places, take note of symmetric designs advised by the manufacturer, like equilateral triangle or other arrangement using tubes.
- Verify that every tube is fastened in its holder to stop any movement during operation and that the overall weight on all opposing sides of the rotor is the same.
- Increase the centrifuge speed gradually while looking for any unusual vibrations or noises that would point to an imbalance; if found, halt the run right away and review the tube configuration.
- Since various models may have different needs, always consult the user handbook for the equipment to get particular balancing instructions and maximum allowed imbalances.
- Record the balancing process in your lab journal to guarantee correct traceability and assist in the troubleshooting of any prospective centrifuge operation problems.
- To guarantee continuous safe functioning, routinely check the rotor and tube holders for wear or damage and follow manufacturer advised routine maintenance.
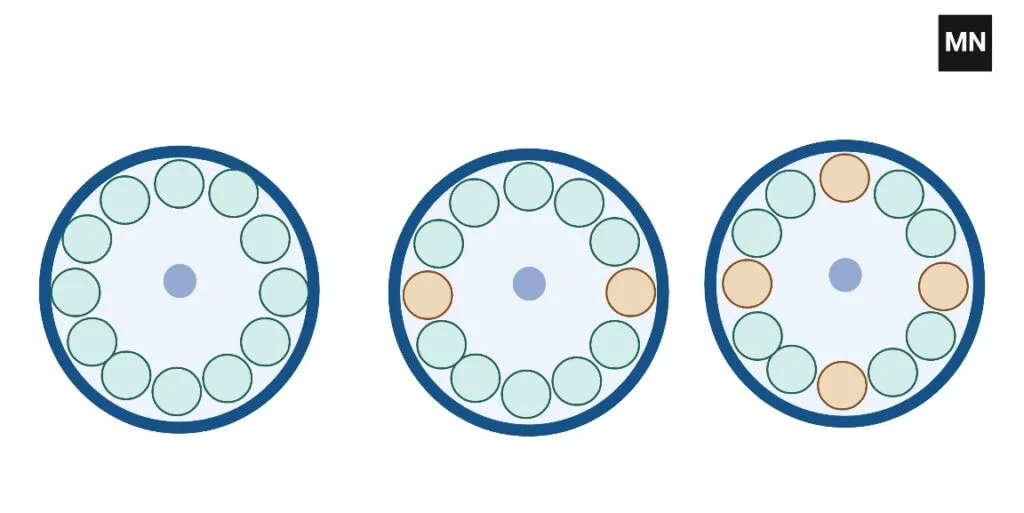
How to balance 3 tubes In Centrifuge
Two methods exist for balancing three tubes. The first alternative is to place three sample tubes side-by-side and three balance tubes directly across from them.
Optionally, three sample tubes can be evenly distributed around the rotor.
How to balance 5 tubes In Centrifuge
To balance five tubes, construct one balance tube and position two sets of three tubes across from one another.
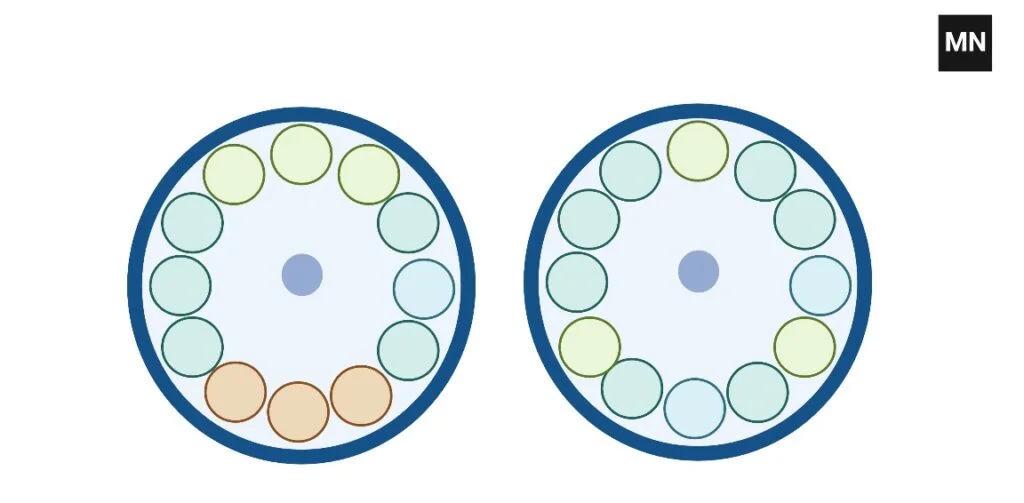
How to balance 7 tubes In Centrifuge
To balance seven tubes, you must construct one balancing tube and put two sets of four tubes across from one another.
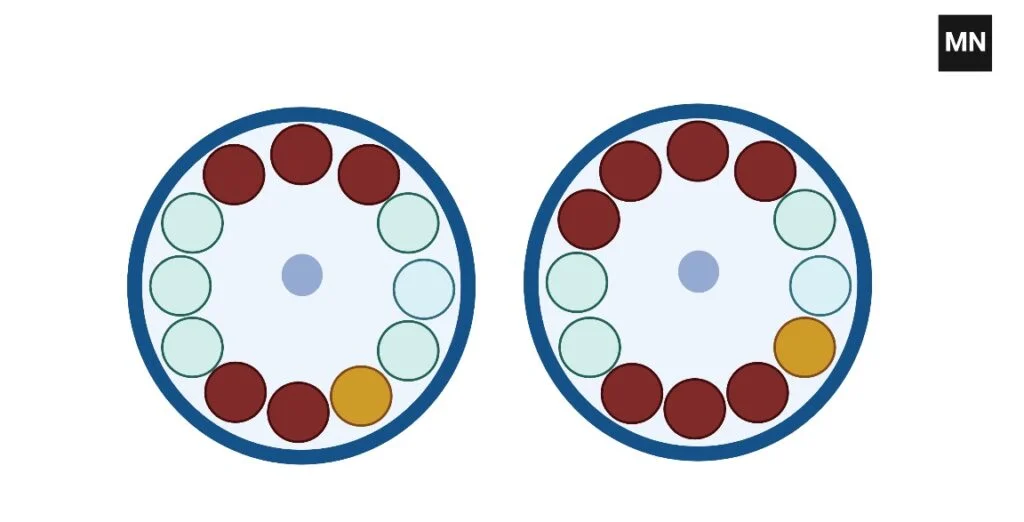
- Check if your rotor allows a triangle configuration for three tubes; if not, add a fourth tube filled with a liquid of equivalent weight to the sample tubes so that the burden is equally divided across paired places.
- If your rotor is built for an even number of tubes—such as 6 or 8—then include an additional dummy tube so that the tubes may be stacked in pairs immediately opposite one another, therefore preserving the center of mass at the rotor’s center. When balancing five tubes,
- Using an eighth tube as a counterweight, arrange the seven tubes equally around the circle to preserve appropriate symmetry and lower the possibility of vibration or rotor damage.
- Verify that every tube—including any fake tubes—contains exactly the same mass using a precision balance; even little variations can generate a major imbalance while the rotor is turning rapidly.
- The tubes should be arranged such that the weight is distributed uniformly around the rotor, therefore distributing the opposite sides equally and so reducing the centrifugal force imbalances during operation.
- prior starting the centrifuge, double-check the placement by visually verifying that the tubes are equidistant from each other and that the center of mass lines the axis of the rotor; if the rotor design permits this, draw up a schematic showing the tube placements prior.
- For any particular advice on balancing odd-numbered weights, see the user manual or standard operating procedures for the centrifuge; various models may have particular needs or extra safety advice.
- To guarantee a stable balance configuration before running the centrifuge under full operating conditions, think about doing a trial run at a slower speed; this helps avoid possible equipment damage or sample loss.
- Record the mass of the tubes, the configuration of the tubes, and any fake tubes placed for future usage so that, should any corrections be made in further runs, they may be made.
- See a lab supervisor or the manufacturer for advice to guarantee both safety and equipment lifetime if ambiguity remains on the optimum way to balance an odd number of tubes.
Care and Maintenance of Centrifuge
- Before starting maintenance, always look for any wear, corrosion, or damage on the centrifuge and its rotor.
- Before cleaning or doing any maintenance, unplug the centrifuge from the power supply to guarantee your safety.
- Using a moderate, nonabrasive detergent, clean the rotor, buckets, and chamber following every usage; steer clear of strong chemicals that can compromise the anodized surfaces.
- To eliminate detergent residue, rinse all cleaned items entirely with deionized or distilled water; then, let them air dry totally before reassembly.
- Review all moving components—including bearings, seals, and interlock systems—regularly and replace any that exhibit wear or degradation.
- As advised by the manufacturer, lubricate all moving parts; be sure only certified lubricants are used to prevent damage or malfunction.
- Examine the rotor closely for cracks, unbalance, or corrosion; if flaws are found, do not use the rotor until they have been fixed or replaced.
- Keep a thorough record of all maintenance activities—including dates of cleaning, components changed, lubrication done, and any repairs done to support continuous preventative care.
- Periodically follow manufacturer recommendations for centrifuge calibration; then, confirm that the speed and timing functions are running within stated tolerances.
- Immediately after a run, clean any spills or residue to avoid accumulation that can compromise the centrifuge’s balance and performance in next usage.
- When not in use, keep the rotor and other removable components in a dry, clean, temperature-regulated environment to reduce corrosion or damage.
- As advised in your laboratory’s standard operating procedures, follow a consistent preventive maintenance plan to guarantee that qualified staff members handle inspections and service.
- Replace broken or worn rubber seals, gaskets, or O-rings right away to preserve appropriate sealing and safety throughout use.
- Once maintenance is finished, test the centrifuge at a reduced speed to guarantee the instrument is running without unusual vibrations or noises.
- Always follow manufacturer advice and refer to the user handbook for particular maintenance directions catered to your centrifuge type.
- During maintenance, use gloves and safety goggles to guard against mechanical dangers or chemical exposure.
- To guarantee responsibility and traceability in the equipment care processes of the laboratory, make sure that each maintenance or repair activity is recorded and checked by a certified supervisor.
Applications of Centrifuge
- Separates blood components in medical labs
- Isolates proteins and nucleic acids in biochemistry
- Concentrates and separates cells in cell culture
- Isolates nanoparticles for research purposes
- Clarifies liquids in industrial processing
- Treats wastewater by separating solids from liquids
- Separates cream from milk in food processing
- Purifies enzymes and drugs in pharmaceutical manufacturing
Advantages of Centrifuge
- Rapid and efficient separation of mixtures
- Shortens separation time compared to gravity methods
- Works with various sample types and densities
- Can be automated for continuous operation
- Produces consistent and reproducible results
- Scalable from lab to industrial use
- Minimizes manual handling of samples
- Improves clarity of separated components
- Preserves sample integrity with controlled conditions
- Compact design fits limited lab space
Limitations of Centrifuge
- High energy consumption increases operating costs
- Excessive noise and vibration can be disruptive
- High-speed operation may damage delicate samples
- Frequent maintenance is required for optimal performance
- High initial capital investment can be a barrier
- Strict balancing needs complicate loading and safety
- Heat generation can affect temperature-sensitive materials
- Limited capacity for some large-scale or unusual samples
Precautions
- Always don personal safety goggles, gloves, and a lab coat.
- Before every usage, make sure the centrifuge is in balance.
- Before loading, look over tubes and containers for damage or cracks.
- Fill tubes to the advised level; do not overfill or underfill.
- Before beginning, check the interlock is engaged and secure the lid.
- Never open the centrifuge under running conditions.
- Watch for any unusual sensations or sounds when using.
- Immediately stop the equipment should an odd behavior or unbalance be found.
- For best functioning, set the centrifuge on a flat, sturdy platform.
- Before servicing or cleaning the centrifuge, unplug it.
What does RCF stand for centrifuge?
- Relative Centrifugal Force, or RCF, is the force acting on a sample during centrifugation.
- It converts the spinning rotor’s delivered effective acceleration on the sample into a multiple of Earth’s gravitational force (g-force).
- More precisely than RPM alone, RCF is computed from the radius from the center of rotation to the sample using the rotating speed of the centrifuge.
- With r in centimeters, the usually used formula is RCF = 1.118 × 10⁻� × r × (RPM)², which lets users translate between RPM and the effective force.
- Since it considers differences in rotor size and shape that RPM misses, using RCF guarantees that tests are uniform and repeatable.
- Many laboratory techniques specify the force applied to samples in terms of RCF instead of RPM to offer a constant metric across several centrifuge types.
- Applications include cell separation, protein isolation, and nucleic acid extraction require RCF as effective separation depends on exact force control.
- Usually showing both RPM and RCF data, modern centrifuges have automatic conversion tools to enable users to create the ideal circumstances for their research.
- Often referred to as g-force, RCF shows how much more times greater the applied centrifugal force is than the force of gravity—for instance, a 1000 g setting denotes the force is 1000 times that of Earth’s gravity.
- Optimizing centrifugation operations in research and industrial environments depends on a basic knowledge of and right use of RCF, therefore guaranteeing that samples are exposed to the optimal force for successful sedimentation and separation.
What is RPM in Centrifuge?
- Revolutions Per Minute, or RPM, is the total number of complete spins the centrifuge rotor completes in one minute.
- Showed immediately on the control panel of the machine, it is the main indication of the centrifuge’s running speed.
- Since RPM ignores the size or radius of the rotor, it does not by itself explain the force applied on the samples. RPM is a measurement of rotating speed.
- Many systems translate RPM into Relative Centrifugal Force (RCF) for accuracy as two centrifuges running at the same RPM may produce varying centrifugal forces depending on their rotor radii.
- Setting up centrifugation settings depends on RPM as it determines the sedimentation rate of particles, therefore affecting processes including protein precipitation and cell separation.
- RPM indicates the speed of the rotor, but the real effective force on the samples—g-force—is computed using a formula using the rotor’s radius as well.
- Many contemporary centrifuges show both RPM and RCF so that users may immediately change the speed and guarantee the required separation force is applied.
- To guarantee repeatability and best separation efficiency in actual laboratory settings, the selected RPM value has to match the experimental needs.
- Knowing RPM is crucial as it creates the foundation for translating to RCF, which is usually the recommended unit mentioned in centrifugation techniques.
- Not only does proper RPM aid to achieve the intended sedimentation but also shields delicate samples from too strong force or damage during the centrifugation process.
How RPM is calculated?
- RPM, or revolutions per minute, gauges the total number of complete revolutions an item completes in one minute.
- RPM in electric motors—especially AC motors—is computed from the supply frequency and motor windings’ pole count.
- To convert seconds to minutes, multiply the line frequency (in Hz) by 60; next, by 2 (to account for both the positive and negative half of the AC cycle); lastly, divide by the number of poles.
- For a 4-pole motor and a 60 Hz supply, the no-load RPM is computed as (60 x 60 x 2) divided by 4, or 1,800 RPM.
- Slip is the phenomena a motor undergoes under load whereby its real full-load RPM is somewhat less than the no-load RPM; the difference is called the RPM slip.
- Manufacturers sometimes offer charts that link voltage to predicted RPM; with DC motors, the RPM is affected by the applied voltage, the number of windings on the armature, and the intensity of the magnetic flux.
- When gears are involved, the gear ratio—derived from the number of teeth on the input gear relative to the output gear—determines the output RPM of a mechanism by multiplying the input RPM.
- For example, the output shaft rotates 600 x (60/12) = 3,000 RPM if a 600 RPM motor powers a gear system whereby a 60-tooth gear meshes with a 12-tooth gear.
- Considering the gearbox ratio and tire size helps one relate the engine’s RPM in automotive applications to the speed of the vehicle; this relationship helps translate rotational speed into linear speed.
- By means of tire circumference, one establishes the relationship between wheel RPM and vehicle speed such that vehicle speed = wheel RPM multiplied by tire circumference (with suitable unit conversions).
- Between angular velocity and RPM is another crucial conversion; the formula RPM = (angular speed × 60) divided by (2π) converts radian-based speeds to RPM. One revolution equals 2π radians.
- In specialist disciplines such as centrifugation, RPM may be computed with formulae modified depending on rotor design and constants particular to the equipment utilizing the radius of the rotor and the required relative centrifugal force.
- Dividing the cutting speed by the product of π and the tool or workpiece diameter determines spindle speed (RPM) for machine tools, including in machining processes; this guarantees the cutter runs at ideal circumstances.
- Linking electrical input straight to rotational speed, brushless motors have a Kv rating indicating the number of RPM per volt applied under no-load conditions.
- Whether it’s figuring the speed of an electric motor, an output shaft in a gear system, or translating rotational speed to linear motion in vehicles, the technique used to calculate RPM depends on the context—and knowledge of these calculations is essential for design, performance tuning, and troubleshooting across many engineering uses.
Relationship between RPM and RCF
- RPM and RCF are connected by a formula wherein the relative centrifugal force experienced by the sample is connected to the rotating speed of a centrifuge, therefore indicating the force exerted relative to the acceleration due to gravity (g).
- With r as the radius from the center of rotation to the sample in centimeters, the normal formula used in centrifugation to translate RPM to RCF is RCF = 1.118 × 10⁻⁵ × r × (RPM)².
- The formula incorporates RPM squared, hence the connection is quadratic—that is, if you double the RPM, the RCF rises by a factor of four, supposing the rotor radius stays constant.
- This quadratic dependency explains why little changes in RPM may produce significant increases in the centrifugal force—essential for effective particle separation in a sample.
- On the other hand, you may reorganize the formula to solve for RPM if you must reach a particular RCF for an experiment: RPM = √(RCF / (1.118 × 10⁻⁵ × r)). This enables centers centrifuges to satisfy experimental criteria.
- The rotor’s radius is a vital aspect; a bigger radius will yield a higher RCF at the same RPM, therefore when setting up a centrifugation process, both the RPM and the rotor radius must be addressed simultaneously.
- Although centrifuges in laboratory environments usually show speeds in RPM, procedures may specify the necessary RCF (measured in multiples of g), so this connection helps scientists to modify settings suitable for their particular use.
- Unit conversions when the radius is measured in centimeters and the necessary force is stated in terms of g produce the constant 1.118 × 10⁻⁵; so, alternative units for radius would need altering this constant.
- Not just in laboratory centrifugation but also in many engineering applications where rotational speed converts into force, therefore assuring that systems are built for the correct performance and safety.
What is the difference between RPM and RCF?
| Parameter | RPM (Revolutions Per Minute) | RCF (Relative Centrifugal Force) |
|---|---|---|
| Definition | Measures the number of complete rotations a centrifuge rotor makes per minute. | Measures the force exerted on samples relative to Earth’s gravity (g-force). |
| Indicates | The speed at which the centrifuge is spinning. | The actual centrifugal force applied to the samples. |
| Formula | Directly read from the centrifuge display. | RCF = 1.118 × 10⁻⁵ × r × (RPM)², where r is the rotor radius in cm. |
| Depends on Rotor Size? | No, RPM is independent of rotor size. | Yes, RCF is affected by both RPM and rotor radius. |
| Use in Protocols | Less reliable for standardization, as different rotors will exert different forces at the same RPM. | More reliable, ensuring consistent experimental conditions across different centrifuges. |
| Centrifuge Settings | Many centrifuges are set by RPM, requiring conversion to RCF when needed. | Many modern centrifuges allow direct input of RCF for precise control. |
| Why It Matters | If two centrifuges have different rotor sizes, the same RPM will produce different forces. | Ensures that the correct force is applied, making results reproducible across different centrifuges. |
Differences between swing bucket rotor and fixed angle rotor
| Feature | Swing Bucket Rotor | Fixed Angle Rotor |
|---|---|---|
| Tube Orientation | Tubes are placed in buckets that swing out horizontally during centrifugation, achieving a 90° angle relative to the axis of rotation. | Tubes are held at a fixed angle, typically between 30° to 45°, relative to the axis of rotation. |
| Pellet Formation | Pellets form at the bottom center of the tube, resulting in a flat and uniform sedimentation. | Pellets form along the side of the tube, corresponding to the fixed angle, which can make it challenging to decant supernatant without disturbing the pellet. |
| Maximum Speed | Generally supports lower maximum speeds due to the mechanical stress on moving parts. | Capable of withstanding higher centrifugal forces, allowing for higher speeds and shorter centrifugation times. |
| Sample Capacity | Offers high vessel capacity and flexibility with various adapter options, accommodating different tube sizes and types. | Typically holds more samples due to efficient tube spacing, making it suitable for high-throughput applications. |
| Applications | Ideal for applications requiring gentle separation, such as density gradient centrifugation, and when precise separation is needed. | Suitable for pelleting particles like cells, bacteria, or cellular debris, and for separating biological macromolecules such as DNA, RNA, and proteins. |
How to use Eppendorf centrifuge?
- Before using the centrifuge for the first time, read the whole operating handbook to grasp all safety measures and operational instructions.
- Place the centrifuge on a sturdy, vibration-free bench in a well-ventilated place with appropriate space from walls and other equipment
- Use only the permitted mains power cable and connector; make sure the power source fits the specs on the name plate of the gadget.
- If the item has been taken from a colder location, let it warm up to ambient temperature to prevent condensation damage.
- Install the centrifuge as directed by the manufacturer to guarantee that all transporting and protective equipment has been taken off.
- Insert the rotor carefully onto the motor shaft and use the supplied rotor key to firmly tighten the rotor nut, ensuring proper alignment and secure fixation
- Load sample tubes or adapters symmetrically in the rotor by using similar tubes with equivalent capacities to ensure balance during operation
- Following the experimental process, set the centrifugation parameters—speed (in rpm or rcf), duration, and temperature—if using a chilled model—on the control panel.
- Pressing the designated start button will initiate the centrifugation process and confirm that the display displays the proper speed, time, and other pertinent settings.
- Track the run across the digital display; many models have automatic rotor detection and imbalance detection mechanisms that stop the process should a problem arise.
- Open the lid only once the rotor has stopped completely; if needed, use the assigned open key or emergency lid release.
- Remove the rotor carefully by holding it with both hands and ensuring that the load remains balanced during removal
- Clean the rotor, buckets, and exterior surfaces regularly with a mild cleaning solution recommended by the manufacturer to prevent contamination and maintain performance
- Follow all maintenance instructions including periodic checks for wear, corrosion, or damage, and replace components only with original Eppendorf accessories
- Keep the operating manual readily available for review of safety guidelines prior to every use and for troubleshooting.
- Adhere to all safety regulations provided in the manual, especially when handling biological samples or hazardous materials, to ensure personal and equipment safety
FAQ
What is a centrifuge?
A centrifuge is a device that uses centrifugal force to separate different components of a mixture based on their size, shape, and density. It consists of a rotating drum or rotor that spins at high speeds, causing the components of the mixture to sediment based on their physical properties.
Centrifuges are commonly used in biochemistry and molecular biology to separate different cellular components, such as organelles or subcellular particles, or to purify and isolate biological molecules, such as DNA, RNA, or proteins. They are also used in a variety of other applications, such as blood banking, where they are used to separate different components of blood, and in industrial processes, where they are used to separate different materials.
There are many different types of centrifuges available, each designed for a specific application. Some centrifuges are designed to spin small samples at high speeds, while others are designed to handle large samples or high volumes of material. Some centrifuges are also equipped with special rotors that allow them to spin samples in different directions or at different angles, which can be useful for separating different components of a mixture.
What does a centrifuge do?
A centrifuge is a device that uses centrifugal force to separate different components of a mixture based on their size, shape, and density. It consists of a rotating drum or rotor that spins at high speeds, causing the components of the mixture to sediment based on their physical properties.
Centrifuges are commonly used in biochemistry and molecular biology to separate different cellular components, such as organelles or subcellular particles, or to purify and isolate biological molecules, such as DNA, RNA, or proteins. They are also used in a variety of other applications, such as blood banking, where they are used to separate different components of blood, and in industrial processes, where they are used to separate different materials.
There are many different types of centrifuges available, each designed for a specific application. Some centrifuges are designed to spin small samples at high speeds, while others are designed to handle large samples or high volumes of material. Some centrifuges are also equipped with special rotors that allow them to spin samples in different directions or at different angles, which can be useful for separating different components of a mixture.
Why is it important to have a balanced centrifuge?
It is important to have a balanced centrifuge because an unbalanced centrifuge can cause serious problems and potentially be dangerous. When a centrifuge is unbalanced, the rotor will not be evenly distributed and will be heavier on one side. This can cause the centrifuge to vibrate excessively, which can damage the equipment and potentially lead to accidents or injuries.
A balanced centrifuge is essential for ensuring the safety of the equipment and the people who use it. It also helps to ensure the accuracy and reliability of the results obtained from the centrifugation process. An unbalanced centrifuge can cause the sample to sediment unevenly, leading to incorrect results or loss of sample material.
To balance a centrifuge, it is important to ensure that the load is evenly distributed in the rotor and that the centrifuge is properly balanced before each use. This can be done by adding or removing samples or using balance tubes to ensure that the rotor is evenly balanced. Proper maintenance and handling of the centrifuge can help to prevent unbalancing and ensure that the equipment is safe and reliable.
How long does a centrifuge spin?
The length of time that a centrifuge spins depends on the specific application and the type of centrifuge being used. Some centrifuges may spin for just a few minutes, while others may spin for several hours. In general, the length of time that a centrifuge spins is determined by the desired separation of the components in the sample, the size and shape of the sample, and the density of the components being separated.
In biochemistry and molecular biology, centrifuges are often used to separate different cellular components or to purify and isolate biological molecules. The length of time that the centrifuge spins will depend on the specific separation being performed and the characteristics of the sample being separated. For example, a sample being separated by isopycnic centrifugation may spin for several hours, while a sample being separated by differential centrifugation may spin for just a few minutes.
It is important to follow the manufacturer’s guidelines for the specific centrifuge being used and to carefully consider the characteristics of the sample being separated when determining the appropriate length of time for the centrifuge to spin.
How much does a centrifuge cost?
The cost of a centrifuge can vary widely depending on the specific model and its features. Centrifuges can range in price from a few hundred dollars for a basic tabletop model to tens of thousands of dollars for a high-end, specialized research-grade centrifuge.
Factors that can affect the cost of a centrifuge include the size and capacity of the rotor, the maximum speed at which the rotor can spin, the type of rotor (e.g. fixed-angle, swinging-bucket), and any additional features or accessories that may be included.
In general, tabletop centrifuges are less expensive than larger, floor-standing models, but they may also have lower capacities and lower maximum speeds. Research-grade centrifuges are typically more expensive than basic models, but they may offer higher performance and greater versatility.
It is important to carefully consider the specific needs and requirements of your application when choosing a centrifuge to ensure that you select a model that is appropriate and cost-effective for your needs.
When do you centrifuge the specimen?
The decision to centrifuge a specimen depends on the specific goals of the experiment or analysis and the characteristics of the specimen being tested. Centrifugation is often used to separate different components of a mixture based on their size, shape, and density, so it may be used when it is necessary to purify or isolate specific components of the specimen.
In general, centrifugation is typically performed after the specimen has been collected and processed, and after any necessary preparatory steps have been completed. For example, in a clinical laboratory, a blood sample may be collected and then processed to remove any clots or debris before being centrifuged to separate the different components of the blood.
It is important to carefully consider the specific goals of the experiment or analysis and the characteristics of the specimen when deciding whether or not to centrifuge the specimen. In some cases, centrifugation may not be necessary or may not be the most appropriate method for separating the components of interest.
Why is blood separated in a centrifuge?
Blood is separated in a centrifuge to separate the different components of the blood based on their size, shape, and density. Blood is made up of several different components, including red blood cells, white blood cells, and plasma. Each of these components has a different density and will sediment at a different rate when the blood is spun in a centrifuge.
By separating the different components of the blood, it is possible to study and analyze them individually or to prepare them for further use. For example, red blood cells can be used for transfusions, while plasma can be used for a variety of purposes, including blood clotting and protein analysis.
Centrifugation is a quick and efficient way to separate the different components of the blood and is commonly used in clinical laboratories and research settings. It is important to follow the manufacturer’s guidelines for the specific centrifuge being used and to carefully consider the characteristics of the sample being separated when determining the appropriate speed and duration for the centrifuge to spin.
What is the purpose of a centrifuge?
The purpose of a centrifuge is to separate different components of a mixture based on their size, shape, and density. It uses centrifugal force to sediment the components of the mixture based on their physical properties, allowing them to be separated and isolated.
Centrifuges are commonly used in biochemistry and molecular biology to purify and isolate biological molecules, such as DNA, RNA, or proteins, or to separate different cellular components, such as organelles or subcellular particles. They are also used in a variety of other applications, such as blood banking, where they are used to separate different components of blood, and in industrial processes, where they are used to separate different materials.
There are many different types of centrifuges available, each designed for a specific application. Some centrifuges are designed to spin small samples at high speeds, while others are designed to handle large samples or high volumes of material. Some centrifuges are also equipped with special rotors that allow them to spin samples in different directions or at different angles, which can be useful for separating different components of a mixture.
How long to centrifuge blood?
The length of time to centrifuge blood depends on the specific separation being performed and the characteristics of the sample. In general, blood samples are typically centrifuged for 10-15 minutes at a relatively low speed, such as around 500-1000 x g, to separate the different components of the blood.
During centrifugation, the red blood cells, which are the heaviest and densest component of the blood, will sediment to the bottom of the tube, while the lighter plasma and white blood cells will remain in the supernatant. By carefully controlling the speed and duration of the centrifugation, it is possible to separate the different components of the blood and prepare them for further analysis or use.
It is important to follow the manufacturer’s guidelines for the specific centrifuge being used and to carefully consider the characteristics of the sample being separated when determining the appropriate length of time for the centrifuge to spin.
How to balance a centrifuge with an odd number of tubes?
To balance a centrifuge with an odd number of tubes, you can follow these steps:
Begin by filling the tubes with the sample and closing the caps securely.
Place the tubes in the rotor, taking care to evenly distribute them around the rotor.
If necessary, use balance tubes to help evenly distribute the weight of the samples in the rotor. Balance tubes are empty tubes that can be added to the rotor to help balance the load.
Check the balance of the rotor by gently tilting it back and forth. If the rotor is evenly balanced, it should remain level and not tilt to one side.
If the rotor is not balanced, adjust the position of the tubes or add or remove balance tubes until the rotor is balanced.
Once the rotor is balanced, close the centrifuge lid and start the centrifuge according to the manufacturer’s instructions.
It is important to ensure that the centrifuge is properly balanced before each use to prevent vibration and damage to the equipment and to ensure the accuracy and reliability of the results obtained from the centrifugation process.
How many revolutions did the centrifuge complete after being turned off?
It is not possible to determine how many revolutions a centrifuge has completed after it has been turned off without additional information. The number of revolutions a centrifuge completes will depend on a variety of factors, including the speed at which it is spinning, the length of time it has been running, and the characteristics of the sample being separated.
To determine the number of revolutions a centrifuge has completed, you would need to know the speed at which the centrifuge was spinning, the length of time it was running, and the size and shape of the rotor. You would also need to know the specific type of centrifuge and its characteristics, as different models may have different rotor sizes and shapes, and may spin at different speeds.
Without this information, it is not possible to determine the number of revolutions a centrifuge has completed after it has been turned off.
What is centrifugation process?
Centrifugation is a process that uses centrifugal force to separate substances of different densities or to separate particles of different sizes in a mixture. It is commonly used in laboratories to separate biological materials such as cells, proteins, and DNA.
The process involves placing a sample in a tube or other container and spinning it at high speeds in a machine called a centrifuge. The centrifugal force generated by the spinning motion causes the heavier or denser materials to settle to the bottom of the tube, while lighter materials remain at the top.
There are several different types of centrifugation, including differential centrifugation, which is used to separate cells and organelles based on their size and density, and sedimentation centrifugation, which is used to separate large molecules such as proteins and DNA based on their size.
Centrifugation is a useful technique for purifying and separating biological materials, and it is an important tool in many areas of research and biotechnology.
What is centrifugation used for?
Centrifugation is a widely used technique in a variety of fields, including biology, chemistry, and medicine. Some common applications of centrifugation include:
Separating cells and organelles: Centrifugation can be used to separate different types of cells or organelles based on their size and density. For example, it can be used to purify red blood cells, white blood cells, or other cell types from a mixed sample.
Separating molecules: Centrifugation can be used to separate molecules such as proteins, DNA, and RNA based on their size. This is often done using a technique called sedimentation centrifugation, which separates molecules based on their sedimentation coefficient, a measure of how fast they move through a liquid when subjected to a centrifugal force.
Purifying proteins: Centrifugation can be used to purify proteins from complex mixtures such as cell lysates or tissue homogenates. This is often done using a technique called ultracentrifugation, which separates molecules based on their size and shape.
Clarifying solutions: Centrifugation can be used to remove solid particles or other contaminants from solutions. For example, it can be used to clarify beer or wine by removing yeast or other solid particles that may be present in the liquid.
Separating mixtures: Centrifugation can be used to separate mixtures of substances based on their density. For example, it can be used to separate heavy and light materials in a sample, or to separate different types of particles based on their size.
Analyzing particles: Centrifugation can be used to analyze the size and shape of particles in a sample, such as bacteria or cells. This can be done by measuring the sedimentation rate of the particles, which is a measure of how fast they settle to the bottom of a tube when subjected to a centrifugal force.
What is centrifugation and examples?
Centrifugation is a process that uses centrifugal force to separate substances of different densities or to separate particles of different sizes in a mixture. It is commonly used in laboratories to separate biological materials such as cells, proteins, and DNA.
Here are a few examples of how centrifugation is used in different fields:
In biology: Centrifugation is often used to separate cells and organelles from tissue homogenates or cell lysates. For example, a researcher might use differential centrifugation to separate cells from a mixed sample, or sedimentation centrifugation to separate proteins or DNA based on their size.
In chemistry: Centrifugation can be used to separate different types of molecules based on their size and density. For example, it can be used to purify proteins from complex mixtures, or to separate different types of particles based on their size.
In medicine: Centrifugation is used in many medical applications, including the separation of red and white blood cells, the purification of proteins for use in therapies, and the analysis of particles such as bacteria or viruses.
In industrial processes: Centrifugation is used in a variety of industrial processes, including the separation of oil and water, the clarification of liquids such as beer and wine, and the purification of products such as drugs and food additives.
In environmental science: Centrifugation is used to analyze and separate particles in environmental samples, such as water or air, to determine the presence of contaminants or other substances of interest.
What is centrifugation also called?
Centrifugation is also known as centrifugal separation. It is a process that uses centrifugal force to separate substances of different densities or to separate particles of different sizes in a mixture.
There are several different types of centrifugation, including differential centrifugation, which is used to separate cells and organelles based on their size and density, and sedimentation centrifugation, which is used to separate large molecules such as proteins and DNA based on their size.
Other terms that are often used in reference to centrifugation include centrifugal filtration, which refers to the use of centrifugation to filter particles from a liquid, and ultracentrifugation, which refers to the use of high-speed centrifugation to separate and purify large molecules such as proteins
What is centrifuge in simple terms?
A centrifuge is a machine that uses centrifugal force to separate substances of different densities or to separate particles of different sizes in a mixture. It consists of a spinning rotor that is placed inside a container or tube holding the sample to be separated. When the rotor is spun at high speeds, the centrifugal force generated by the spinning motion causes the heavier or denser materials to settle to the bottom of the container, while lighter materials remain at the top.
Centrifuges are commonly used in laboratories to separate biological materials such as cells, proteins, and DNA. They are also used in a variety of industrial and medical applications, including the separation of oil and water, the clarification of liquids such as beer and wine, and the purification of products such as drugs and food additives.
What causes centrifugation?
Centrifugation is caused by the application of a centrifugal force to a sample that is placed in a container or tube and spun at high speeds. The centrifugal force is generated by the spinning motion of the sample and acts on the particles in the sample, causing them to move away from the center of the container.
The magnitude of the centrifugal force is determined by the speed of the spinning motion and the radius of the sample. As the speed increases or the radius decreases, the centrifugal force increases, causing the particles in the sample to move further away from the center.
Centrifugation is used to separate substances or particles of different densities or sizes in a mixture by exploiting the differences in the way that they respond to the centrifugal force. Heavier or denser materials will tend to settle to the bottom of the container, while lighter materials will remain at the top. This allows the substances or particles to be separated based on their density or size.
Who is the father of centrifugation?
The credit for the development of centrifugation is often given to the French scientist Antoine César Becquerel, who first described the use of centrifugal force to separate substances in 1827. However, the first practical application of centrifugation was developed by the English chemist and industrialist James Small, who used a spinning motion to separate cream from milk in the early 19th century.
The modern centrifuge, which uses a spinning rotor to generate centrifugal force, was developed in the early 20th century by the German chemist and inventor Theodor Svedberg. Svedberg’s work laid the foundation for the development of many different types of centrifuges, which are now widely used in a variety of fields including biology, chemistry, and medicine.
What is the maximum speed of centrifuge?
The maximum speed of a centrifuge is determined by the design of the centrifuge and the materials used in its construction. In general, the maximum speed of a centrifuge will depend on the size and type of rotor being used, the size and shape of the sample being spun, and the materials that the rotor and sample are made of.
Some high-speed centrifuges are capable of spinning at speeds of up to 100,000 RPM or more, while others are designed to spin at more moderate speeds of a few thousand RPM. The maximum speed of a centrifuge is typically limited by factors such as the strength and durability of the materials used in its construction, the stability of the rotor, and the safety of the operator.
It is important to use caution when operating a centrifuge at high speeds, as the forces generated by the spinning motion can be dangerous if the centrifuge is not properly designed or maintained. It is also important to follow the manufacturer’s guidelines for the safe use of a centrifuge, as well as any relevant safety regulations.
Why do we centrifuge at 4 degrees?
It is common to store biological samples, such as cells or proteins, at low temperatures, such as 4°C (39°F), to preserve their integrity and prevent degradation. Centrifugation is often used to separate or purify these samples, and it is important to maintain the sample at a low temperature during the centrifugation process to avoid damaging the sample.
Maintaining the sample at a low temperature during centrifugation can also help to reduce the risk of sample contamination, as some contaminants may be less stable at low temperatures. Additionally, low temperature can help to prevent the formation of ice crystals or other forms of damage that may occur if the sample is allowed to warm up during the centrifugation process.
In general, it is recommended to store biological samples at 4°C or lower to preserve their integrity and to minimize the risk of degradation or contamination. This is especially important for samples that are sensitive or prone to damage, such as proteins or DNA.
What happens if you centrifuge too fast?
Spinning a sample too fast in a centrifuge can cause a number of problems, including:
Damage to the sample: The high speeds and forces generated by centrifugation can cause mechanical damage to the sample, leading to the denaturation or degradation of proteins, DNA, or other molecules in the sample. This can affect the accuracy and reliability of the results of any experiments or analyses performed on the sample.
Risk of contamination: Operating a centrifuge at high speeds can generate aerosols or other forms of contamination, which can affect the integrity of the sample and the accuracy of the results.
Risk of accidents: Centrifugation at high speeds can be dangerous if the centrifuge is not properly designed or maintained, as the forces generated by the spinning motion can be hazardous to the operator or bystanders.
Inaccurate results: Spinning a sample too fast in a centrifuge can cause the sample to become too concentrated, leading to inaccurate results. This can be especially problematic if the sample contains particles or molecules of different sizes, as the separation may not be as effective at high speeds.
It is important to follow the manufacturer’s guidelines and any relevant safety regulations when operating a centrifuge to ensure that the sample is spun at an appropriate speed for the specific application.
What happens if you centrifuge for too long?
Spinning a sample for too long in a centrifuge can cause a number of problems, including:
Damage to the sample: The high speeds and forces generated by centrifugation can cause mechanical damage to the sample, leading to the denaturation or degradation of proteins, DNA, or other molecules in the sample. This can affect the accuracy and reliability of the results of any experiments or analyses performed on the sample.
Risk of contamination: Operating a centrifuge for a long period of time can increase the risk of contamination, as the sample may be exposed to the environment for an extended period.
Risk of accidents: Centrifugation for a long period of time can be hazardous if the centrifuge is not properly designed or maintained, as the forces generated by the spinning motion can be dangerous to the operator or bystanders.
Inaccurate results: Spinning a sample for too long in a centrifuge can cause the sample to become too concentrated, leading to inaccurate results. This can be especially problematic if the sample contains particles or molecules of different sizes, as the separation may not be as effective after an extended period of time.
It is important to follow the manufacturer’s guidelines and any relevant safety regulations when operating a centrifuge to ensure that the sample is spun for an appropriate amount of time for the specific application.
What is the most common error when using a centrifuge?
One of the most common errors when using a centrifuge is not following the manufacturer’s guidelines or relevant safety regulations. It is important to carefully read and understand the instructions for operating the centrifuge and to follow all recommended procedures to ensure the safety of the operator and the integrity of the sample.
Other common errors when using a centrifuge include:
Overloading the centrifuge: Putting too much weight or volume in the centrifuge can cause the rotor to become unbalanced, leading to vibrations and potentially damaging the centrifuge or the sample.
Using the wrong type of rotor: Using a rotor that is not designed for the specific sample or application can lead to inaccurate results or damage to the sample.
Using the wrong speed or time: Spinning the sample at an inappropriate speed or for too long can cause damage to the sample or lead to inaccurate results.
Improperly sealing the tubes or containers: If the tubes or containers holding the sample are not properly sealed, the sample may become contaminated or the results of the separation may be inaccurate.
Not maintaining the centrifuge: Failing to properly maintain the centrifuge, including cleaning it regularly and checking for wear and damage, can lead to problems with the performance of the centrifuge and the accuracy of the results.
How long do you centrifuge for serum?
The optimal time for centrifuging a sample of serum (the clear, straw-colored liquid component of blood) will depend on a number of factors, including the size and type of rotor being used, the volume and density of the sample, and the specific goals of the separation.
In general, it is recommended to spin serum samples at a moderate speed (e.g., 1,500-2,000 RPM) for a period of 10-15 minutes. This should be sufficient to separate the serum from any solid components present in the sample, such as cells or clotting factors.
It is important to follow the manufacturer’s guidelines for the specific centrifuge being used, as well as any relevant protocols or procedures, to ensure the safety of the operator and the integrity of the sample. In some cases, it may be necessary to adjust the speed or time of the centrifugation based on the specific needs of the application.
Can you centrifuge blood twice?
It is generally not recommended to centrifuge blood more than once, as the high speeds and forces generated by centrifugation can cause damage to the cells and other components of the blood. Additionally, repeated centrifugation can increase the risk of contamination and may lead to inaccurate results.
However, there are some specific situations in which it may be necessary to centrifuge blood more than once. For example, if the sample is very large or if it contains a high concentration of cells or other solid components, it may be necessary to perform multiple rounds of centrifugation to effectively separate the components of the blood.
If it is necessary to centrifuge blood more than once, it is important to follow the manufacturer’s guidelines for the specific centrifuge being used, as well as any relevant protocols or procedures, to ensure the safety of the operator and the integrity of the sample. In addition, it may be necessary to use protective measures, such as wearing gloves and a face mask, to minimize the risk of contamination.
What does G stand for in centrifugation?
In centrifugation, “G” stands for “gravity.” Centrifugation is a process that uses centrifugal force to separate substances or particles in a mixture based on their size, density, or other characteristics. Centrifugal force is generated by the spinning motion of the sample and acts in a direction that is perpendicular to the axis of rotation.
The strength of the centrifugal force is typically measured in units of “G” or “g-force,” which represents the acceleration of the sample due to the centrifugal force. The g-force is equal to the acceleration of gravity (9.81 m/s^2) multiplied by the radius of the sample divided by the radius of the Earth (6,371 km).
For example, if a sample is spinning at a speed of 1,000 RPM in a centrifuge with a radius of 10 cm, the g-force would be approximately 1,000 G. This is much stronger than the force of gravity that we experience on Earth, which is typically around 1 G.
The g-force applied to a sample during centrifugation can affect the separation of the components in the sample, as well as the stability and integrity of the sample itself. It is important to carefully consider the g-force when selecting a centrifuge and to follow the manufacturer’s guidelines for the specific centrifuge being used to ensure the safety of the operator and the accuracy of the results.
What is maximum speed of high speed centrifuge?
The maximum speed of a high-speed centrifuge will depend on the specific design and capabilities of the centrifuge. Some high-speed centrifuges are capable of spinning at speeds of up to 100,000 RPM or more, while others are designed to spin at more moderate speeds of a few thousand RPM.
High-speed centrifuges are typically used to separate small particles or molecules, such as proteins or DNA, or to purify samples for analysis. They are often used in research and industrial applications where high speed and high separation efficiency are required.
It is important to carefully consider the specific needs of the application and the capabilities of the centrifuge when selecting a high-speed centrifuge. It is also important to follow the manufacturer’s guidelines and any relevant safety regulations when operating the centrifuge to ensure the safety of the operator and the integrity of the sample.
- Moran, S. (2017). Centrifuges. Process Plant Layout, 389–397. doi:10.1016/b978-0-12-803355-5.00027-5
- Bridges, S., & Robinson, L. (2020). Centrifuges. A Practical Handbook for Drilling Fluids Processing, 475–488. doi:10.1016/b978-0-12-821341-4.00021-x
- Berk, Z. (2009). Centrifugation. Food Process Engineering and Technology, 217–232. doi:10.1016/b978-0-12-373660-4.00009-0
- https://www.ehs.uci.edu/sop/hazardous-operations/centrifuge-sop.pdf
- https://www.mls.be/en/p/lab-apparatus/centrifuges/centrifuges-hettich/hematocrit-centrifuges/
- https://www.sigmaaldrich.com/IN/en/technical-documents/technical-article/protein-biology/protein-pulldown/centrifugation-separations
- https://www.iqsdirectory.com/articles/centrifuge.html
- https://www.tec2med.com/centrifuge-rotor-rcf-rpm/
- https://www.toppr.com/guides/chemistry/is-matter-around-us-pure/centrifugation/
- https://www.biocompare.com/Lab-Equipment/Laboratory-Centrifuges/
- https://druckerdiagnostics.com/knowledge/how-a-centrifuge-works/
- https://study.com/academy/lesson/what-is-centrifugation-definition-process-uses.html
- https://www.genfollower.com/centrifuges-types-uses-in-laboratories/
- https://www.labmanager.com/product-focus/the-basics-of-centrifuge-operation-and-maintenance-1433
- https://www.coleparmer.in/c/centrifuges
- https://microbenotes.com/centrifuge-principle-parts-types-uses-examples
- https://www.labtron.com/centrifuge
- https://www.vedantu.com/chemistry/uses-of-centrifuge