What is Bacterial Growth Curve?
A bacterial growth curve is the graphical representation that shows the increase and decrease in number of bacterial cells in a population with respect to time. It is studied when bacteria are grown in a closed system or batch culture where no fresh nutrients are added and waste products are not removed. Under these conditions the bacterial population shows a typical S–shaped pattern of growth. The growth curve is divided into four phases, namely lag phase, log phase, stationary phase and death phase. In the lag phase the bacterial cells are metabolically active and they synthesize enzymes and other substances but the cell division is not started.
After this the cells enter the log or exponential phase where rapid cell division takes place and the number of bacteria increases very quickly. As nutrients become limited and toxic metabolic products accumulate the growth slows down and the culture enters the stationary phase where the number of new cells formed is equal to the number of cells dying. Finally the death phase occurs when the rate of cell death becomes higher than the rate of cell division and the number of living bacterial cells gradually decreases.
Definition of Bacterial Growth Curve
The bacterial growth curve is a graphical representation of the different phases of bacterial growth over time, including the lag phase, log phase, stationary phase, and death phase.
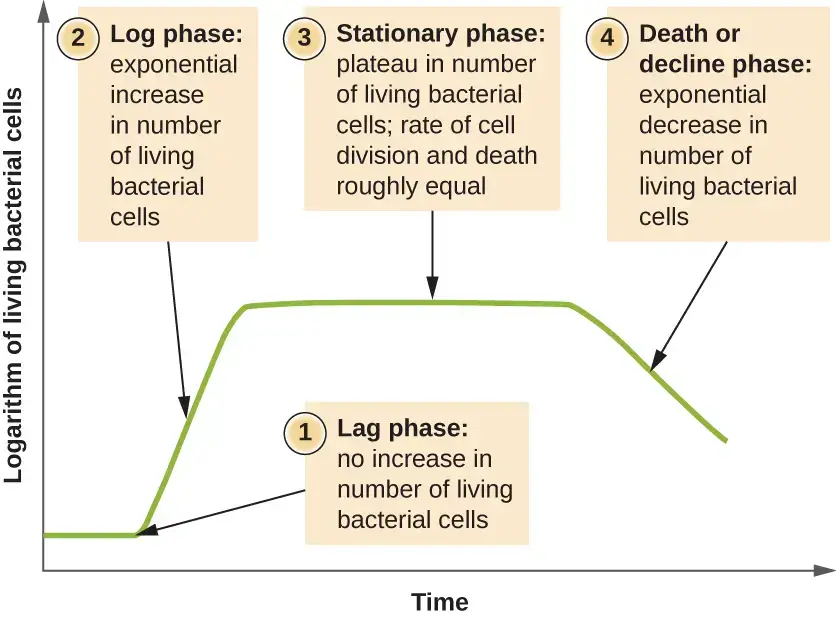
Phases of Bacterial Growth Curve
Some of the main phases of the bacterial growth curve are–
- Lag Phase– It is the initial stage of the bacterial growth curve. In this phase the bacterial cells are metabolically active but the cells do not divide. The cells increase in size and different enzymes, RNA and metabolites are synthesized which are necessary for the growth of bacteria.
- Log Phase (Exponential Phase)– It is the phase of active growth. In this phase the bacterial cells divide rapidly and the population increases exponentially. The number of bacterial cells doubles at a constant rate and the growth of bacteria is maximum during this phase.
- Stationary Phase– In this phase the growth of bacteria becomes constant. The nutrients present in the culture medium become limited and toxic metabolic wastes accumulate in the medium. The number of new cells produced becomes equal to the number of cells dying.
- Death Phase (Decline Phase)– It is the final phase of the bacterial growth curve. In this phase the environmental conditions become unfavorable for the bacterial cells. The rate of cell death becomes higher than the rate of cell division and the number of living bacterial cells gradually decreases.
1. Lag phase of Bacterial Growth Curve
Some of the main characteristics of lag phase are–
- It is the initial stage of the bacterial growth curve where the bacterial cells are introduced into a new culture medium.
- In this phase the bacterial cells do not divide and the number of cells remains constant.
- The bacterial cells remain metabolically active during this phase.
- The cells increase in size and become physiologically active.
- Different enzymes, proteins, RNA and other metabolites are synthesized which are necessary for bacterial growth.
- The bacterial cells adapt themselves to the new environmental conditions of the culture medium.
- If the bacterial cells are damaged or injured they repair themselves during this phase before starting cell division.
- The duration of lag phase is variable and it depends on the type of bacteria and the environmental conditions of the culture medium.
2. Log phase or exponential phase
Some of the main characteristics of log phase are–
- It is also referred to as the exponential phase or logarithmic phase of the bacterial growth curve.
- In this phase the bacterial cells divide rapidly by binary fission.
- The number of bacterial cells increases exponentially and the population doubles at a constant rate.
- The bacterial cells show maximum growth and metabolic activity during this phase.
- The cells remain physiologically active and show uniform metabolic activities.
- The bacterial cells synthesize DNA, proteins and cell wall materials actively during this phase.
- The cells are more sensitive to antibiotics and disinfectants during this stage.
- This phase continues only when nutrients are available and environmental conditions such as temperature and pH remain favorable.
- When nutrients become limited and toxic metabolic products accumulate the log phase gradually changes into the stationary phase.
3. Stationary Phase
Some of the main characteristics of stationary phase are–
- It is the phase where the growth of bacterial population becomes constant.
- In this phase the number of new cells formed is equal to the number of cells dying.
- The bacterial population remains stable and no net increase in cell number is observed.
- This phase occurs when the nutrients in the culture medium become limited and space becomes insufficient.
- Toxic metabolic waste products accumulate in the culture medium which affect bacterial growth.
- The metabolic activity of bacterial cells decreases and the cells enter a survival condition.
- Some bacteria may produce secondary metabolites such as antibiotics during this phase.
- Certain bacterial species may form spores to survive under unfavorable environmental conditions.
- The bacterial cells show increased resistance to environmental stress during this phase.
4. Death Phase
Some of the main characteristics of death phase are–
- It is the final stage of the bacterial growth curve.
- In this phase the rate of cell death becomes higher than the rate of cell division.
- The number of living bacterial cells decreases gradually with time.
- This phase occurs due to exhaustion of nutrients in the culture medium.
- Toxic metabolic waste products accumulate in the medium which makes the environment unfavorable for bacterial growth.
- The pH of the culture medium may change which further affects the survival of bacterial cells.
- Many bacterial cells die and may undergo cell lysis releasing their cellular contents into the medium.
- Some bacterial cells may form spores or enter a dormant condition to survive under unfavorable conditions.
- A few bacterial cells may remain viable and survive until favorable environmental conditions return.
Factors Influencing Bacterial Growth Curve
Some of the main factors influencing bacterial growth are–
- Nutrient Availability– It is the important factor for bacterial growth. Bacteria require nutrients such as carbon, nitrogen, minerals and vitamins for metabolism and cell division. Adequate nutrients support rapid bacterial growth while depletion of nutrients slows the growth.
- Temperature – Temperature influences the rate of bacterial growth. Each bacterial species grows within a specific temperature range such as psychrophiles, mesophiles and thermophiles. Temperature outside the optimum range slows the growth or may cause death of bacterial cells.
- pH of Medium– The pH of the culture medium also affects bacterial growth. Most bacteria grow best at neutral pH conditions. Extreme acidic or alkaline conditions inhibit the growth of bacterial cells.
- Oxygen Availability– Oxygen is an important factor affecting bacterial growth. Some bacteria require oxygen for growth while others grow in the absence of oxygen. Based on oxygen requirement bacteria are classified as aerobic, anaerobic and facultative bacteria.
- Moisture and Osmotic Pressure– Water availability is necessary for bacterial metabolism and cell division. Low moisture conditions inhibit bacterial growth. The osmotic pressure of the environment also affects the growth of bacterial cells.
- Light– Light may influence bacterial growth. Certain wavelengths of light such as ultraviolet light can damage bacterial DNA and inhibit bacterial growth.
- Inhibitory Substances– Certain substances such as antibiotics, disinfectants and toxic chemicals inhibit bacterial growth. Accumulation of toxic metabolic waste products also affects the growth of bacteria.
- Physical Space– Availability of space in the culture medium influences bacterial growth. In a closed culture system the limited space restricts further cell division and leads to stationary phase.
Significance of Bacterial Growth Curve
Some of the important significance of bacterial growth curve are–
- It helps in determining the generation time or doubling time of bacterial population during growth.
- It helps in predicting and controlling the growth of bacteria under different environmental conditions.
- It provides information about the nutritional and physical requirements of bacterial cells during growth.
- It helps in identifying the exponential phase where bacterial cells are most active and suitable for laboratory experiments.
- It is useful in industrial microbiology for maintaining optimum conditions for bacterial growth during fermentation processes.
- It helps in studying bacterial pathogenesis and the development of infectious diseases.
- It is useful in understanding the mechanism of antibiotic resistance in bacteria.
- It helps in monitoring bacterial growth in food and helps in preventing food spoilage and maintaining food safety.
Lecture Video
What is a bacterial growth curve?
A bacterial growth curve represents the growth pattern of a population of bacteria over time, typically plotted as the logarithm of the cell count or optical density against time.
What are the phases of a bacterial growth curve?
Bacterial growth curves typically consist of four phases: lag phase, exponential (log) phase, stationary phase, and death phase.
What happens during the lag phase of a bacterial growth curve?
The lag phase is an initial period of adaptation, where bacteria adjust to the environment and prepare for growth. Little to no increase in cell count occurs during this phase.
What characterizes the exponential (log) phase of a bacterial growth curve?
The exponential phase is a period of rapid bacterial growth, where the population size increases exponentially. Cells divide at their maximum rate during this phase.
What occurs during the stationary phase of a bacterial growth curve?
The stationary phase is a plateau phase where the growth rate slows down and the number of dividing cells equals the number of dying cells. Limited nutrients and accumulation of waste products contribute to this phase.
Why does the growth rate stabilize in the stationary phase of a bacterial growth curve?
In the stationary phase, nutrient depletion and waste product accumulation inhibit further cell division, leading to a balance between cell growth and death.
What happens during the death phase of a bacterial growth curve?
The death phase occurs when the number of dying cells exceeds the number of dividing cells. Conditions become unfavorable for growth, and the population size declines.
Can bacterial growth curves differ depending on the bacterial species and environmental conditions?
Yes, bacterial growth curves can vary depending on the bacterial species, nutrient availability, temperature, pH, oxygen levels, and other environmental factors.
How can bacterial growth curves be used in research or industrial applications?
Bacterial growth curves provide insights into microbial physiology, kinetics of growth, and response to environmental conditions. They are used in various fields, including microbiology, biotechnology, food science, and medicine.
What are the applications of bacterial growth curves in antimicrobial research?
Bacterial growth curves help evaluate the effectiveness of antimicrobial agents by monitoring changes in bacterial growth patterns in the presence of different concentrations or combinations of drugs.
- AJC. (2002). Bacterial growth: Page 17/30.
- Allen, R. J., & Waclaw, B. (2019). Bacterial growth: A statistical physicist’s guide. Reports on Progress in Physics, 82(1), 016601.
- Blue-Ray Biotech. (2024, June 1). OD600 measurement: A key technique in the microbiology laboratory.
- BMG LABTECH. (n.d.). How to optimise OD600 measurements.
- Bose, P. (2026, January). Bacterial growth curve phases and assessment methods. The Scientist.
- Breakwell, D., Merkel, S., Haggart, J., & Pfiffner, S. (2006, October 24). An in-class growth curve critical-thinking active-learning activity. MicrobeLibrary.
- Bruslind, L. (2021, January 3). 9: Microbial growth. Biology LibreTexts. Oregon State University.
Choudhary, A. (2024, April 17). Physical parameters for bacterial growth and growth curve. Pharmaguideline. - Cornette, J. L., Ackerman, R. A., & Nykamp, D. Q. (n.d.). Doubling time and half-life of exponential growth and decay. Math Insight.
- Creative Biogene. (n.d.). Bacterial growth curve protocol.
- Dadlani, M. (2024, February 1). A guide to microbial growth stages & sequential order. CosmosID.
- Damron, M. (2024, August 12). Aerobic vs. anaerobic blood culture. Magnolia Medical Technologies.
- DeNovix. (2025, December 22). TN 168 OD600 measurements.
- Diaz, S. O., & Viplav, A. (2023, May 11). OD600 measurement: 4 facts and tips to know for bacterial culture growth. Byonoy.
- Douglas College. (n.d.). Unit 5.3 Measuring bacterial growth. In Introduction to Microbiology for Health Sciences.
- Eppendorf. (2026). How to quantify bacterial cultures – From CFU and OD to counting chamber.
GeeksforGeeks. (2025, July 23). Bacterial growth curve – Phases, & its factors. - Hall, B. G., Acar, H., Nandipati, A., & Barlow, M. (2014). Growth rates made easy. Molecular Biology and Evolution, 31(1), 232–238.
- Hardo, G. (2016, August 4). Prevent clumping of bacteria in my media so that I can measure turbidity? Stack Exchange.
- Huntress, A. (2024, September 12). Is your bacterial culture still growing? A primer on OD 600 measurements. Bitesize Bio.
Implen. (n.d.). The OD600 basics. - JG134, aaronsaunders, & darkzephyrZ. (2019). How to calculate bacterial growth rate using a graph log10 OD600 vs time. Reddit.
- Kamiya, A., & Robb, A. (2026). Aerobic vs. anaerobic bacteria | Definition & differences. Study.com.
- Kisiala, A. (2013, August 15). How to measure cell density of bacteria with tendency to form aggregates in liquid culture? ResearchGate.
- Kobayashi, H., Oethinger, M., Tuohy, M. J., Procop, G. W., & Bauer, T. W. (2009). Improved detection of biofilm-formative bacteria by vortexing and sonication: A pilot study. Clinical Orthopaedics and Related Research, 467(5), 1360–1364.
- Krumm, A. (2019, October 11). Measure microbial growth using the OD600. BMG LABTECH.
Leibniz Institute DSMZ. (n.d.). Special instructions – Cultivation of anaerobes. - Matlock, B. C. (2019). Bacterial growth curves: Analysis through OD600 measurements / Differences in bacterial optical density measurements between UV-Visible spectrophotometers. Thermo Fisher Scientific.
- Mumtaz, P. T. (2022, March 23). How to make L-cysteine-HCL solution for supplementing media for anaerobic bacteria? ResearchGate.
- Nieto, C., Igler, C., & Singh, A. (2024, September 25). Bacterial cell size modulation along the growth curve across nutrient conditions. bioRxiv.
- Northern Arizona University. (n.d.). Microbiology 130 — Study guide for chapters 6,7,20.
OpenStax. (n.d.). How microbes grow. In Microbiology. Lumen Learning. - Pan, H., Zhang, Y., He, G.-X., Katagori, N., & Chen, H. (2014). A comparison of conventional methods for the quantification of bacterial cells after exposure to metal oxide nanoparticles. BMC Microbiology, 14(222).
- Peñuelas-Urquides, K., Villarreal-Treviño, L., Silva-Ramírez, B., Rivadeneyra-Espinoza, L., Said-Fernández, S., & de León, M. B. (2013). Measuring of Mycobacterium tuberculosis growth. A correlation of the optical measurements with colony forming units. Brazilian Journal of Microbiology, 44(1), 287–289.
- Rolfe, M. D., Rice, C. J., Lucchini, S., Pin, C., Thompson, A., Cameron, A. D. S., Alston, M., Stringer, M. F., Betts, R. P., Baranyi, J., Peck, M. W., & Hinton, J. C. D. (2012). Lag phase is a distinct growth phase that prepares bacteria for exponential growth and involves transient metal accumulation. Journal of Bacteriology, 194(3), 686–701.
- SBT Instruments. (2021). Application note OD600 vs BactoBox.
- Sigma-Aldrich. (n.d.). Microbial growth protocols.
- Snoeyenbos-West, O. L., Guerrero, C. R., Valencia, M., & Carini, P. (2023, October 10). Cultivating efficiency: High-throughput growth analysis of anaerobic bacteria in compact microplate readers. bioRxiv.
- Soyer, O., de Wolf, C., & Großkopf, T. (2016, June 16). Protocol sheet: OSP01_01 Anaerobic media preparation protocol. OSS Lab.
- Trunk, T., Khalil, H. S., & Leo, J. C. (2018). Bacterial autoaggregation. AIMS Microbiology, 4(1), 140–164.
- Uchendu, S. N., Rafalowski, A., Cohn, E. F., Davoren, L. W., & Taylor, E. A. (2018). Anaerobic protein purification and kinetic analysis via oxygen electrode for studying DesB dioxygenase activity and inhibition. Journal of Visualized Experiments, 140, 58307.
- Wagner, A. O., Markt, R., Mutschlechner, M., Lackner, N., Prem, E. M., Praeg, N., & Illmer, P. (2019). Medium preparation for the cultivation of microorganisms under strictly anaerobic/anoxic conditions. Journal of Visualized Experiments, 150, 60155.
- Workman, J., Jr. (2025, September 24). Beyond linearity: Identifying and managing nonlinear effects in spectroscopic data. Spectroscopy.
- (Unknown Author). (n.d.). Comprehensive analysis of bacterial growth dynamics: Protocols, quantitative methodologies, and kinetic modeling in microbial systems.