What are Biomolecules?
- Biomolecules are fundamental molecules produced by living organisms that play a crucial role in sustaining life. These molecules primarily consist of organic compounds, which means they typically contain carbon atoms covalently bonded to other elements, including hydrogen, oxygen, and nitrogen. The four major categories of biomolecules are polysaccharides, proteins, nucleic acids (such as DNA and RNA), and lipids. Each of these biomolecule groups has distinct structures and functions that contribute to the biological processes essential for life.
- Polysaccharides are complex carbohydrates formed by long chains of monosaccharides, which serve as energy sources and structural components. For instance, starch and glycogen are vital energy storage forms, while cellulose provides structural support in plant cell walls. Proteins, another significant class of biomolecules, are composed of amino acids linked by peptide bonds. They perform a myriad of functions, including catalyzing biochemical reactions as enzymes, facilitating communication as hormones, and providing structural support as collagen in connective tissues.
- Nucleic acids, which include DNA and RNA, are polymers of nucleotides that store and transmit genetic information. DNA encodes the instructions for building and maintaining an organism, while RNA plays various roles in protein synthesis and gene expression. Lastly, lipids, which include fats, oils, and phospholipids, are crucial for energy storage, cellular membrane structure, and signaling. Their hydrophobic nature allows them to form barriers in biological membranes, thus regulating the internal environment of cells.
- The term “polymer” is particularly relevant when discussing biomolecules, as many of them are formed by the polymerization of smaller units known as monomers. For example, proteins are polymers made up of amino acid monomers, and polysaccharides consist of sugar monomers. This structural diversity allows biomolecules to perform a wide range of biological functions.
- Overall, biomolecules are indispensable for the maintenance and metabolic activities of living organisms. They exist in various forms, ranging from small metabolites and hormones to large macromolecules like proteins and nucleic acids. Therefore, understanding biomolecules is essential for comprehending the intricate processes that sustain life, from cellular metabolism to genetic inheritance.
Definition of Biomolecule
A biomolecule is any organic molecule that is essential for life and is involved in the structure, function, and regulation of the cells and tissues in living organisms. Common types of biomolecules include carbohydrates, lipids, proteins, and nucleic acids.
Types of Biomolecules – Four Major Types of Biomolecules
Biomolecules are essential components of living organisms, classified into four major categories: carbohydrates, proteins, nucleic acids, and lipids. Each class possesses unique structures and functions that are integral to biological processes.
- Carbohydrates
Carbohydrates are chemically categorized as polyhydroxy aldehydes or ketones, or as compounds that yield these upon hydrolysis. Commonly referred to as sugars, they encompass a group known as saccharides, derived from the Greek term “sakcharon,” meaning sugar. Carbohydrates can be classified based on the number of sugar units they contain:- Monosaccharides: These are the simplest carbohydrates, consisting of a single sugar unit. Examples include glucose and fructose.
- Oligosaccharides: Comprising 2 to 10 sugar units, oligosaccharides can be found in various food sources and are often involved in cell recognition processes.
- Polysaccharides: These consist of more than ten sugar units and serve multiple roles, including energy storage and structural support. For instance, cellulose is a vital structural component in plant cell walls, providing rigidity and strength. Carbohydrates serve as the primary dietary energy source, underscoring their significance in nutrition and metabolism.
- Proteins
Proteins constitute about 50% of the cellular dry weight, making them critical biomolecules. They are polymers formed by the linking of amino acids into polypeptide chains. The structure of proteins is organized into four levels of complexity:- Primary Structure: The linear sequence of amino acids in a polypeptide chain.
- Secondary Structure: Local folding patterns, such as alpha helices and beta sheets, formed through hydrogen bonding.
- Tertiary Structure: The overall three-dimensional shape of a protein, determined by interactions among various side chains.
- Quaternary Structure: In some proteins, multiple polypeptide chains come together to form a functional complex.
Proteins have diverse functions, including providing structural support (as seen in collagen), facilitating movement (such as myosin in muscle contraction), and catalyzing biochemical reactions as enzymes. Their dynamic roles are essential for various biological processes.
- Nucleic Acids
Nucleic acids are critical for storing and transmitting genetic information within cells. There are two primary types:- Deoxyribonucleic Acid (DNA): This molecule carries hereditary information from parents to offspring. Its structure is characterized by a double-helix formation, formed by the hydrogen bonding between nitrogenous bases on two antiparallel strands. The four nitrogenous bases in DNA are adenine, guanine, cytosine, and thymine.
- Ribonucleic Acid (RNA): This nucleic acid plays a pivotal role in protein synthesis and is structurally similar to DNA but differs in that it contains uracil instead of thymine.
The fundamental unit of nucleic acids is the nucleotide, composed of a nitrogenous base, a pentose sugar, and a phosphate group. Nucleotides are connected through phosphodiester bonds, forming the backbone of the nucleic acid strands. Nucleic acids are essential for processes such as transcription and translation, which are crucial for gene expression and protein synthesis.
- Lipids
Lipids are organic molecules that are primarily hydrophobic, meaning they do not dissolve in water. Instead, they are soluble in organic solvents and are generally associated with fatty acids. Lipids encompass a variety of substances, including:- Fats and oils, which serve as energy storage molecules.
- Waxes, which provide protective coatings for plants and animals.
- Sterols and fat-soluble vitamins, which are important for various physiological functions.
- Phospholipids, which are key components of cell membranes, creating a barrier that separates cellular compartments.
Unlike carbohydrates, proteins, and nucleic acids, lipids are not polymers. However, they play crucial roles in cellular structure and function, primarily as energy sources and as integral parts of cell membranes.
Carbohydrates
Carbohydrates are essential biomolecules that play a pivotal role in the diet of living organisms. They are primarily recognized for providing energy necessary for various physiological processes. Scientifically, carbohydrates are classified as polyhydroxy aldehydes or polyhydroxy ketones, making them the most abundant biomolecules on Earth.
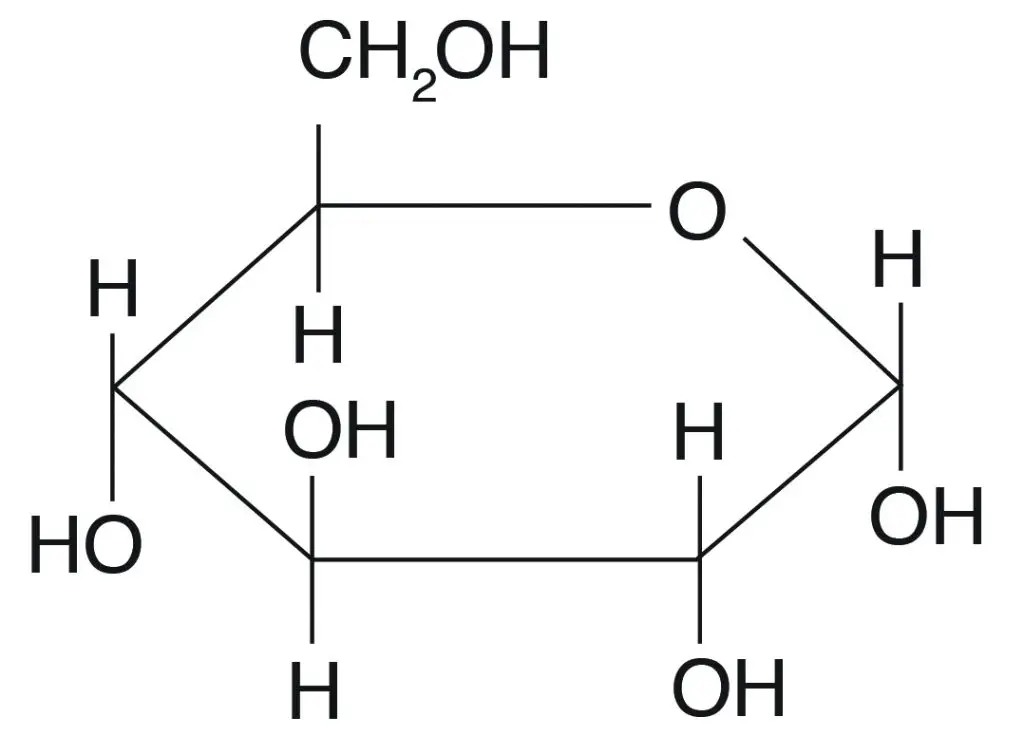
Types of Carbohydrates and Their Functions
Scientifically, carbohydrates can be classified as polyhydroxy aldehydes or polyhydroxy ketones, marking them as the most abundant biomolecules found on Earth. The classification of carbohydrates is based on the number of products formed after hydrolysis, leading to three main groups: monosaccharides, disaccharides, and polysaccharides. Each group has distinct characteristics and functions.
- Monosaccharides:
- Composed of a single unit of polyhydroxy aldehyde or ketone, monosaccharides are fundamental building blocks of carbohydrates.
- They appear as colorless, crystalline solids and are completely soluble in water.
- Monosaccharides play a vital role in energy generation for the body.
- Common examples include:
- Glucose: A primary energy source for cells.
- Fructose: Found in many fruits, it serves as a sweetener.
- Ribose: A component of nucleic acids, critical for genetic information.
- Arabinose: Often found in plant cell walls.
- Disaccharides:
- Formed by the combination of two sugar units linked through O-glycosidic bonds, disaccharides play various roles in energy supply and metabolism.
- The following are notable disaccharides, along with their monomer units and functions:
- Sucrose: Comprising glucose and fructose, it is a product of photosynthesis and serves as a common sweetener.
- Lactose: Made up of galactose and glucose, this disaccharide is a major energy source for many animals, particularly in the form of milk.
- Maltose: Consisting of two glucose units (alpha-1,4 linkage), it acts as an important intermediate in the digestion of starch and glycogen.
- Trehalose: Comprising two glucose units (alpha-1, alpha-1 linkage), it serves as an energy source, particularly for insects.
- Cellobiose: Formed from glucose units (beta-1,4 linkage), it is essential for carbohydrate metabolism.
- Gentiobiose: Comprising glucose units (beta-1,6 linkage), it is found in plant glycosides and some polysaccharides.
- Polysaccharides:
- Consisting of more than two sugar monomer units, polysaccharides, also known as glycans, serve various structural and storage functions in living organisms. They can be categorized into two types:
- Homopolysaccharides:
- Composed of a single type of sugar unit, they are further classified based on their functions:
- Structural Polysaccharides:
- Provide mechanical stability to cells, organs, and entire organisms. Examples include:
- Chitin: Integral to the construction of fungal cell walls.
- Cellulose: An essential dietary component for ruminants, forming a major part of plant cell walls.
- Provide mechanical stability to cells, organs, and entire organisms. Examples include:
- Storage Polysaccharides:
- Act as carbohydrate stores, releasing sugar monomers when needed. Key examples include:
- Starch: Serves as the primary energy reserve for plants. It is catalyzed by the enzyme amylase (found in saliva) in animals for energy requirements.
- Glycogen: The primary food reserve in animals, bacteria, and fungi, providing energy when required.
- Inulin: A storage form of carbohydrate in certain plants.
- Act as carbohydrate stores, releasing sugar monomers when needed. Key examples include:
- Structural Polysaccharides:
- Composed of a single type of sugar unit, they are further classified based on their functions:
- Heteropolysaccharides:
- Comprising two or more different types of sugar units, these complex carbohydrates have diverse functions. Examples include:
- Glycosaminoglycans: Such as hyaluronic acid, heparan sulfate, keratan sulfate, and murein, which contribute to various physiological processes.
- Heparin: Functions as an anticoagulant, preventing blood clotting.
- Hyaluronic Acid: Acts as a shock absorber and lubricant within tissues.
- Peptidoglycans (Mureins): Found in bacterial cell walls, providing structural integrity.
- Glycosaminoglycans: Such as hyaluronic acid, heparan sulfate, keratan sulfate, and murein, which contribute to various physiological processes.
- Comprising two or more different types of sugar units, these complex carbohydrates have diverse functions. Examples include:
Functions of Carbohydrates
Below are detailed explanations of the primary functions of carbohydrates:
- Energy Provision:
- Carbohydrates are the main source of energy for the body and the nervous system. When ingested, they are metabolized to produce glucose, the primary energy substrate for cellular functions. This energy is essential for all forms of physical activity, mental function, and overall metabolic processes.
- Basic Food Component:
- Carbohydrates are classified into various categories, including sugars, starch, and dietary fiber. They are abundantly found in staple foods such as grains, fruits, and dairy products. These carbohydrates contribute significantly to daily caloric intake, providing not only energy but also essential nutrients.
- Involvement in Fat Metabolism:
- Carbohydrates play a crucial role in fat metabolism, facilitating the breakdown and utilization of fats for energy. Adequate carbohydrate intake prevents the body from entering a state of ketosis, which occurs when fat stores are used excessively for energy. In this state, the body produces ketones, which can lead to metabolic disturbances.
- Protein Preservation:
- One of the essential functions of carbohydrates is their ability to inhibit the breakdown of proteins for energy. When carbohydrates are available, they serve as the primary source of energy, thus preserving protein stores for their vital functions, such as tissue repair and muscle maintenance.
- Digestive Process Assistance:
- The enzyme amylase plays a key role in the digestion of carbohydrates. It catalyzes the breakdown of starch into simpler sugars, primarily glucose. This process is vital for converting dietary carbohydrates into a form that can be easily utilized by the body, thereby facilitating metabolism and energy production.
Examples of Carbohydrates
Below are some important examples of carbohydrates, detailing their types and functions.
- Glucose:
- Glucose is a monosaccharide, often referred to as blood sugar, and is a crucial source of energy for cells. It is readily absorbed into the bloodstream and utilized for immediate energy needs or stored as glycogen for future use.
- Galactose:
- Another monosaccharide, galactose is a component of lactose, the sugar found in milk. It is less sweet than glucose and plays a role in the synthesis of glycoproteins and glycolipids, which are important for cellular recognition and signaling.
- Maltose:
- Maltose is a disaccharide formed from two glucose units linked by an alpha-1,4 glycosidic bond. It is produced during the digestion of starch and is found in malted foods and beverages. Maltose serves as an intermediate in the breakdown of starch and glycogen.
- Fructose:
- Fructose, also a monosaccharide, is found naturally in fruits and honey. It is sweeter than glucose and is often used as a sweetener in processed foods. Fructose is absorbed quickly and can be converted into glucose in the liver for energy production.
- Sucrose:
- Sucrose is a common disaccharide composed of glucose and fructose. It is obtained from sugarcane and sugar beets and is widely used as table sugar. Sucrose is a product of photosynthesis and serves as an important energy source in the diet.
- Lactose:
- Lactose, known as milk sugar, is a disaccharide made up of galactose and glucose. It is primarily found in dairy products and provides energy, particularly in infants. Some individuals lack the enzyme lactase, which is necessary for lactose digestion, leading to lactose intolerance.
- Starch:
- Starch is a polysaccharide that serves as a storage form of energy in plants. It consists of numerous glucose units linked together. When consumed, starch is broken down into glucose by enzymes such as amylase, providing a sustained energy source for humans and other animals.
- Cellulose:
- Cellulose is a structural polysaccharide found in the cell walls of plants. Composed of glucose units linked by beta-1,4 glycosidic bonds, it is indigestible for humans but serves as an important source of dietary fiber, promoting digestive health.
- Chitin:
- Chitin is a structural polysaccharide found in the exoskeletons of arthropods, such as insects and crustaceans, as well as in the cell walls of fungi. Composed of N-acetylglucosamine units, chitin provides mechanical strength and protection to these organisms.
Proteins
Proteins are large, complex molecules made up of chains of amino acids. They play a critical role in the body by serving as structural components of cells, facilitating biochemical reactions as enzymes, regulating bodily functions as hormones, and providing immune defense as antibodies. Proteins are essential for growth, repair, and maintenance of tissues in living organisms.
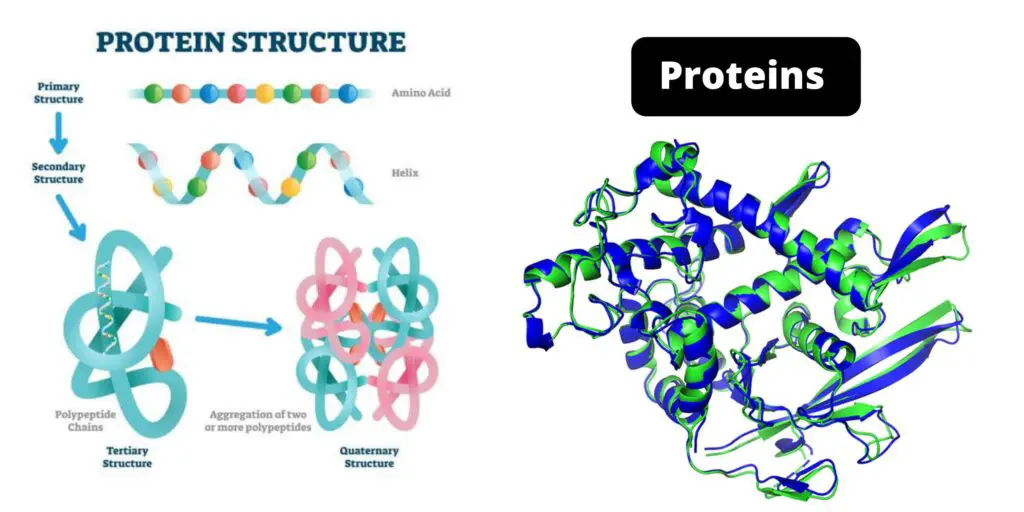
Protein Structure
Protein structure is a fundamental aspect of molecular biology that describes the various levels of organization in protein molecules. Understanding the hierarchy of protein structure is essential for grasping how proteins function in biological systems. Proteins can be categorized into two main types: fibrous proteins and globular proteins. Each type plays distinct roles in biological processes, and their structures contribute significantly to their functions.
- Fibrous Proteins: These proteins are characterized by their insolubility and elongated shapes. They typically serve structural roles within cells and tissues. Examples include collagen and keratin, which provide strength and support in connective tissues and hair, respectively.
- Globular Proteins: In contrast, globular proteins are soluble and compact. They often function as enzymes, hormones, and antibodies. Their spherical shape allows them to interact more easily with other molecules in the aqueous environment of cells.
Proteins exhibit four primary levels of structure, each crucial for their overall functionality:
- Primary Structure: This level refers to the specific sequence of amino acids that make up a protein. The order of these amino acids is dictated by the genetic information stored in an organism’s DNA. This sequence determines how the protein will fold and function.
- Secondary Structure: This involves the local three-dimensional form of segments of the polypeptide chain, stabilized by hydrogen bonds between atoms along the backbone of the chain. Common secondary structures include alpha helices and beta sheets, which provide structural stability and support.
- Tertiary Structure: The tertiary structure refers to the overall three-dimensional shape of a protein, which is determined by interactions among the side chains (R-groups) of the amino acids. This level of structure is crucial for the protein’s function, as it dictates how the protein interacts with other molecules.
- Quaternary Structure: This level describes the arrangement of multiple folded protein subunits into a larger multi-subunit complex. Not all proteins exhibit quaternary structure, but those that do rely on this level for functional interactions. Hemoglobin, for example, consists of four subunits that work together to transport oxygen.
Classifications of Proteins
Based on their structural characteristics and solubility properties, proteins can be broadly classified into two main categories: fibrous proteins and globular proteins. Understanding these classifications provides insights into the diverse roles that proteins play in biological systems.
- Fibrous Proteins: Fibrous proteins are characterized by their elongated, thread-like structures. This form is achieved when protein chains run parallel and are stabilized through hydrogen and disulfide bonds. Due to their structural composition, fibrous proteins are typically insoluble in water. Their primary functions often relate to providing strength and support within various biological structures.
- Examples:
- Keratin: This protein is a critical component of hair, wool, and silk. It provides rigidity and protection, contributing to the structural integrity of hair and skin.
- Myosin: Found in muscle tissue, myosin plays a fundamental role in muscle contraction and movement. Its fibrous structure allows it to interact effectively with actin filaments, facilitating the contraction process.
- Examples:
- Globular Proteins: In contrast to fibrous proteins, globular proteins exhibit a more compact and spherical shape due to the twisting and folding of their amino acid chains. These proteins are generally soluble in water, which is essential for their diverse biological functions. Globular proteins often participate in enzymatic activities, transportation, and signaling processes within cells.
- Examples:
- Insulin: This hormone regulates blood sugar levels by facilitating the uptake of glucose into cells. Its globular structure allows it to interact with specific receptors on cell surfaces, initiating various metabolic processes.
- Albumins: These proteins are found in blood plasma and serve multiple functions, including the transport of hormones, fatty acids, and other compounds. Their solubility in water enables them to play a vital role in maintaining osmotic pressure and fluid balance in the bloodstream.
- Examples:
Functions of Proteins
- Enzymes: Proteins primarily function as enzymes, which are biological catalysts that facilitate and accelerate chemical reactions within cells. By lowering the activation energy required for reactions, enzymes such as DNA polymerase and lipase enable vital processes, including DNA replication and metabolism. Enzymes play a pivotal role in regenerating DNA molecules and executing complex biochemical pathways.
- Hormones: Another significant role of proteins is in the formation of hormones, which are regulatory molecules that influence numerous physiological functions. For instance, hormones like insulin regulate blood sugar levels, while others, such as secretin, are involved in digestion and the formation of digestive juices. Hormones are crucial for maintaining homeostasis in the body, affecting growth, development, reproduction, and overall metabolic balance.
- Antibodies: Also known as immunoglobulins, antibodies are specialized proteins produced by the immune system to identify and neutralize foreign invaders such as bacteria and viruses. They work in conjunction with other immune cells to target antigens, facilitating the repair and healing of tissues. Antibodies are vital for the adaptive immune response, ensuring that the body can effectively combat pathogens.
- Energy Source: Proteins can also serve as an energy source, particularly when carbohydrates and fats are scarce. They are essential for bodily movements and various metabolic activities. When consumed in excess, proteins can be converted into fat, subsequently becoming part of adipose tissue. Thus, maintaining an appropriate intake of proteins is critical for energy balance.
- Structural Proteins: Proteins provide structural support throughout the body, forming the framework of cells and tissues. Fibrous proteins, such as collagen, elastin, and keratin, are key components of connective tissues, bones, tendons, and other structural elements. These proteins contribute to the physical integrity and resilience of various tissues.
- Respiratory Pigments: Globular proteins, such as hemoglobin and myoglobin, function as respiratory pigments that transport oxygen throughout the body. Hemoglobin carries oxygen from the lungs to tissues and organs, while myoglobin provides oxygen to working muscles. This oxygen transport is essential for cellular respiration and energy production.
- Transport Proteins: Certain proteins function as transport proteins that facilitate the movement of molecules across cell membranes. These proteins form channels and carriers within the plasma membrane, selectively allowing specific substances to enter or exit cells. For example, serum albumin transports fatty acids and hemin, while channel proteins enable the passage of ions and small molecules.
- Motor Proteins: Motor proteins, such as actin and myosin, play crucial roles in muscle contraction and movement. They are responsible for the mechanical actions of muscles, enabling organisms to move and interact with their environment. Additionally, proteins like kinesin and dynein are involved in intracellular transport, moving cellular components along cytoskeletal filaments.
- Storage Proteins: Proteins also serve as storage reserves for amino acids and metal ions within cells. Examples include ferritin, which stores iron, and ovalbumin, found in egg whites. These proteins provide essential nutrients that can be mobilized when needed, supporting growth and development.
- Toxins: Some proteins function as toxins produced by bacteria, aiding in their survival and pathogenicity. These toxins, such as diphtheria toxin, exert cytotoxic effects that can harm host organisms. Understanding the role of these proteins is essential for developing treatments and preventive measures against bacterial infections.
Examples of Proteins
Here are several examples of different types of proteins:
- Enzymes:
Enzymes are biological catalysts that speed up chemical reactions in the body.- DNA Polymerase: An enzyme that synthesizes DNA molecules by assembling nucleotides, crucial for DNA replication.
- Amylase: An enzyme that helps digest carbohydrates by breaking down starch into sugars.
- Lactase: An enzyme that breaks down lactose, the sugar found in milk.
- Structural Proteins:
These proteins provide support and shape to cells and tissues.- Collagen: A major structural protein found in connective tissues, skin, bones, and cartilage.
- Keratin: A protein that forms hair, nails, and the outer layer of skin, providing strength and protection.
- Elastin: A protein that allows tissues in the body to resume their shape after stretching or contracting.
- Transport Proteins:
These proteins facilitate the movement of substances across cell membranes or within the bloodstream.- Hemoglobin: A protein in red blood cells that binds and transports oxygen from the lungs to the tissues.
- Albumin: A protein found in blood plasma that maintains osmotic pressure and transports various substances, including hormones and fatty acids.
- Glucose Transporters: Proteins that facilitate the transport of glucose across cell membranes.
- Hormonal Proteins:
These proteins act as hormones, which are chemical messengers in the body.- Insulin: A hormone produced by the pancreas that regulates blood sugar levels.
- Growth Hormone: A hormone that stimulates growth, cell reproduction, and regeneration in humans and other animals.
- Follicle-Stimulating Hormone (FSH): A hormone involved in the regulation of reproductive processes.
- Antibodies:
Also known as immunoglobulins, these proteins are critical for the immune response.- IgG: The most abundant type of antibody in blood circulation, providing protection against pathogens.
- IgA: An antibody found in mucosal areas, such as the gut, respiratory tract, and urogenital tract, as well as in saliva and breast milk.
- Motor Proteins:
These proteins are responsible for movement in cells and organisms.- Myosin: A motor protein that interacts with actin filaments to cause muscle contraction.
- Kinesin: A motor protein that transports cellular cargo along microtubules within cells.
- Dynein: Another motor protein that moves along microtubules and is involved in intracellular transport and ciliary motion.
- Storage Proteins:
These proteins store amino acids or other substances for future use.- Ferritin: A protein that stores iron in a soluble, non-toxic form, crucial for iron metabolism.
- Casein: A milk protein that serves as a source of amino acids and calcium for young mammals.
- Ovalbumin: The main protein found in egg whites, serving as a nutrient source for developing embryos.
Nucleic Acids
Nucleic acids are biomolecules composed of nucleotide monomers that store and transmit genetic information within cells. The two primary types of nucleic acids are deoxyribonucleic acid (DNA), which carries the genetic blueprint for an organism, and ribonucleic acid (RNA), which plays a crucial role in protein synthesis and gene expression.
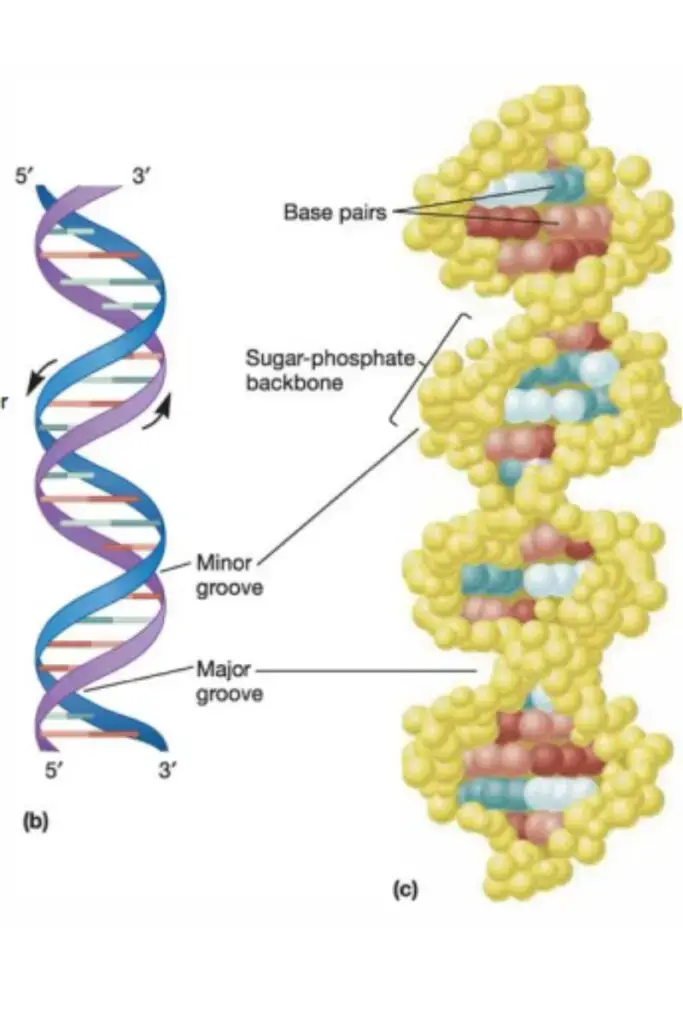
Structure of Nucleic Acids
Nucleic acids are vital macromolecules that play crucial roles in the storage and transmission of genetic information. The structure of nucleic acids, which includes DNA and RNA, is based on polymers of nucleotides, also referred to as polynucleotides. These nucleotides are composed of three fundamental components that together form the backbone and coding mechanism of nucleic acids.
- Nucleotides: The basic units of nucleic acids are nucleotides, which consist of the following components:
- Nitrogenous Base: These are heterocyclic, planar, and aromatic molecules. Nitrogenous bases are categorized into two groups: purines and pyrimidines.
- Purines: This group includes adenine (A) and guanine (G), both of which are present in DNA and RNA. Their structure consists of a two-ring system, which contributes to their stability and pairing ability.
- Pyrimidines: This group consists of thymine (T), which is exclusive to DNA, cytosine (C), which is found in both DNA and RNA, and uracil (U), which is unique to RNA. Pyrimidines have a single-ring structure and are essential for base pairing in the nucleic acid double helix.
- Five-Carbon Sugar: The sugar component of nucleotides is a pentose sugar. There are two types of pentose sugars:
- Ribose: Present only in RNA, ribose has a hydroxyl group attached to its 2′ carbon, making it more reactive and less stable compared to deoxyribose.
- Deoxyribose: Found in DNA, deoxyribose lacks an oxygen atom at the 2′ position, which contributes to the overall stability of the DNA molecule. The sugars in nucleic acids have the D-stereoisomeric configuration, which is important for their biological functions.
- Phosphoric Acid Ion: This component consists of a phosphate group that is crucial for the formation of nucleotides. The phosphate group facilitates the polymerization of nucleotides through phosphodiester bonds, which connect the 5′ carbon of one sugar to the 3′ carbon of another sugar. This linkage leads to the formation of a long chain of nucleotides, known as polynucleotides, which constitute the backbone of nucleic acids.
- Nitrogenous Base: These are heterocyclic, planar, and aromatic molecules. Nitrogenous bases are categorized into two groups: purines and pyrimidines.
- Polynucleotides: Nucleic acids, such as DNA and RNA, are formed by long chains of nucleotides linked together by phosphodiester bonds. The sequence of nitrogenous bases along the polynucleotide strand encodes genetic information. In DNA, two strands are intertwined in a double helix formation, stabilized by hydrogen bonds between complementary bases (A with T, and G with C). In RNA, the single-stranded structure allows for various configurations, enabling its role in protein synthesis and other cellular functions.
Types of Nucleic Acids
Nucleic acids are fundamental biomolecules responsible for the storage and transmission of genetic information. Based on their nature, structure, and function, nucleic acids can be categorized into two primary groups: Deoxyribonucleic acid (DNA) and Ribonucleic acid (RNA). Each type of nucleic acid possesses unique structural characteristics and functions, which are essential for the biology of living organisms.
- Deoxyribonucleic Acid (DNA):
- Structure: DNA is primarily located within the nucleus of cells and serves as the hereditary material. Its structure was first elucidated as a double helix by Watson and Crick in 1953. The DNA double helix comprises two polynucleotide strands coiled around a central axis. These strands are antiparallel, meaning they run in opposite directions, and they are held together by hydrogen bonds formed between complementary base pairs.
- Forms of DNA: DNA exists in three structural forms: the most common is the B-form, while A and Z forms are also recognized. The conformation that DNA adopts can vary based on several factors, including the hydration level, DNA sequence, chemical modifications to the bases, and the concentration of metal ions present in the solution.
- Genetic Role: As the genetic material, DNA stores all the information necessary for the development and functioning of living organisms and viruses. It specifies the biological characteristics that can be passed on to progeny and is crucial for the processes of replication and gene expression.
- Ribonucleic Acid (RNA):
- Presence and Function: RNA is present in all living cells and fulfills various roles depending on the organism. In some viruses, RNA serves as the genetic material, while in other organisms, it exhibits enzymatic activity, in which case it is referred to as a ribozyme. RNA is involved in the transfer of genetic information for protein synthesis and plays a significant role in regulating gene expression.
- Types of RNA: Three primary types of RNA exist, each with distinct functions:
- Ribosomal RNA (rRNA): This type of RNA is a structural component of ribosomes, the cellular machinery responsible for protein synthesis.
- Messenger RNA (mRNA): mRNA serves as the intermediary that conveys genetic information from DNA to the ribosome, where it is translated into proteins.
- Transfer RNA (tRNA): tRNA is responsible for transporting specific amino acids to the ribosome during protein synthesis, facilitating the assembly of proteins according to the mRNA template.
- Structure: RNA can exist in both single-stranded (primary structure) and double-stranded (secondary structure) forms. The double-helical structure of RNA, when present, is typically in the A form. This structural versatility allows RNA to perform a variety of functions in cellular processes.
Functions of Nucleic Acids
Here are the key functions of nucleic acids:
- Storage of Genetic Information:
- DNA serves as the hereditary material in most organisms. It contains the genetic instructions required for the development, functioning, growth, and reproduction of living organisms and viruses. The sequences of nucleotides in DNA encode the information necessary for building proteins, which are crucial for various cellular functions.
- Transmission of Genetic Information:
- DNA is responsible for passing genetic information from one generation to the next during reproduction. This process occurs through DNA replication, ensuring that each new cell receives an accurate copy of the genetic material.
- Protein Synthesis:
- RNA plays a vital role in translating the genetic information stored in DNA into proteins. This process involves several types of RNA:
- Messenger RNA (mRNA): This RNA type carries the genetic code from DNA to the ribosome, where proteins are synthesized. The sequence of nucleotides in mRNA is translated into a specific sequence of amino acids to form proteins.
- Ribosomal RNA (rRNA): rRNA is a component of ribosomes, the cellular machinery that facilitates protein synthesis. It helps in the assembly of amino acids into polypeptides.
- Transfer RNA (tRNA): tRNA is responsible for transporting specific amino acids to the ribosome during protein synthesis, matching them with the corresponding codons on the mRNA strand.
- RNA plays a vital role in translating the genetic information stored in DNA into proteins. This process involves several types of RNA:
- Enzymatic Activity:
- Certain RNA molecules, known as ribozymes, possess enzymatic functions, enabling them to catalyze biochemical reactions. This capability highlights the versatility of RNA beyond its role in protein synthesis.
- Regulation of Gene Expression:
- RNA molecules are involved in regulating gene expression, determining when and how much of a particular protein is produced. This regulation is crucial for cellular processes, allowing cells to respond to environmental changes and developmental signals.
- Genetic Variation and Evolution:
- The sequences of nucleotides in DNA can undergo mutations, leading to genetic diversity. This genetic variation is fundamental to the process of evolution, allowing species to adapt to changing environments.
Examples of Nucleic Acids
Nucleic acids are essential biomolecules that play critical roles in genetic information storage and transmission. There are two primary types of nucleic acids: deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). Here are examples of each type:
- Deoxyribonucleic Acid (DNA):
DNA serves as the hereditary material in most organisms and is responsible for encoding the genetic instructions necessary for development and functioning.- Genomic DNA: This is the DNA found within the chromosomes of a cell, containing the complete set of genes necessary for the organism.
- Mitochondrial DNA (mtDNA): This is a small circular DNA found in mitochondria, distinct from genomic DNA, and is inherited maternally. It encodes genes essential for mitochondrial function.
- Plasmid DNA: Small, circular DNA molecules found in bacteria and some eukaryotes, often carrying genes that provide advantages, such as antibiotic resistance.
- Ribonucleic Acid (RNA):
RNA plays various roles in the expression of genes and the synthesis of proteins. It can act as genetic material in some viruses and has enzymatic properties in others.- Messenger RNA (mRNA): This type of RNA is synthesized from DNA and carries the genetic code from the nucleus to the ribosomes, where proteins are synthesized.
- Transfer RNA (tRNA): tRNA is involved in translating the mRNA sequence into a specific amino acid sequence during protein synthesis. Each tRNA molecule carries a specific amino acid corresponding to the mRNA codon.
- Ribosomal RNA (rRNA): rRNA is a structural and functional component of ribosomes, facilitating the assembly of amino acids into proteins during translation.
- MicroRNA (miRNA): Small non-coding RNA molecules that play a role in regulating gene expression by binding to mRNA and preventing translation.
- Ribozyme: An RNA molecule that acts as an enzyme, catalyzing biochemical reactions.
Lipids
Lipids are a diverse group of hydrophobic organic compounds, including fats, oils, waxes, and sterols, that are insoluble in water but soluble in organic solvents. They play essential roles in energy storage, cellular structure, insulation, and signaling within biological systems.
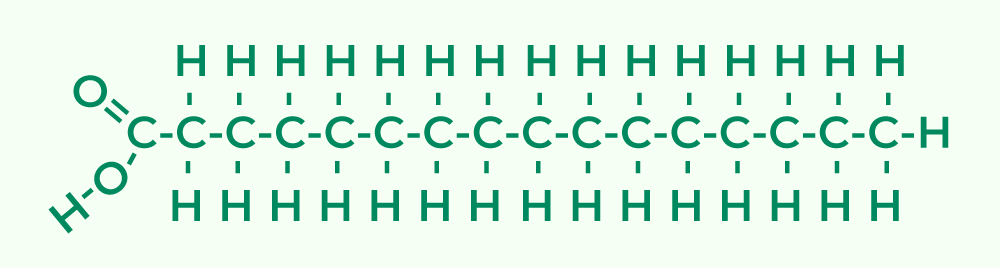
Structure of Lipids
Lipids are vital organic compounds that play essential roles in various biological processes. They are primarily composed of long, non-polar hydrocarbon chains along with a small polar region containing oxygen. This unique structure contributes to their diverse functions within living organisms. Below are the key structural characteristics and features of lipids:
- Polymers of Fatty Acids:
- Lipids are considered polymers formed from fatty acids. Fatty acids consist of long hydrocarbon chains that are hydrophobic (water-repelling) and a polar carboxyl group that can interact with water. This duality allows lipids to perform a variety of functions in biological systems.
- Non-Polar and Hydrophobic Nature:
- Lipids are predominantly nonpolar molecules, which makes them oily or greasy. This property is crucial for their role in forming biological membranes and storing energy. The hydrophobic nature means they do not mix well with water, a characteristic that defines many of their functions in cells.
- Heterogeneous Group of Compounds:
- Lipids represent a heterogeneous group of compounds, primarily composed of hydrocarbon chains. This variety includes different types of lipids such as triglycerides, phospholipids, sterols, and waxes, each with distinct structures and functions.
- Energy-Rich Molecules:
- Lipids are energy-rich organic molecules, serving as a significant energy source for organisms. They yield high energy upon oxidation, making them vital for various life processes, including metabolism and cellular function. Their ability to store energy efficiently allows organisms to sustain longer periods without food.
- Solubility Characteristics:
- One of the defining characteristics of lipids is their solubility in nonpolar solvents and insolubility in water. This property is critical for their function in biological systems, as it allows lipids to form structures like membranes that separate aqueous environments.
- Formation of Biological Membranes:
- Lipids play a significant role in the formation of cell membranes, acting as a mechanical barrier that divides a cell from its external environment. This barrier is composed primarily of phospholipids, which arrange themselves into a bilayer, allowing for selective permeability and compartmentalization of cellular functions.
Types of Lipids
Based on their structure and functions, lipids can be categorized into several classes, each exhibiting unique characteristics and contributing to various physiological processes. Below are the primary types of lipids:
- Fatty Acids:
- Fatty acids represent the simplest forms of lipids, consisting of hydrocarbon chains that range from 4 to 36 carbon atoms long, terminating with an acidic group. These molecules can be either linear or branched. Fatty acids serve as fundamental building blocks for other lipid types, playing a crucial role in energy storage and membrane formation.
- Waxes:
- Waxes are esters formed from fatty acids and long-chain alcohols, typically containing hydrocarbon chains of 14 to 36 carbons. These lipids are synthesized by numerous plants and animals for protective functions. A well-known example of wax is beeswax, which consists of an ester derived from palmitic acid and triacontanol alcohol. Waxes serve to provide a waterproof barrier, protecting surfaces from water loss and environmental damage.
- Phospholipids:
- Phospholipids are composed of fatty acids, an attachment platform, a phosphate group, and an alcohol bonded to the phosphate. These molecules are critical components of cell membranes, forming bilayers that create barriers between the cell’s internal environment and the external surroundings. The amphipathic nature of phospholipids—having both hydrophilic (water-attracting) and hydrophobic (water-repelling) parts—allows for the formation of the lipid bilayer essential for cellular integrity.
- Glycolipids:
- Glycolipids contain saccharide (sugar) groups and are important constituents of cell membranes. They play significant roles in cell recognition and signaling, participating in various cellular communication processes. By attaching to proteins or other lipids, glycolipids contribute to the functionality of the cell membrane and assist in signal transduction pathways.
- Steroids:
- Steroids are complex derivatives of triterpenes, characterized by a multi-ring structure. Cholesterol is a prominent example of a steroid; it is a crucial component of cell membranes and acts as a precursor for the biosynthesis of steroid hormones and bile acids. Steroids play vital roles in regulating various biological functions, including metabolism and immune responses.
- Eicosanoids:
- Eicosanoids are derived from polyunsaturated fatty acids containing 20 carbon atoms. These lipids have diverse physiological roles. For instance, prostaglandins are involved in stimulating uterine contractions and regulating blood pressure, while leukotrienes are essential for chemotaxis and inflammation. Thromboxanes play a role in vasoconstriction and platelet aggregation, demonstrating the critical functions of eicosanoids in mediating inflammatory responses and physiological processes.
Functions of Lipids
- Energy Storage:
- Lipids, particularly triglycerides, serve as a major source of energy. They store more energy per gram than carbohydrates or proteins, making them efficient for energy storage in adipose tissue. During periods of energy demand, lipids are metabolized to provide fuel for cellular activities.
- Structural Components of Cell Membranes:
- Phospholipids and cholesterol are fundamental components of cell membranes. The amphipathic nature of phospholipids allows them to form bilayers, creating a barrier that separates the interior of the cell from its external environment. Cholesterol contributes to membrane fluidity and stability, ensuring that membranes maintain their integrity under varying temperatures.
- Insulation and Protection:
- Lipids provide insulation to maintain body temperature and protect vital organs from physical shock. The adipose tissue, composed mainly of lipid cells, serves as a cushion for internal organs and helps to regulate thermal insulation.
- Hormone Production:
- Many hormones, particularly steroid hormones, are derived from lipids. Cholesterol is the precursor for steroid hormones such as cortisol, testosterone, and estrogen, which play crucial roles in regulating metabolism, growth, and reproductive functions.
- Signal Transduction:
- Certain lipids, such as eicosanoids, function as signaling molecules in the body. They are involved in various physiological processes, including inflammation, immune responses, and blood clotting. Lipids can also act as secondary messengers in cellular signaling pathways, facilitating communication between cells.
- Vitamin Absorption:
- Lipids are essential for the absorption of fat-soluble vitamins (A, D, E, and K) in the intestines. These vitamins require the presence of lipids for efficient uptake into the bloodstream, underscoring the importance of dietary fats in overall nutrition.
- Formation of Lipoproteins:
- Lipids combine with proteins to form lipoproteins, which transport lipids through the bloodstream. These complexes play a vital role in lipid metabolism, facilitating the delivery of triglycerides and cholesterol to tissues for energy use and cellular functions.
- Cell Recognition and Signaling:
- Glycolipids, which contain carbohydrate groups, are critical for cell recognition and signaling. They contribute to the formation of glycoproteins and play important roles in immune responses, cellular communication, and tissue recognition.
Examples of Lipids
Below are examples of different types of lipids, categorized based on their structures and functions:
- Fatty Acids:
- Saturated Fatty Acids: These contain no double bonds between carbon atoms and are typically solid at room temperature. Examples include:
- Palmitic Acid: Commonly found in palm oil and animal fats.
- Stearic Acid: Present in beef fat and cocoa butter.
- Unsaturated Fatty Acids: These have one or more double bonds in their hydrocarbon chain and are usually liquid at room temperature. Examples include:
- Oleic Acid: A monounsaturated fatty acid found in olive oil.
- Linoleic Acid: A polyunsaturated fatty acid present in sunflower oil.
- Saturated Fatty Acids: These contain no double bonds between carbon atoms and are typically solid at room temperature. Examples include:
- Waxes:
- Beeswax: Composed of long-chain fatty acids esterified to long-chain alcohols, commonly used by bees to construct honeycomb.
- Carnauba Wax: Obtained from the leaves of the carnauba palm, used in cosmetics and food products.
- Phospholipids:
- Phosphatidylcholine: A major component of biological membranes, often found in egg yolk and soybeans.
- Phosphatidylserine: Important for cell signaling and found in brain tissues.
- Glycolipids:
- Cerebrosides: Found in nerve tissue and contribute to the myelin sheath surrounding nerve fibers.
- Gangliosides: Complex glycolipids present in the cell membranes of nerve cells, involved in cell recognition and signaling.
- Steroids:
- Cholesterol: A crucial component of cell membranes that also serves as a precursor for steroid hormones and bile acids.
- Testosterone: A steroid hormone responsible for the development of male characteristics.
- Cortisol: A steroid hormone involved in the stress response and regulation of metabolism.
- Eicosanoids:
- Prostaglandins: Involved in various physiological processes, including inflammation and pain response.
- Leukotrienes: Play a role in immune responses and are involved in conditions like asthma.
Significance of Biomolecule
Biomolecules are essential components of all living organisms and play critical roles in maintaining life. Their significance can be summarized in the following points:
- Building Blocks of Life:
Biomolecules, including carbohydrates, lipids, proteins, and nucleic acids, are the fundamental building blocks of cells and tissues. They provide the structure necessary for cellular organization and function. - Energy Storage and Transfer:
Biomolecules such as carbohydrates and lipids serve as energy sources for living organisms. Carbohydrates, like glucose, are quickly metabolized for energy, while lipids provide long-term energy storage. This energy is crucial for cellular activities and metabolic processes. - Genetic Information Storage and Transfer:
Nucleic acids (DNA and RNA) are vital for storing and transmitting genetic information. DNA encodes the genetic instructions necessary for the growth, development, and reproduction of organisms, while RNA plays a key role in protein synthesis and gene expression. - Catalysis and Regulation:
Proteins function as enzymes, which catalyze biochemical reactions, facilitating essential metabolic processes. Additionally, proteins regulate various biological functions, including hormone signaling and immune responses. - Cellular Communication and Signaling:
Biomolecules are involved in communication between cells. For instance, hormones (proteins or steroids) act as signaling molecules that convey messages to target cells, coordinating physiological responses. - Structural Support and Protection:
Certain biomolecules, such as collagen and keratin, provide structural integrity and protection to cells and tissues. Lipids, particularly phospholipids, form the cell membrane, creating a barrier that regulates the movement of substances in and out of the cell. - Immune Defense:
Biomolecules play a critical role in the immune system. Antibodies, which are specialized proteins, identify and neutralize pathogens, providing defense against infections and diseases. - Cellular Metabolism:
Biomolecules are involved in metabolic pathways that generate energy and synthesize essential compounds. They contribute to catabolic and anabolic reactions, ensuring that the cell maintains homeostasis. - Adaptation and Evolution:
The diversity of biomolecules allows organisms to adapt to different environments and challenges. Variations in biomolecular structures and functions have evolved over time, leading to the vast array of life forms on Earth.
- https://conductscience.com/biomolecules-types-and-functions/?srsltid=AfmBOoqc6gWpdRSl0BQ85P_rg-2S_3p613xZ81krOgXNNjLHM8nlhZqI
- https://www.biologyonline.com/dictionary/biomolecule
- https://www.geeksforgeeks.org/biomolecules-types-and-structure/
- https://www.majordifferences.com/2023/03/four-biomolecules-structure-and.html
- https://ncert.nic.in/textbook/pdf/kebo109.pdf
- https://nios.ac.in/media/documents/SrSec313NEW/313_Chemistry_Eng/313_Chemistry_Eng_Lesson29.pdf
- https://sist.sathyabama.ac.in/sist_coursematerial/uploads/SBC1101.pdf
- https://nios.ac.in/media/documents/SrSec313NEW/313_LG_E/313_LG_E_L29.pdf
- https://vtputkal.odisha.gov.in/subjectwise/biomolecules-and-cell-biology/
- https://ncert.nic.in/textbook/pdf/lech205.pdf