What is Bright Field Microscope?
Bright field microscope is an optical microscope that produce a dark image of the specimen against a bright background, and it is the most common compound light microscope used in biological laboratories. It is the system where the specimen is illuminated from below by a uniform beam of light, and the light pass directly through the specimen so the contrast is formed mainly by differential absorption and differential refraction of the transmitted light.
It is the process in which the stained specimen show more absorption because the refractive index of the stained parts is higher than the surrounding medium, so the image appear darker than the bright field around it.
The microscope is specially designed with a light source, condenser, objective lenses and eyepiece lenses, and these parts together modify the transmitted light to produce the magnified image. It is the condenser that focus the beam of light onto the slide, and this step is important for forming a high-resolution image because the specimen must be uniformly illuminated.
The objective lens is made up of several glass lenses which enlarge and collect the transmitted rays, and then the eyepiece further magnify the image for viewing. It is the standard microscope used to observe fixed and live samples, but staining is usually required for proper contrast because unstained transparent cells absorb very little light and show very poor visibility. In many biological preparations the specimen is first stained with basic dyes, because the staining introduce color, and this color provide a clear refractive difference from the surrounding medium.
The specimen is placed on the stage, often covered with a coverslip, and can be viewed under low or high power objectives, including oil immersion when needed. The image produced is bright field because the background appear bright, and the specimen appear dark, and the quality of the image is controlled by the diaphragm and condenser adjustment which regulate the amount and angle of light entering the objective lens.
It is mainly used to view cells, tissues, microorganisms and other microscopic structures in routine laboratory studies, and it remains the most essential and widely used transmitted-light system for basic biological examination.
Bright Field Microscope Definition
The Brightfield Microscope, often termed the Compound Light Microscope, is an optical instrument that utilizes light rays to produce a dark image against a bright background, primarily used in biological studies to observe stained specimens.
Principle of Brightfield Microscope

The principle of a bright field microscope is based on transmitted light, where a uniform beam of visible light is passed from the light source to the condenser and then through the specimen so the image is formed mainly by differential absorption of light. It is the process in which different parts of the specimen attenuate the incoming light to different degrees because of variation in thickness, density or composition, and these regions absorb more light so they appear darker against the bright background of the field.
The condenser focus the light onto the specimen placed on the stage, and the transmitted light then enter the objective lens which is the primary magnifying part, and later the image is further enlarged by the eyepiece for viewing. It is a simple light path that does not require any special component because the microscope rely completely on the contrast formed by absorption and refraction inside the specimen.
The limitation of this principle is that most living and transparent biological material do not absorb much light, so the contrast produced is very low and the specimen is not clearly visible unless stained. Staining introduce artificial color and increase the absorption contrast which allow the structural details to be seen clearly. For optimum result, the microscope must be aligned by Köhler illumination so the specimen plane receive uniform and glare-free illumination which help in improving the resolution and contrast.
Light Path of Brightfield Microscope
In Brightfield Microscope the illumination light travel through different optical parts until the final image is produced. In this Microscope the transillumination light source is placed at the base of the microscope stand and the beam is directed upward in a uniform manner although slight fluctuation in brightness is sometimes noticed due to the condition of the lamp.
At the beginning of this step the light moves toward the condenser lens which is fitted just beneath the stage. The aperture diaphragm of the condenser adjust the cone of illumination so that more focused rays strike the stained specimen surface. It is known that small misalignment of the condenser can influence the clarity of the field.
The stained specimen kept on the stage is usually placed under a coverslip or under an oil immersion interface. During this process the denser or pigmented region absorb part of the beam and this differential absorption is the reason for contrast formation in brightfield observation. These are required for distinguishing the structural region of the specimen.
After the light passes through the specimen it is collected by the objective lens placed above the stage. The objective forms the enlarged intermediate image and it is sensitive to small shifting of the focal plane and sometimes the field is slightly distorted when the slide is tilted.
In the next step this intermediate image is transmitted upward through the body tube to the ocular lens or to the camera system. The overall quality in this step is affected when dust particles or smear is present on the lens giving stray glare on the formed image.
This light-path is referred to as a simple optical path but it is connected with the use of Critical or Köhler illumination. Köhler illumination is the method which is applied for obtaining more uniform field brightness although any method can be used depending on the detail the observer want to visualize.
Parts of Brightfield Microscope
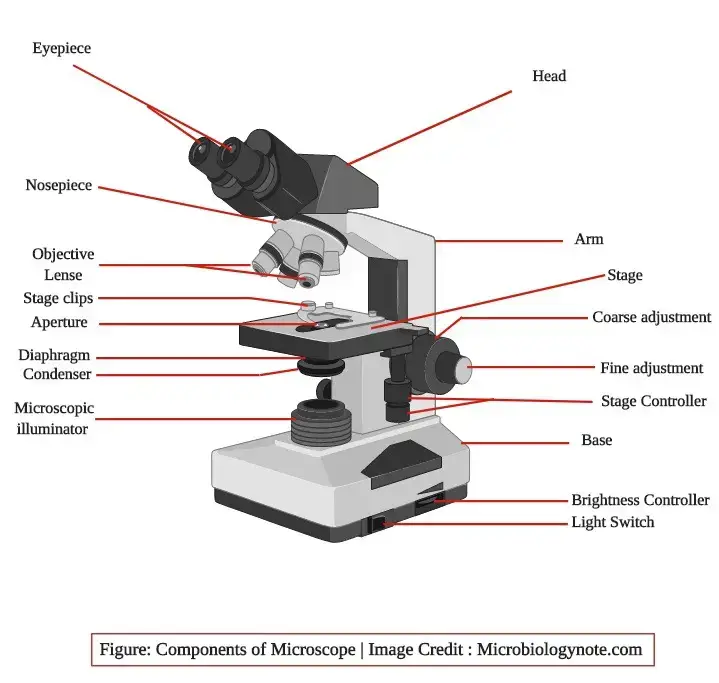
The brightfield microscope is made up of different parts which together help in forming and viewing the image. These are arranged in such a way that the light is focused on the specimen and the magnified image is seen through the eyepiece.
1. Eyepiece (Ocular lens)
It is present at the top of the microscope and it has one or two lenses which focus the image formed by the objective lens. This is the part from where the observer see the final image.
2. Objective lenses
These lenses are attached to the revolving nosepiece and each objective is made up of several glass lenses. It is the part which make the clear enlarged image from the specimen and different magnifying power is used depending on the requirement.
3. Focusing knobs
There are two focusing knobs known as coarse adjustment knob and fine adjustment knob. These are found on the arm of the microscope. The coarse adjustment knob move the stage or the nosepiece for initial focusing while the fine adjustment knob is used to get a sharp image.
4. Stage
The stage is located just below the objectives and the specimen slide is placed on it. The stage allow movement of the slide in different directions and the light is concentrated on this part.
5. Condenser
The condenser is mounted below the stage. It focus the beam of light onto the specimen. The condenser may be fixed or movable and the adjustment of the condenser decide the quality of illumination.
6. Arm
The arm is a strong metallic support which join the base and the upper parts of the microscope. It is used to hold and carry the microscope from one place to another and it support the whole structure.
7. Light illuminator or mirror
The illuminator is generally found on the base and it provide the light needed for observation. In some microscopes a mirror is used in place of the illuminator and it reflect external light to the condenser.
8. Nosepiece
The nosepiece hold two to five objective lenses and it can rotate to bring any lens in position. It help in selecting the required magnification for viewing the specimen image.
9. Aperture diaphragm
This diaphragm is present below the condenser and it control the diameter of the beam of light which pass into the condenser. When it is nearly closed the contrast of the image is high and when it is open widely the image appears bright with low contrast.
Total Magnification power of Brightfield Microscope
The combined magnifying power produced when the ocular lens and the objective lens work together. The total magnification is obtained by multiplying the power of the eyepiece with the power of the objective lens which is used at that time. This is referred to as the basic rule for calculating magnification in a brightfield microscope.
The ocular lens generally has 10X magnifying power and this value remain constant in most microscopes. The objective lenses are placed on the revolving nosepiece and they have different magnification like 4X, 10X, 40X and 100X. These are the lenses which form the primary enlarged image from the specimen.
In this system the total magnification is calculated as
Total magnification = Ocular lens power × Objective lens power
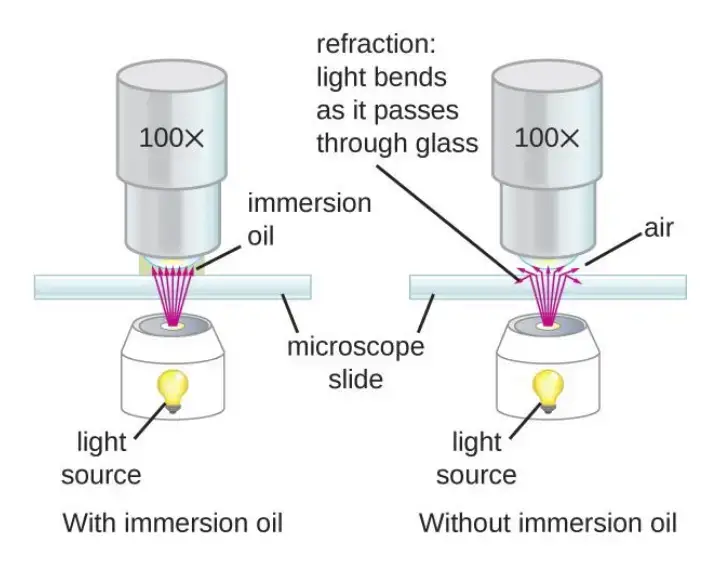
and the magnification change depending on the selected objective. For example, when 4X objective is used, the total magnification is 40X. At 10X objective the magnification is 100X, and at 40X objective the magnification is 400X. The highest magnification is obtained by using the oil immersion lens which is 100X and it gives 1000X total magnification.
It is known that some objectives may have higher power, but the practical limit for clarity in brightfield microscope is around 1300X. Beyond this limit the resolution is restricted because the wavelength of visible light can only resolve structures up to about 200 nm and further magnification does not give additional detail.
Operating Procedure of Brightfield Microscope
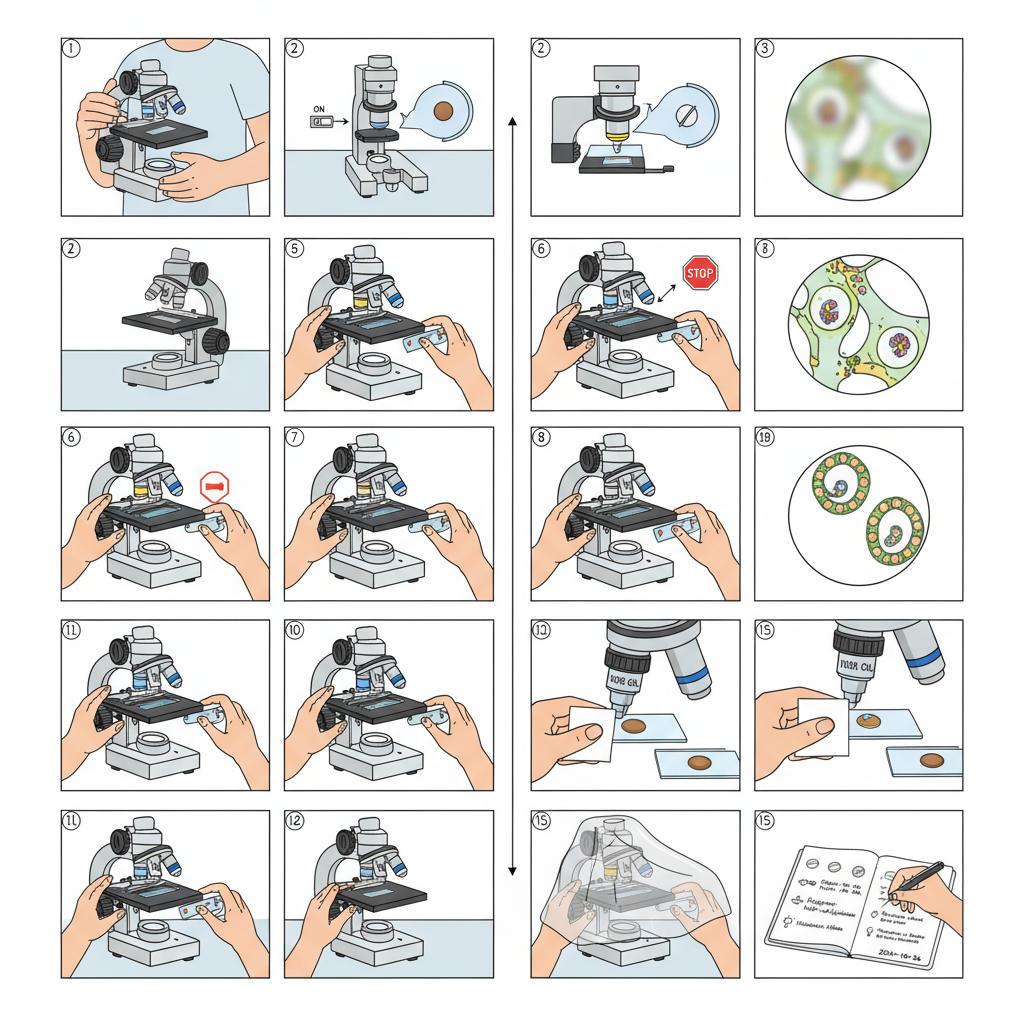
- The microscope is carried by using two hands, one hand holding the arm and the other supporting the base. It is then placed on a stable bench so that vibration does not disturb the observation.
- The illuminator is switched on and the light intensity is kept at a moderate level. When a mirror is used, it is adjusted to reflect sufficient light. Too bright illumination can wash out the contrast and too dim light decrease visibility.
- The condenser is kept in its starting position, usually in a lowered or central level. The aperture diaphragm is opened halfway so that a balanced cone of light is obtained at the beginning.
- The clean slide containing the specimen is placed on the stage and fixed with the stage clips. The required region of the specimen is kept over the central opening of the stage.
- The nosepiece is rotated to bring the low power objective (4X or 10X) into position and the lens is checked so that it clicks into place. These objectives are parfocal and the focus is roughly maintained during shifting.
- The coarse adjustment knob is used to move the stage slowly upward. When the specimen begins to form a fuzzy image, the movement is stopped in order to avoid hitting the slide with the objective.
- The fine adjustment knob is then used to sharpen the image. Slight changes in condenser height and diaphragm opening is made because small adjustments improve clarity of the field.
- The specimen is centered by using the X–Y stage controls. If the sample is unstained or thin, the illumination may be reduced or the diaphragm may be slightly closed to produce more contrast.
- When higher magnification is required, the next objective is selected, such as 40X. Only the fine adjustment knob is used for focusing because the parfocal arrangement already keeps the specimen nearly in focus.
- If oil immersion is required (100X objective), the lens is rotated into position first. A drop of immersion oil is placed on the coverslip. The oil immersion lens is lowered gently into the oil and then focused with fine adjustment only because coarse focusing can damage the lens.
- For imaging through the eyepiece or camera, the diopter adjustment is made until the virtual image becomes sharp. Illumination is also balanced so that the entire field is evenly bright. Köhler illumination is used whenever available.
- After observation the oil immersion objective is lifted from the oil. The oil is cleaned from the lens and slide using lens tissue. The microscope is returned to a low power objective before the slide is removed.
- The lamp intensity is reduced and the illuminator is switched off. The stage is lowered and the slide is removed. The instrument is cleaned properly, then covered with a dust cover and kept in a dry place.
- Important observation settings like objective used, condenser level, diaphragm opening and illumination condition are recorded in the lab note because these details help in future comparison of results.
Application of Brightfield Microscope
The brightfield microscope is mainly used for general observation of stained and unstained specimens. It is the most common microscope used in teaching and laboratory work because it provide a simple method for viewing cellular structures.
- It is applied for examining tissues, cells and microorganisms which are stained with basic dyes. The staining increase the contrast so that different parts of the specimen is clearly visible.
- Bacteria, fungi and protozoa present in cultures are viewed under this microscope to check their shape, arrangement and general morphology. It is the routine tool in microbiology laboratory.
- Sections of plant tissues like root tip, leaf epidermis or animal tissues like blood smear, muscle fibers and epithelial cells are commonly studied because the microscope show their basic structure.
- Blood films, urine sediments, sputum smears and other clinical samples are examined for the presence of cells, parasites or crystals. It is the first step of many diagnostic procedures.
- It is used to estimate cell number in simple counting chambers. Some microorganisms or cells are identified based on their general appearance.
- In laboratory experiments, this microscope is used for checking slide preparation, studying mitosis stages, observing temporary mounts and viewing small organisms like algae or pollen grains.
- It is the most widely used instrument in schools and colleges for teaching the basic principles of microscopy. Students learn image formation, focusing and identification of general specimens.
Advantages of Brightfield Microscope
- It is easy to operate and does not require complex adjustment for routine observation.
- The instrument is inexpensive compared to other advanced microscopes and is commonly available.
- Stained specimens show clear contrast, helping in identifying different structures.
- The microscope allow observation of both living and fixed specimens prepared on simple glass slides.
- It produce a natural colour appearance of the specimen when unstained preparations are used.
- The focusing system is simple, and the image can be viewed quickly for teaching and demonstration work.
- Maintenance of the microscope is easy because the parts are durable and do not need frequent calibration.
- It is suitable for general biological studies like morphology, cell arrangement and tissue sections.
Limitations of Brightfield Microscope
- It cannot provide clear details of transparent or unstained specimens because the contrast is very low.
- The resolution is limited by the wavelength of visible light, so very fine structures cannot be distinguished.
- High magnification often results in dim image and requires strong illumination which may wash out important details.
- The microscope does not show depth information, and thick specimens appear blurred.
- Most internal structures of cells cannot be seen without staining, and staining may sometimes distort the specimen.
- It is not suitable for observing very small microorganisms like viruses because their size is below the resolving limit.
- Brightfield viewing is affected by glare, dust and improper light adjustment, which reduce image clarity.
Difference between dark field and bright field Microscope

Bright Field Microscope
- It uses direct light which pass through the specimen to form the image.
- The background appear bright while the specimen appear darker because of staining or absorption.
- It is mainly used for stained specimens or naturally pigmented samples.
- Internal structures are not clearly visible in unstained preparations as the contrast is low.
- Illumination and focusing system is simple and easy to operate in general laboratory work.
- It is suitable for routine observation of cells, tissues, microorganisms and prepared slides.
Dark Field Microscope
- It uses oblique light which does not enter the objective directly but only the light scattered by the specimen is collected.
- The background appear completely dark while the specimen appear bright or glowing.
- It is used for observing unstained, transparent and living specimens.
- Very thin structures like spirochetes become clearly visible because scattering of light increase contrast.
- The condenser arrangement is special and require careful alignment for proper viewing.
- It is suitable for examining small particles, microorganisms and delicate structures which cannot be seen clearly under bright field.
| Bright field Microscope | Dark field Microscope |
|---|---|
| Background appears bright, and most transmitted light is collected, which sometimes cause’s low contrast for unstained specimen’s. | Background appear’s black because only scattered light reach the Objective, producing a glowing outline, which leads to Strong edge visibility. |
| Direct illumination passes through sample; staining is usually needed for clarity, however illumination may look uneven. | Oblique Rays are utilized, and a special stop/disc block’s central light, forming a dark background even when sample is transparent. |
| It shows general morphology but fine transparent structures become hard to see, resulting in washed-out details. | It highlights very small structures (e.g. thin bacteria), although glare halos may occur in thick specimen’s. |
| Condenser is centrally aligned (NA matched), and the Optical Path is simple. | Condenser uses Dark field stop, in to change the angle of light, creating scattered-beam Imaging. |
| Works reasonably with thicker tissues, though clarity drops. | Works best for thin specimen’s, because thick layers create halo / noise artifacts, and the field flicker’s easily. |
| Photomicrography is easy, exposure stable and also predictable. | Photomicrography require’s careful tuning— sometimes overexposed Glare appears, producing noisy images. |
| Common in teaching labs, routine histology etc.. | Useful for delicate organisms like spiral-shaped Treponema pallidum, giving a look into motility that bright field rarely capture’s. |

FAQ
Why is it called bright field microscope?
A bright field microscope is so named because it illuminates the specimen from below, causing the field of view to appear bright against a dark background. This type of microscope is the most basic and most commonly used, and it is suitable for examining transparent or semi-transparent specimens that are not naturally fluorescent. The light source is usually a lamp or an LED, and the specimen is placed on a transparent glass stage. The image is formed by light that is transmitted through the specimen and then focused by the objective lens and eyepiece. The brightness of the field depends on the intensity of the light source and the transparency of the specimen.
Which of the following can be examined with an ordinary bright-field microscope?A. Wet mountsB. Heat-fixed specimensC. Chemical-fixed specimensD. All of the choices are correct.
D. All of the choices are correct.
What is bright field microscope?
A bright field microscope is a type of optical microscope that uses visible light to create an image of a specimen. It is called a bright field microscope because the field of view appears bright against a dark background. This is achieved by illuminating the specimen from below and using a condenser lens to focus the light onto the specimen. The light is then transmitted through the specimen and focused by the objective lens and eyepiece, creating an image that can be viewed through the eyepieces or captured with a camera.
Bright field microscopes are the most basic and most commonly used type of microscope. They are suitable for examining transparent or semi-transparent specimens that are not naturally fluorescent, such as biological tissues, cells, and microorganisms. Bright field microscopes can be used to observe the morphological features of a specimen, such as its shape, size, and internal structure. They are widely used in research, education, and industry for a variety of applications, including biological research, pathology, and quality control.
How does a bright-field microscope form its image?
A bright field microscope forms its image by using visible light to illuminate the specimen from below and then focusing the transmitted light onto a viewing plane or detector. The light is first directed through a condenser lens, which focuses it onto the specimen. The light then passes through the specimen and is focused by the objective lens onto the eyepiece or camera detector.
The objective lens is a high-powered lens that is mounted on a rotating nosepiece, allowing the user to switch between different magnifications. The eyepiece, also known as the ocular lens, magnifies the image further and allows the user to view it. The eyepieces are usually mounted on a binocular or trinocular head, which allows the user to view the image through both eyes or through one eye while using a camera to capture the image.
The brightness of the field depends on the intensity of the light source, the transparency of the specimen, and the quality of the optics. The contrast of the image can be enhanced by using special stains or dyes to highlight specific features of the specimen.
Bright Field microscope image


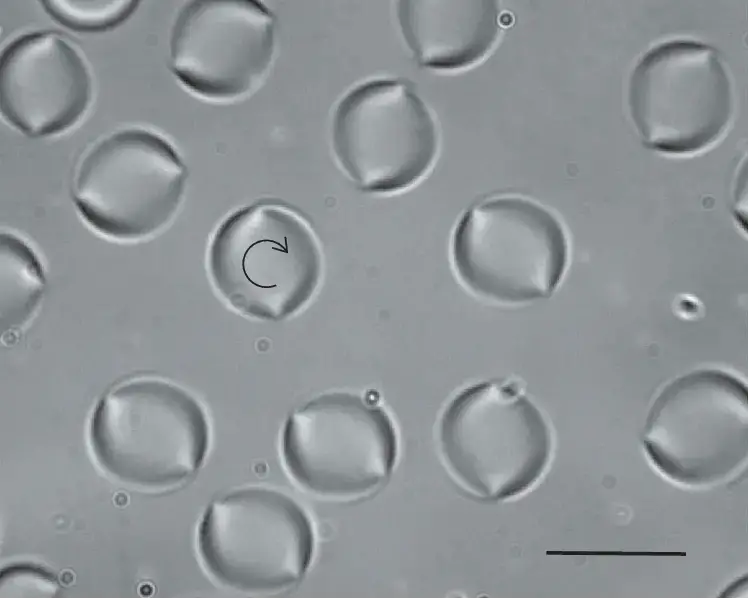
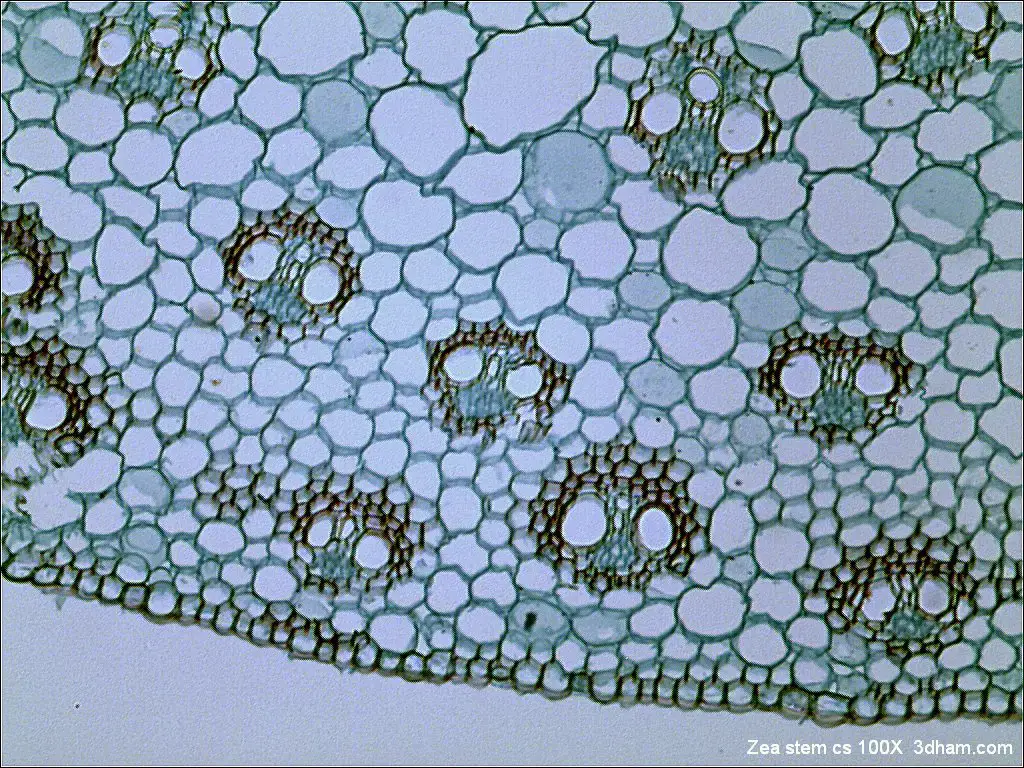
- https://www.microscopemaster.com/brightfield-microscopy.html
- https://en.wikipedia.org/wiki/Bright-field_microscopy
- https://www.med.unc.edu/microscopy/files/2018/06/lm-ch-8-bright-field.pdf
- https://www2.hawaii.edu/~johnb/micro/m140/syllabus/week/handouts/m140.2.4.html
- https://www.rsc.org/publishing/journals/prospect/ontology.asp?id=CMO:0002529&MSID=C2LC21116E
- https://science.umd.edu/CBMG/faculty/wolniak/wolniakmicro.html