The immune system is a complex network of organs, tissues, and cells that work together to keep the body healthy and protect it against outside intruders. Among the numerous components of the immune system, immune system cells play an important role in identifying and removing dangerous chemicals.
Primary lymphoid organs and secondary lymphoid organs are the two basic types of immunological organs. The major lymphoid organs are where lymphocytes, a type of white blood cell that plays an important role in immunological responses, form and mature. The thymus and bone marrow are examples of primary lymphoid organs.
Secondary lymphoid organs, on the other hand, are in charge of removing antigens (foreign substances) from tissues and vascular spaces. They also operate as meeting points for lymphocytes and antigens. Lymph nodes, spleen, tonsils, and mucosa-associated lymphoid tissue (MALT) are examples of secondary lymphoid organs. The lymphatic system and blood arteries connect these organs, forming a functional unit for immunological responses.
Various forms of white blood cells, known as leukocytes, can be found in the blood and lymph. These cells are the mainstays of the immune system, protecting the body from outside invaders. White blood cells are classified into four types: lymphocytes, neutrophils, monocytes, and macrophages.
Lymphocytes are a type of white blood cell with distinct properties such as diversity, specificity, memory, and self/nonself recognition. These characteristics are typical of adaptive immune responses, which enable the immune system to mount targeted defenses against specific antigens. T-lymphocytes, B-lymphocytes, and Natural Killer (NK) cells are the three types of lymphocytes.
T-lymphocytes, often known as T cells, are critical components of cell-mediated immunity. They detect and fight contaminated or abnormal cells in the body. B-lymphocytes, also known as B cells, are in charge of manufacturing antibodies, which are protein molecules that target and neutralize antigens. NK cells play a role in the innate immune response and are especially effective in destroying virus-infected and cancer cells.
Other types of white blood cells, in addition to lymphocytes, contribute to immune responses. Neutrophils are the most numerous form of white blood cell and play an important role in the first line of defense against bacterial infections via phagocytosis, which is the process of engulfing and eliminating foreign particles. Monocytes are progenitors to macrophages, which are multifunctional cells capable of engulfing and digesting foreign substances, dead cells, and detritus. Macrophages are also important in stimulating lymphocytes and secreting immune-effector chemicals.
Furthermore, the immune system generates necessary proteins to aid in its defense systems. Cytokines are protein molecules released by white blood cells that function as immunoregulators, modifying and coordinating immune responses. Antibodies are specialized proteins produced by B-lymphocytes that detect and attach to certain antigens, designating them for destruction by other immune cells. Complement proteins, which are activated by antibodies, also help the immune response by boosting pathogen death.
To summarize, the immune system’s cells are diverse and specialized, and they work together to protect the body against outside intruders. Lymphocytes, with their distinct properties, play a critical part in adaptive immune responses, whereas other white blood cells support and augment immune functions through phagocytosis, immune-effector molecule secretion, and antibody and complement protein synthesis. The intricate interplay of these cells, as well as their connections with immunological organs, ensures the body’s ability to develop effective immune responses and preserve overall health.
Cells of the Lymphoreticular System/Cells of the Immune System
It is important for the immune system to distinguish between its own molecules, cells, and organs (self) and those of foreign origin (non-self) (nonself). The innate immune system accomplishes this by expressing pattern recognition receptors encoded in the germline on the surface of its cells. These receptors recognise features on potentially invading bacteria. Adaptive immunity, on the other hand, employs epitope-specific T-cell receptors (TCRs) and B-cell receptors created somatically (BCRs). Prior to encountering antigens, these receptors are created randomly and anew in each T and B cell through gene recombination.
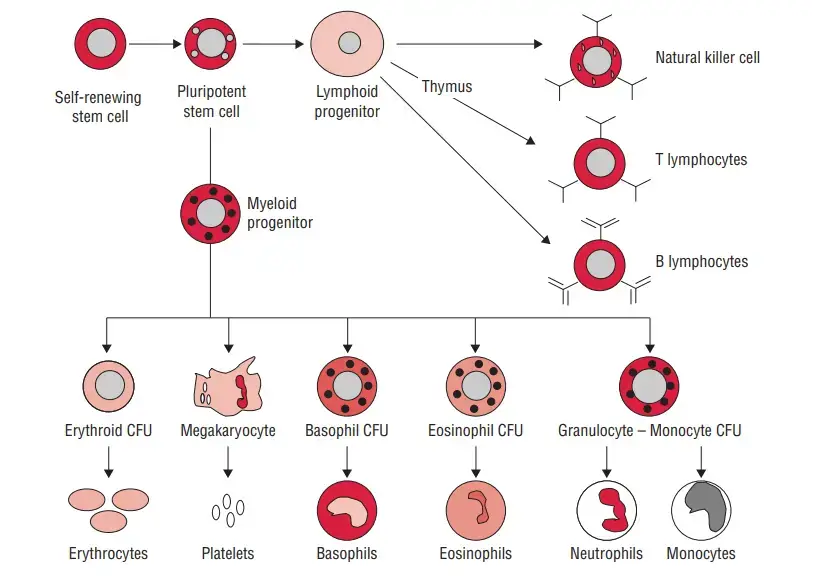
1. Lymphoid Cells
- Lymphocytes, a subset of white blood cells, are essential components of the immune system. They account for approximately 20%–40% of the body’s white blood cells and make up 99% of the cells found in the lymph. It is estimated that there are about 1011 lymphocytes present in the human body.
- These cells possess the remarkable ability to circulate continuously in both the blood and the lymph, allowing them to migrate into various body tissues and lymphoid organs. This mobility enables lymphocytes to integrate the immune system and respond to immune challenges effectively.
- Based on their functions and cell-membrane components, lymphocytes are broadly divided into three distinct populations:

a. Thymus-derived cells
- T lymphocytes, often known as T cells, are so named because the thymus plays a crucial role in their maturation.
- They are the essential components of adaptive immunity.
- In addition to participating directly in immune responses, they orchestrate and regulate the actions of other cells.
- 65–80% of the circulating population of tiny lymphocytes are T cells.
- They are present in the inner subcortical regions but not in the lymph node germinal centres.
- They have a longer lifespan than B lymphocytes (months or years).
- T lymphocytes can be induced to divide when exposed to particular mitogens, including phytohemagglutinin and concavalin A.
- Most human T cells contain surface receptors for sheep erythrocytes and the ability to form rosettes with them; this trait is used to identify T cells in a population of mixed cells.
- Two significant classes of T lymphocyte activities are as follows:
- Regulation of immune responses: Helper (CD4) T cells, which produce interleukins, play a vital role in the regulation of immunological responses.
- Different effector functions: The majority of effector activities are mediated by cytotoxic (CD8) T cells, which eliminate allografts, tumour cells, and virus-infected cells. T cells are split into two primary types, CD4 T cells and CD8 T cells, based on the CD4 and CD8 proteins present on their surface. CD4 or CD8 proteins are always present on mature T cells, but never both.
i. CD4+ T cells
- CD4 cells are also known as helper T (Th) cells. They constitute about 65% of peripheral T cells and are found mainly in the thymic medulla, tonsils, and blood. CD4 displayed on the surfaces of these T cells recognize a nonpeptide-binding portion of MHC class II molecules.
- Hence, CD4 T cells are restricted to the recognition of pMHC class II complexes.
- Helper T lymphocytes are involved in the induction and regulation of immune responses. CD4 T cells perform following helper functions:
- They facilitate the transformation of B cells into plasma cells.
- They facilitate the activation of CD8 T cells into cytotoxic T cells.
- They assist macrophages in mediating hypersensitivity reactions of the delayed kind.
- Th-1 cells and Th-2 cells, the two subpopulations of CD4 T cells, mediate these functions:
- By generating IL-2, Th-1 cells activate cytotoxic T lymphocytes. They contribute to the development of hypersensitive responses by generating IL-2 and gamma interferon mostly.
- Th-2 cells serve as B-cell helper cells by primarily generating IL-4 and IL-5.
- IL-12 and gamma interferon govern the equilibrium between Th-1 and Th-2 cells. Gamma interferon limits the formation of Th-2 cells, while IL-12 raises the number of Th-1 cells, hence enhancing the host’s defence against pathogens governed by a delayed hypersensitive reaction.
Main function of helper T cells
- Contribute to the antigen-specific activation of B and effector T lymphocytes.
- Th-1 cytokines induce cytotoxic inflammation and delayed sensitization.
- Th-2 cells aid in the synthesis of interleukins, which stimulate antibody formation, particularly IgE.
- Th-2 cytokines are associated with robust antibody and allergy response control.
ii. CD8+ T cells
- CD8 T cells are also referred to as cytotoxic T cells (Tc) and suppressor T cells (Ts).
- They represent roughly one-third of all mature CD3 cells. They are more prevalent in human bone marrow and intestinal lymphoid tissue.
- CD8 T glycoprotein expressed on the surface of these T cells recognises a part of MHC class I molecules that does not bind peptides.
- Consequently, CD8 T cells can only recognise pMHC class I complexes. CD8 T cells are predominantly cytotoxic.
- They eliminate (a) cells infected by a virus, (b) allograft cells, and (c) tumour cells.
- T-cell mediated cytotoxicity appears to be an apoptotic mechanism mediated by two distinct routes.
- One mechanism involves the release of perforin proteins, which insert themselves into the membranes of target cells to form channels. These channels allow granzymes, which are serine esterases, to diffuse into the cytoplasm. The precise mechanism by which granzymes induce apoptosis has not been determined, but it is Ca2-dependent.
- The alternative pathway is dependent on signals supplied by the cytotoxic cell to the target cell, which require direct cell-cell interaction. This route is independent of Ca2.
- The ratio of CD4 to CD8 T lymphocytes in normal human peripheral blood is approximately 2:1. This may be drastically affected by immunodeficiency diseases, autoimmune diseases, and other conditions.
Activation of T cells
- For the activation of helper T cells, the recognition of a complex on the surface of APCs, such as macrophages and dendritic cells, consisting of both the antigen and a class II MHC protein by TCR present on T cells is essential. Two signals are necessary for T cell activation:
- The initial signal required for the activation process is the interaction between the antigen and the MHC protein with the T-cell-receptor-specific antigen. Macrophage-secreted IL-1 is also required for effective helper T-cell activation.
- A costimulatory signal is the second signal necessary for T cell activation. In this signal, the B7 protein on APC must connect with the CD28 protein on T cell helper cells. Helper T cells release IL-2 in response to a costimulatory signal, which is essential for generating helper T cells capable of regulatory, effector, and memory activities.
- After T cell activation, a new protein termed CTLA-4 develops on the cell surface of T cells, displaces CD28, and binds to B7.
- CTLA-4 and B7 interact to limit T-cell activation via inhibiting IL-2 production. This maintains the quiescent state of T cells and thus plays a crucial function in T-cell homeostasis.
- Conversely, mutant T cells that lack CTLA-4 and so cannot be deactivated are more frequently associated with autoimmune illnesses.
iii. Memory T cells
Memory As their name suggests, T cells offer host immunity with the capacity to respond promptly and robustly for many years after initial exposure to a bacterium or other external agents. The memory generated in response to a particular antigen exhibits the following characteristics:
- Memory cells have a long lifespan or the ability to replicate.
- Due to the production of a large number of memory cells, the secondary response is strengthened and stronger than the main response.
- Memory cells are activated by less antigen and require less costimulation than naive and unactivated T cells. 4
- First-activated naive T cells release a smaller number of interleukins than activated memory T cells.
T-cell receptor
- Alpha and beta polypeptides make up the antigen-specific T-cell receptor (TCR). CD3 proteins are related with these two peptides.
- Each T cell has a unique TCR on its surface, meaning that each individual possesses hundreds of millions of distinct T cells.
- Both activated T cells and activated B cells create a large number of antigen-specific cells.
- In the following respects, T-cell alpha and beta polypeptides resemble immunoglobulin heavy chain.
- Multiple DNA regions are rearranged to generate the genes that code for T-cell polypeptides.
- V (variable), D (diversity), J (joining), and C (constant) segments rearrange to produce diversity, resulting in over 107 distinct receptor proteins.
- RAG-1 and RAG-2 are the genes encoding the recombinase enzymes that catalyse these gene rearrangements in T and B cells, respectively.
- However, T cells are distinct from immunoglobulins in the following ways:
- T cells have two immunoglobulin chains as opposed to four.
- T lymphocytes can only identify antigen in association with MHC proteins, whereas immunoglobulins can recognise antigens on their own.
Effect of superantigens on T cells
- Certain proteins, including staphylococcal enterotoxins, toxic shock syndrome toxins, and mouse mammary tumour virus, are referred to be superantigens.
- These are referred to as “super” because they activate a high number of helper T cells, as opposed to “antigens,” which only activate a few helper T cells.
- The superantigens play a crucial role in the development of Staphylococcus aureus-caused staphylococcal toxic shock syndrome.
- In this scenario, S. aureus-produced toxic shock syndrome toxin attaches directly to class II MHC proteins without undergoing internal processing.
- This toxin then interacts with variable component of the beta chain (V) of various T-cell receptors.
- T cell activation results in the release of interleukins, including IL-2 from T cells and tumour necrosis factor (TNF) from macrophages.
- These interleukins are responsible for many of the clinical manifestations reported in staphylococcal illnesses mediated by toxins.
Effector functions of T cells
T cells have two crucial roles: (a) cytotoxicity and (b) delayed hypersensitivity.
Cytotoxicity
T cell cytotoxicity is required largely for the destruction of virus-infected cells and tumour cells. In addition, it plays a significant role in graft rejection. By injecting perforins and granzymes (degrading enzymes) into the infected cell, by the Fas–Fas ligand (FasL) connection, and by antibody-dependent cellular cytotoxicity (ADCC mechanism), cytotoxic T cells eliminate virus-infected cells.
- By inserting perforins and granzymes: The insertion of perforins into the cells results in the creation of a channel through the membrane. This causes the loss of cell content and, ultimately, cell death. Granzymes are proteins that destroy cell membrane proteins, resulting in the loss of cell contents. These enzymes also trigger caspases, which results in cell death by apoptosis.
- By the Fas–Fas ligand (FasL) interaction: By interacting with FasL, virus-infected cells are eliminated by cytotoxic T cells. FasL is a protein found on the surface of several cells. FasL occurs on cytotoxic T cells when a cytotoxic TCR detects an epitope on the surface of virus-infected cells. When Fas and FasL connect, target cells die or undergo apoptosis. FasL interaction can also destroy target cells with NK cells.
- By antibody-dependent cellular cytotoxicity (ADCC): ADCC is capable of killing virus-infected cells. In this procedure, IgG and phagocytic cells work together to eliminate target cells. Antibody attached to the surface of infected cells is identified by the IgG receptor on the surface of phagocytic cells (e.g., macrophages, NK cells), resulting in the destruction of the infected cell. After destroying virus-infected cells, cytotoxic T cells remain unharmed and are able to continue killing other cells infected with the same virus. However, cytotoxic T cells have no effect on virus-free cells; they only affect virus-infected cells. By use of a mechanism known as immune surveillance, cytotoxic T cells eliminate tumour cells. Typically, new antigens emerge on the surface of many tumour cells. These antigens linked to class I proteins are detected by proliferating cytotoxic T lymphocytes stimulated by IL-2. The clone of cytotoxic T lymphocytes that result can kill tumour cells. In graft rejection, cytotoxic T cells also play a crucial role. Foreign cells’ class I MHC molecules are recognised by cytotoxic CD8 lymphocytes. Helper CD4 cells identify the foreign class II molecules on specific graft cells, such as macrophages and lymphocytes. The activated helper cells secrete IL-2, which stimulates the cytotoxic cells to form a clone that kills the transplant cells.
Delayed hypersensitivity
- CD4 cells, namely Th-1 subset cells and macrophages, mediate delayed hypersensitivity reactions against the antigens of numerous intracellular infections.
- Interleukins, including gamma interferon, macrophage activation factor, and macrophage inhibition factor, which mediate delayed hypersensitivity reactions, are produced by CD4 cells.
- Th-1 cells generate IL-12-gamma interferon, which activates macrophages and boosts their ability to fight Mycobacterium TB.
- Therefore, gamma interferon is crucial to the ability of host immunity to control infections produced by M. tuberculosis, Listeria monocytogenes, and other intracellular microorganisms.
- A lack of CMI renders a person extremely susceptible to infection by these bacteria.
Regulatory functions of T cells
T cells play a crucial function in the regulation of antibody synthesis and inhibition of specific immune responses.
- Regulation of antibody production: The production of antibodies by B cells can be (a) T-cell dependent, requiring the cooperation of helper T cells (T-cell-dependent response), or (b) independent of T cells (T-cell-independent response). In the T-cell-dependent response, all classes of immunoglobulins are produced, including IgG, IgM, IgA, IgE, and IgD. The T-cell-dependent reaction generates B-cell memories. Only IgM antibody is generated during the non-T-cell reliant response (T-cell-independent response). There are no memory cells produced by this response. Therefore, there is no secondary antibody reaction. In this response, multivalent macromolecules, such as bacterial capsule polysaccharide, are not properly digested and presented by APCs, and consequently do not activate helper T cells. Polysaccharides do not interact with class II MHC proteins, whereas peptide antigens do.
- Stimulation of helper and cytotoxic T cells to participate in the CMI: Antigen is processed by macrophages and delivered along with class II MHC molecules on the surface during CMI. These engage with the receptor on the helper T cells, activating them to release IL-2, a T-cell growth factor that encourages the proliferation and participation of particular helper and cytotoxic T cells in the CMI.
- Suppression of certain immune responses: It has been demonstrated that T cells prevent various immune-mediated illnesses in animals. Regulatory T cells (TR), also known as suppressor T cells, are a subgroup of T cells linked with inhibiting specific immunological responses. TR cells, also known as suppressor T cells, exhibit the CD25 marker and account for 5–10% of CD4 cells. Unknown is the precise method through which regulatory cells decrease the immune response. An imbalance in the number or activity of CD4 and CD8 cells also impairs the host’s cellular immune response.
2. Bone marrow-derived cells
- The B lymphocyte derives its letter identification from its place of maturation in the bursa of Fabricius in birds; nevertheless, bone marrow is its primary site of maturation in numerous mammalian species, such as humans and mice.
- By synthesising and displaying membrane-bound immunoglobulin (antibody) molecules that function as antigen receptors, mature B cells are conclusively separated from other lymphocytes.
- Each of the approximately 1.5 x 105 antibody molecules on the membrane of a single B cell has an identical antigen-binding site.
- The following molecules are also expressed on the membrane of mature B cells: B220 (a variant of CD45) is commonly employed as a marker for B cells and their progenitors.
- Unlike antibody, however, it is not expressed exclusively by B-lineage cells.
- Class II MHC molecules allow B cells to behave as antigen-presenting cells (APC).
- Both CR1 (CD35) and CR2 (CD21) are complement product receptors.
- FcRII (CD32) is the receptor for the antibody IgG.
- B7-1 (CD80) and B7-2 (CD86) are molecules that interact with CD28 and CTLA-4, which are essential regulatory molecules on the surface of various T cell subtypes, including TH cells.
- CD40 is a molecule that interacts with CD40 ligand on helper T cell surfaces. In the majority of instances, this contact is essential for the survival of antigen-stimulated B cells and their differentiation into antibody-secreting plasma cells or memory B cells.
- The interaction between antigen and membrane-bound antibody on a mature, naive B cell, as well as interactions with T cells and macrophages, produces selective activation and differentiation of B-cell clones with the matching specificity.
- In this phase, the B cell divides and differentiates multiple times over the course of four to five days, establishing a population of plasma cells and memory cells.
- Plasma cells, which contain less antibody attached to their membranes than B cells, produce and secrete antibody.
- All clonal descendants of a given B cell secrete antigen-binding-specific antibody molecules. Plasma cells are terminally differentiated cells, and the majority of them die within one to two weeks.
3. Natural Killer Cells
- In 1976, it was demonstrated that the body harbours a tiny population of large, granular lymphocytes that exhibit cytotoxic activity against a wide variety of tumour cells in the absence of prior inoculation with the tumour.
- NK cells were subsequently demonstrated to play a crucial part in the host’s defence against tumour cells and certain virus-infected cells, but not all.
- These cells, which make up 5–10% of lymphocytes in human peripheral blood, lack the membrane components and receptors that differentiate T- and B-cell lineages.
- Despite the absence of T-cell receptors or immunoglobulin in their plasma membranes, NK cells can recognise prospective target cells in two distinct ways.
- In certain instances, NK cells use NK cell receptors to identify anomalies, such as a reduction in the presentation of class I MHC molecules and the atypical profile of surface antigens shown by some tumour cells and cells infected with specific viruses.
- Some tumour cells and cells infected by certain viruses display antigens against which the immune system has produced an antibody response, so that antitumor or antiviral antibodies are linked to their surfaces, allowing NK cells to identify potential target cells.
- Due to the fact that NK cells contain CD16, a membrane receptor for the carboxy-terminal end of the IgG molecule, also known as the Fc region, they can bind to these antibodies and then destroy the target cells.
- This is an illustration of antibody-dependent cell-mediated cytotoxicity (ADCC).
- Several observations indicate that NK cells play a crucial role in the host’s antitumor response. In humans, the autosomal recessive Chediak-Higashi syndrome is associated with impaired neutrophils, macrophages, and NK cells as well as an increased prevalence of lymphomas.
- Similarly, animals with the autosomal mutation beige lack NK cells; these mutants are more prone to tumour formation following injection with live tumour cells than are normal mice.
- The NK1-T cell is increasingly recognised as a cell type that shares characteristics with both T cells and NK cells.
- NK1-T cells, like T cells, possess T cell receptors (TCRs). Unlike other T cells, NK1-T cell TCRs engage with CD1 molecules, which are MHC-like molecules, rather than class I or class II MHC molecules.
- Similar to NK cells, they exhibit varying amounts of CD16 and other NK-specific receptors and can kill cells.
- A population of activated NK1-T cells can rapidly release significant quantities of the cytokines required to stimulate antibody synthesis by B cells, inflammation, and the growth and expansion of cytotoxic T cells.
- Some immunologists see this cell type as a form of quick response mechanism that has developed to provide assistance while traditional TH responses are still maturing.
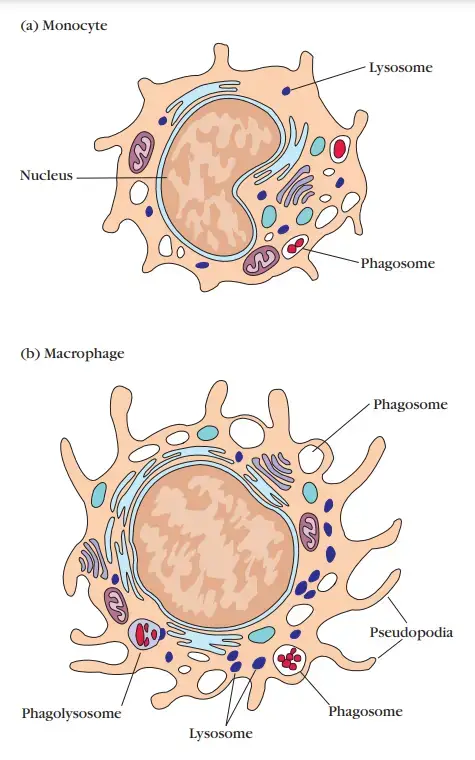
Mononuclear Phagocytes
- The mononuclear phagocytic system is composed of circulating monocytes and tissue-dwelling macrophages.
- During hematopoiesis in the bone marrow, granulocyte-monocyte progenitor cells develop into promonocytes, which then leave the bone marrow and enter the blood, where they mature into monocytes.
- Monocytes circulate in the bloodstream for around eight hours, during which time they expand; they then move into the tissues and develop into tissue-specific macrophages or, as will be detailed further on, dendritic cells.
- The transformation of a monocyte into a tissue macrophage requires several alterations: The cell increases in size by five to tenfold, the quantity and complexity of its intracellular organelles grow, and it has enhanced phagocytic ability, produces more hydrolytic enzymes, and begins to exude a variety of soluble substances.
- Macrophages are distributed over the body. Others stay motile and are referred to as free or wandering macrophages because they are mobile.
- Free macrophages traverse the tissues by amoeboid migration. Macrophage-like cells serve distinct tasks in various tissues and are designated by their tissue location. Lung macrophages are alveolar
- Histiocytes in connective tissues.
- Kupffer cells in the liver.
- Mesangial cells in the kidney
- Microglial cells in the brain
- Osteoclasts in bone
- In the course of an immune response, macrophages are triggered by a number of stimuli notwithstanding their normal resting state.
- Particle antigen phagocytosis serves as the initial activation stimulation. Nevertheless, cytokines released by activated TH cells, mediators of the inflammatory response, and bacterial cell wall components might further stimulate macrophage activity.
- Interferon gamma (IFN-), which is released by activated TH cells, is one of the most powerful macrophage activators.
- Activated macrophages are more effective than resting macrophages at eliminating potential pathogens due to their increased phagocytic activity, ability to destroy ingested microorganisms, release of inflammatory mediators, and capacity to activate T cells.
- In addition, activated macrophages, but not resting ones, secrete several cytotoxic proteins that enable them to kill a wide variety of pathogens, including as virus-infected cells, tumour cells, and intracellular bacteria.
- Activated macrophages also express greater amounts of class II MHC molecules, allowing them to function as antigen-presenting cells more efficiently.
- During the immunological response, macrophages and TH cells encourage each other’s activation.
Granulocytic Cells
- On the basis of their cellular morphology and cytoplasmic staining features, granulocytes can be classed as neutrophils, eosinophils, or basophils.
- The neutrophil possesses a multilobed nucleus and granulated cytoplasm that stains with both acidic and basic dyes; it is commonly referred to as a polymorphonuclear leukocyte (PMN) due to its multilobed nucleus.
- The eosinophil has a bilobed nucleus and cytoplasm that stains with eosin red (hence its name).
- The basophil has a nucleus with lobes and cytoplasm that stains with methylene blue, a basic dye.
- Neutrophils and eosinophils are both phagocytic, unlike basophils. Neutrophils, which account for 50–70% of the white blood cells in circulation, are far more prevalent than eosinophils (1–3%) or basophils (1%)
1. Neutrophils
- Neutrophils are created in the bone marrow during hematopoiesis. They are discharged into the peripheral circulation, where they circulate for 7–10 hours before migrating into the tissues, where they live for only a few days.
- In response to numerous types of infections, the bone marrow releases an abnormally high number of neutrophils, which are typically the first cells to reach a site of inflammation.
- Leukocytosis, the resultant transitory rise in the number of circulating neutrophils, is a medical indicator of infection.
- The movement of circulating neutrophils into tissues, known as extravasation, involves a series of steps: first, the cell adheres to the vascular endothelium, then it penetrates the space between adjacent endothelial cells lining the vessel wall, and finally it penetrates the vascular basement membrane, moving into the tissue spaces.
- A number of chemicals produced during an inflammatory response serve as chemotactic agents that increase neutrophil accumulation at the site of inflammation.
- Among these chemotactic factors are certain complement components, blood-clotting system components, and a number of cytokines released by activated TH cells and macrophages. Neutrophils, like macrophages, are active phagocytic cells.
- Phagocytosis by neutrophils is comparable to that described for macrophages, with the exception that lytic enzymes and bactericidal chemicals are housed within primary and secondary granules.
- Primary granules are a form of lysosome containing peroxidase, lysozyme, and numerous hydrolytic enzymes.
- The secondary granules, which are smaller, contain collagenase, lactoferrin, and lysozyme. Similar to macrophages, both main and secondary granules fuse with phagosomes, whose contents are ultimately digested and removed.
- Neutrophils create antimicrobial compounds via both oxygen-dependent and oxygen-independent mechanisms.
- Neutrophils are far more likely than macrophages to eliminate ingested pathogens.
- Neutrophils have a greater respiratory burst than macrophages, allowing them to produce more reactive oxygen and reactive nitrogen intermediates.
- In addition, neutrophils have higher defensin levels than macrophages.

2. Eosinophils
- Similar to neutrophils, eosinophils are mobile phagocytic cells that can migrate from the blood into tissue voids.
- It is believed that they have a role in the defence against parasitic organisms, although their phagocytic function is substantially less essential than that of neutrophils.
- The contents of eosinophilic granules may cause membrane damage to the parasite.
3. Basophils
- Basophils are nonphagocytic granulocytes whose cytoplasmic granules release pharmacologically active chemicals.
- These chemicals have a significant role in a variety of allergic reactions.
4. Mast Cells
- Mast-cell precursors are released into the blood as undifferentiated cells; they do not differentiate until they leave the circulation and enter the tissues.
- The epidermis, connective tissues of numerous organs, and mucosal epithelial tissue of the respiratory, genitourinary, and digestive systems all include mast cells.
- These cells, like circulating basophils, contain many cytoplasmic granules containing histamine and other pharmacologically active chemicals.
- Together with blood basophils, mast cells play a significant role in the development of allergies.
5. Dendritic Cells
- The dendritic cell (DC) derives its name from its membrane extensions, which resemble nerve cell dendrites.
- Dendritic cells can be challenging to isolate due to the tendency of traditional cell separation techniques to harm their lengthy extensions.
- The introduction of isolation procedures involving enzymes and softer dispersion has aided the in vitro isolation of these cells.
- There are numerous varieties of dendritic cells, however the majority of mature dendritic cells have the same primary function, which is antigen presentation to TH cells.
- Langerhans cells, interstitial dendritic cells, myeloid cells, and lymphoid dendritic cells are the four known types of dendritic cells.
- Each originates from hematopoietic stem cells via distinct mechanisms and distinct sites.
- Despite their variations, they all express significant quantities of class II MHC molecules and co-stimulatory B7 family members constitutively.
- Therefore, they are more effective antigen-presenting cells than macrophages and B cells, which must be activated before they can operate (APCs).
- By phagocytosis or endocytosis, immature or precursor forms of each of these dendritic cell types acquire antigen; the antigen is processed, and adult dendritic cells present it to TH cells.
- Following microbial invasion or during inflammation, mature and immature forms of Langerhans cells and interstitial dendritic cells migrate into draining lymph nodes, where they offer antigen to TH cells, which is essential for the initiation of immune responses by these important cells.
- The follicular dendritic cell is not derived from bone marrow and has a distinct role than the antigen-presenting dendritic cells described previously.
- The absence of class II MHC molecules on follicular dendritic cells precludes their role as antigen-presenting cells for TH-cell activation.
- These dendritic cells were given their name due to their exclusive presence in B-cell-rich lymph node formations known as lymph follicles.
- Despite the fact that they do not express class II molecules, follicular dendritic cells exhibit a significant number of membrane receptors for antibodies, allowing for the binding of antigen-antibody complexes.
- This interaction between B cells and the attached antigen can have significant impact on B cell responses.
References
- https://www.cancer.gov/publications/dictionaries/cancer-terms/def/immune-cell#:~:text=Immune%20cells%20develop%20from%20stem,B%20cells%20and%20T%20cells).
- https://primaryimmune.org/immune-system-and-primary-immunodeficiency
- https://www.medicalnewstoday.com/articles/320101
- https://www.healio.com/hematology-oncology/learn-immuno-oncology/the-immune-system/components-of-the-immune-system
- https://www.healio.com/hematology-oncology/learn-immuno-oncology/the-immune-system/components-of-the-immune-system
- https://info.gbiosciences.com/blog/cells-of-the-immune-system
- https://www.ncbi.nlm.nih.gov/books/NBK27092/
- https://www.ibiology.org/immunology/cells-immune-system/
- https://www.betterhealth.vic.gov.au/health/conditionsandtreatments/immune-system
- https://my.clevelandclinic.org/health/articles/21196-immune-system
- https://www.ncbi.nlm.nih.gov/books/NBK21070/
- https://medlineplus.gov/ency/article/000821.htm
- https://www.hopkinsmedicine.org/health/conditions-and-diseases/the-immune-system
- https://www.chop.edu/centers-programs/vaccine-education-center/human-immune-system/parts-immune-system
- https://www.msdmanuals.com/en-in/home/immune-disorders/biology-of-the-immune-system/overview-of-the-immune-system
- https://www.intechopen.com/books/8798
- https://www.khanacademy.org/science/high-school-biology/hs-human-body-systems/hs-the-immune-system/a/hs-the-immune-system-review