Antigen Presentation Definition
- Antigen presentation is the expression of antigen molecules on the surface of a macrophage or other antigen-presenting cell in conjunction with MHC class II molecules when the antigen is being presented to a CD4+ helper T cell or with MHC class I molecules when the antigen is being presented to a CD8+ cytotoxic T cell.
- It is crucial that peptides bind tightly to MHC class II molecules for proper presentation, as those that do not bind or bind very weakly are not presented and fail to generate an immune response.
- After antigen and MHC class II molecules connect with the CD4+ helper T cell receptor, the CD4+ lymphocyte is activated, IL-2 is secreted, and IL-2 receptors are expressed on the CD4+ lymphocyte surface.
- The IL-2 released by an active cell activates both its own receptors and those of mononuclear phagocytes, thereby enhancing their microbicidal action. IL-2 also promotes B cell antibody synthesis.
- T cells only identify peptides that result from antigen processing in the context of major histocompatibility complex components, whereas B cells can recognise protein antigens in their pristine state.
Antigen-Presenting Cells Definition
- Antigen-presenting cells (APC) are cells that can take a protein antigen, break it down into peptides, and display it with class II MHC molecules on the cell surface, where it can connect with the proper T cell receptors.
- Professional APCs include dendritic cells, macrophages, and B cells, while thymic epithelial cells and vascular endothelial cells are nonprofessional APCs that act in antigen presentation for only limited durations.
- Dendritic cells, macrophages, and B cells are the most important antigen-presenting cells for T cells, while follicular dendritic cells are the most important for B cells.
- There are three types of antigen-presenting cells in the immune system: macrophages, dendritic cells, and B cells.
Types of Antigen-Presenting Cells
Antigen-presenting cells fall into two categories:
- Professional Antigen-Presenting Cells
- Non-professional Antigen-Presenting Cells
Antigen-presenting cells that contain MHC class II molecules, co-stimulatory molecules, and pattern recognition receptors are often referred to be “professional.” Non-professional APCs express molecules of MHC class I.
1. Professional Antigen-Presenting Cells
- Professional APCs are specialised in antigen presentation to T cells. Internalizing antigens by phagocytosis (e.g. macrophages) or receptor-mediated endocytosis (B cells), digesting the antigen into peptide fragments, and then displaying those peptides (attached to a class II MHC protein) on their membranes.
- The T cell identifies the antigen-class II MHC molecule complex on the membrane of the antigen-presenting cell and interacts with it.
- The antigen-presenting cell subsequently produces an extra co-stimulatory signal, which activates the T cell.
- Co-stimulatory molecule expression and MHC class II expression are defining characteristics of professional APCs.
- Additionally, all professional APCs express MHC class I molecules.
- The principal types of antigen-presenting cells are:
- dendritic cells
- Macrophages
- B cells
a. Macrophages
- The mononuclear phagocytic system is composed of circulating monocytes and tissue-dwelling macrophages.
- The monocyte is considered a leukocyte when circulating in the blood, but it transforms into a macrophage once it becomes fixed in a tissue.
- The ability of monocytes, macrophages, and granulocytes to consume particulate matter (microorganisms, cells, inert particles) is referred to as phagocytic function.
- The phagocytic activity of macrophages is greater than that of monocytes, particularly after activation by soluble mediators released during immunological reactions.
- The transformation of a monocyte into a tissue macrophage entails the following changes:
- The cell multiplies 5–10 times.
- Its internal organelles multiply and become more complicated.
- It gains enhanced phagocytic capability.
- It generates greater quantities of hydrolytic enzymes.
- It begins to release several soluble components.
- According to their tissue location, macrophage-like cells serve various tasks in various tissues and are designated by a variety of names. (a) alveolar macrophages in the lung, (b) histiocytes in connective tissues, (c) Kupffer cells in the liver, (d) mesangial cells in the kidney, (e) microglial cells in the brain, and (f) osteoclasts in the bone are a few examples.
Activation of Macrophages
For macrophages to participate in the immune response, they must be stimulated and enter a “active state.”
- Various cytokines, components of the bacterial cell wall, and mediators of the inflammatory response can activate macrophages.
- Helper T cell-produced gamma interferon is a potent macrophage activator and is secreted by diverse cells in response to relevant stimuli. Also activating macrophages include bacterial lipopolysaccharides (endotoxin), bacterial peptidoglycan, and bacterial DNA.
- Activated macrophages are more potent than normal macrophages in a number of ways, including their enhanced phagocytic and microbe-killing capabilities. They are superior APCs and more effectively stimulate T-cell activation. By secreting numerous cytotoxic proteins, they aid in the elimination of a wide variety of pathogens, such as virus-infected cells, tumour cells, and intracellular bacteria.
Functions of macrophages
Macrophages have three principal functions: phagocytosis, antigen presentation, and cytokine generation.
i. Phagocytosis
- The most important function of macrophages is phagocytosis of bacteria, viruses, and other foreign particles.
- The Fc receptors on the cell surfaces of macrophages interact with the Fc component of IgG, allowing the absorption of opsonized organisms. Additionally, they feature receptors for C3b, an additional essential opsonin.
- The phagosome holding the bacterium unites with a lysosome following ingestion.
- In the phagolysosome, reactive oxygen, reactive nitrogen molecules, and lysosomal enzymes destroy the bacteria.
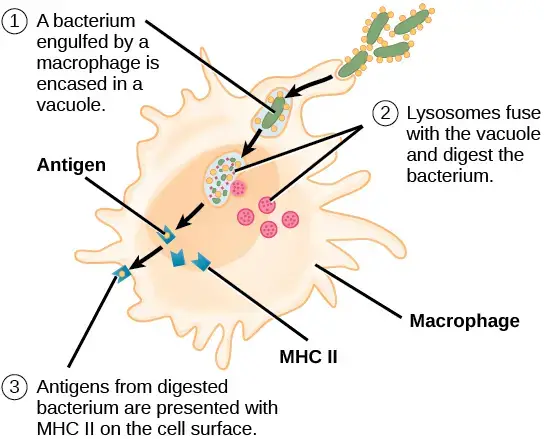
ii. Antigen presentation
- Antigen fragments are displayed on the cell surface of macrophages along with class II MHC proteins for interaction with the TCR of CD4 helper T cells following the intake and breakdown of foreign materials.
- Antigen’s interaction with class II MHC proteins in the cytoplasm prevents the degradation of the foreign protein.
- After then, transporter proteins move the complex to the cell surface.
iii. Cytokine production
- Macrophages generate several cytokines, such as IL-1, TNF, and IL-8. IL-1 is essential for the activation of helper T cells, while TNF is a key mediator in inflammatory responses.
- Neutrophils and T lymphocytes are drawn to the site of an infection by IL-8.
2. Dendritic cells
- Dendritic cells derive their name from their numerous long, thin projections that resemble neuronal dendrites, which enable them to establish contact with external substances very efficiently.
- They are primarily found in the skin (e.g., Langerhans cells) and mucosa, and move to local lymph nodes to present antigen to helper T cells.
- Dendritic cells are essential for antigen presentation to T cells during the first immunological response.
- They are bone marrow-derived cells that deliver antigen to CD4+ T lymphocytes by expressing class II MHC proteins.
- There is minimal to no phagocytic activity.
- Additionally, they act as professional APCs, while macrophages and B cells are the most important APCs.
Types of Dendritic cells
Langerhans cells, interstitial dendritic cells, myeloid cells, and lymphoid dendritic cells are the four known types of dendritic cells. All of these cells express significant quantities of class II MHC molecules and costimulatory B7 family members constitutively. Following microbial invasion or inflammation, mature and immature forms of Langerhans cells and interstitial dendritic cells migrate into draining lymph nodes, where they offer antigen to TH cells, which is essential for the initiation of immune responses by these important cells.
Follicular dendritic cells
- Dendritic cells are identical to follicular dendritic cells except for their locations and functions.
- These cells are found in the germinal centres of B-cell-containing follicles in the spleen and lymph nodes.
- These cells do not present antigen to helper T cells, but their surface Fc receptors combine with antigen–antibody complexes.
- FDCs do not digest antigen for presentation to T lymphocytes, unlike other DCs. Rather, they trigger a CD4Th2 response and preserve memory through a distinct mechanism.
- FDCs bind processed and unprocessed antigens to beaded, three-dimensional structures known as iccosomes using a range of receptors.
- Antigens may be stored in iccosomes for several months or even years. Antigen is slowly released, digested by B cells, and given to T cells in the context of class II molecules in order to induce antibody formation.
- The production of cytokines by FDCs also contributes to the activation and differentiation of B cells into plasma cells.
- As a result of constant B cell stimulation, antibodies and memory cells are continuously created.
Interstitial Dendritic Cells
- With the exception of the brain and the eye, interstitial dendritic cells are found in most tissues. These cells act as sentinels for recognising antigenic or foreign substances.
- The largest reservoir of IDCs is found in the skin, where resident Langerhans cells (LC) occupy 25 percent of the skin’s surface area but only 2 to 3 percent of skin cells.
- Resting or immature LCs occur in the suprabasilar epidermis and are distinguished by the presence of cytoplasmic Birbeck granules, CD1, class I, and class II molecules, and numerous receptors for pathogen-associated microbial patterns (PAMPs) and mannose.
- When antigens are collected, immature LCs move to the paracortex or T cell zones of the regional lymph nodes.
- LCs undergo a metamorphosis into “veiled cells” during migration. After entering the lymph node, “veiled cells” take the function of resident interdigitating dendritic cells that offer antigen to CD4 cells that are not yet activated.
- Resident lymph node IDCs are also capable of processing soluble antigens that enter the lymph node via lymph fluid.
- Lymph-borne soluble antigens diffuse through the lymph node in a manner that guarantees contact with IDCs. Antigen is given to naive and effector CD4 T cells, which are in close contact with IDCs, after internalisation and processing.
Plasmacytoid Dendritic Cells
- Plasmacytoid dendritic cells (pDCs) resemble plasma cells that produce antibodies and are thought to originate from a lymphoid progenitor.
- Blood and lymphoid tissues, such as lymph nodes, tonsils, spleen, thymus, and Peyer’s patches, contain pDCs.
- Activated pDCs connect the innate and adaptive immune responses to viruses. Toll-like receptors 7 and 9 bind viral DNA and RNA from herpes simplex virus (HSV), Sendai virus, human immunodeficiency virus type 1 (HIV-1), and influenza virus during the innate immune response.
- Activation of pDC receptors causes the production of – and -interferon. Interferon inhibits the virus’ transmission to uninfected cells and stimulates natural killer cells. pDCs present antigens to T cells during the adaptive response.
- pDC-produced cytokines also stimulate the growth of antigen-specific CD8 memory cells and unique CD4Th1 cells that respond to endogenous antigens.
3. B Cells
- Under specific conditions, B cells can present antigens to T cells.
- Antigens attach to an antibody-modified B cell receptor (BCR) that is antigen-specific.
- Antigens are degraded in the cytoplasm and presented to T lymphocytes in conjunction with class II markers after BCR cross-linking.
- B cells present antigens at concentrations 100 to 10,000 times lower than those required by macrophages.

Properties of specialized antigen-presenting cells
Tissue surveillance
- The majority of pathogens penetrate the host through the mucosa or skin, whereas antigen-naive T cells are activated in secondary lymphoid tissue. Therefore, a method is necessary to bring together antigen, APC, and T lymphocytes in such tissue.
- Dendritic cells are responsible for this mechanism. They are found in the majority of bodily tissues, where they receive antigen and convey it to the draining lymph nodes, where they can interact with cells from the recirculating pool of naive T lymphocytes.
- Due to this activity, DC are frequently referred to as the immune system’s sentinels. Under normal conditions, DC are present in the tissues in tiny numbers; however, extra cells are rapidly attracted in response to inflammatory signals, especially chemokines such as IL-8, MIP-1a, MCP-1, and RANTES.
- In the blood, dendritic cell progenitors are continuously connected to and rolling along vascular endothelia, prepared to enter the tissues in response to such signals. These progenitors may contain monocytes and undifferentiated precursors in addition to the peripheral blood-identifiable circulating DC population.
- The chemicals involved in the interaction between DC and vascular endothelia are not well described, however P-selectin glycoprotein ligand (PSGL)-1 has been implicated in binding to endothelial P- and E-selectin in the skin.
Antigen uptake and processing
- Tissue-dwelling immature dendritic cells are highly adapted for an additional crucial need of specialised APC, namely effective antigen absorption. Immature DC are phagocytically active and macropinocytose antigens in the fluid phase.
- These receptors enhance the absorption of opsonized particles by dendritic cells.
- In addition, they express receptors that enable them to identify microbial compounds or “patterns.”
- In this manner, they are able to discriminate potentially hazardous nonself antigens from self antigens. These receptors may contain molecules that identify mannose and other carbohydrates, as well as the unmethylated CpG patterns that are typical of bacterial DNA.
- Probably one of these receptors is the molecule DEC205, which is identified by the DC-reactive monoclonal antibody NLDC-145.
- Dendritic cells are also adept in acquiring the antigens carried by apoptotic cells. This permits DC to mediate the cross-stimulation or cross-priming phenomenon.
- The cell biology of antigen processing has been studied extensively elsewhere.
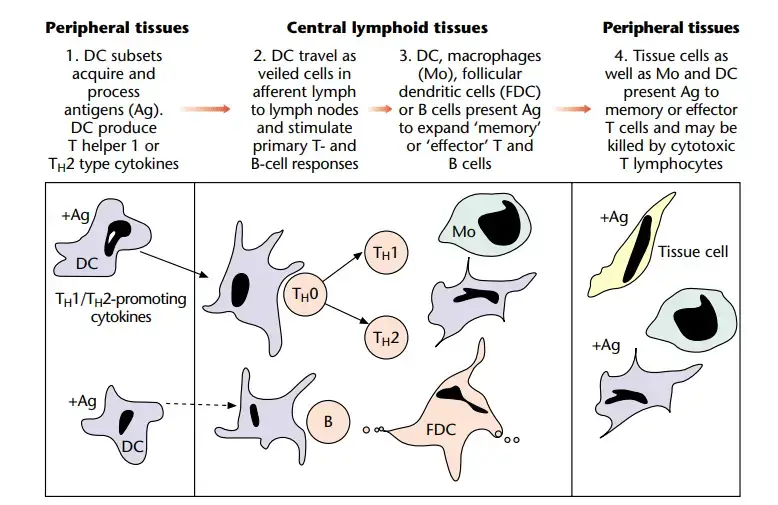
Transport to lymphoid tissue
- After antigen exposure, DC move to the draining lymph node. During this phase, T cells mature, downregulate the machinery required for antigen absorption, and gain the ability to cluster and excite other T cells.
- This process involves increase of MHC class II expression, redistribution of MHC– antigen complexes to the cell surface, and overexpression of molecules such as CD80, CD86, and CD40.
- This requirement for maturation and migration from peripheral tissues to lymph nodes prior to the acquisition of stimulatory activity likely provides protection against excessive inflammation or autoimmunity induced in tissues by stimulation of memory T cells by self-antigens on DC that are cross-reactive.
- The cues that stimulate DC maturation or migration are not fully understood, but include danger-signaling cytokines such as tumour necrosis factor a (TNFa) and the direct action of microbial products such as lipopolysaccharide or double-stranded RNA (dsRNA).
- In response to maturational cues, DC alter the expression of chemokine receptors on their cell surfaces from those that bind inflammatory chemokines to those that bind constitutively generated chemokines.
- Thus, the synthesis of secondary lymphoid chemokine (SLC) by the endothelial cells of lymphatic arteries and Epstein–Barr virus-induced molecule 1 ligand (ELC) by mature DC that have previously migrated to the lymph node aids in directing maturingDC into the T cell regions of the lymph node.
Shaping of the immune response by cytokines of APC
- APC, and specifically DC, have a crucial position at the junction of innate and acquired immunity, and are thus perfectly positioned to control the developing immune response.
- These APC’s cytokines determine the type of immune response that is generated. However, the ‘default’ pattern of DC cytokine production differs between cells of distinct lineages and cells separated from distinct organs.
- This ‘default’ pattern can also be altered in response to cytokines or microbial stimulus. T helper cells of type 1 (TH1) favour cell-mediated immune responses, such as the activation of macrophages and the production of cytotoxic T lymphocytes, whereas TH2 cells favour antibody-mediated immunological responses.
- DC may support the development of TH1 and TH2 responses, respectively, by producing cytokines such as IL-12 and IL-10.
- Although IL-12 was once thought to be a macrophage-derived cytokine, current investigations indicate that DC may be the primary source of IL-12 in the early phases of an immune response.
APC and nonresponsiveness
- Specialized APC may be implicated in both ‘central’ and ‘peripheral’ tolerance by sending signals that cause nonresponsiveness to self-antigens during T-cell development in the thymus and later outside of the thymus.
- In the thymus, epithelial cells and, under certain conditions, dendritic cells (DC) are responsible for the positive selection of T-cell populations for export to the circulation to produce the peripheral pool of T cells. In addition, self-antigen-specific T lymphocytes are eliminated through a process called as negative selection.
- This phase of T-cell repertoire negative selection is typically mediated by DC. The positive or negative effects of APC interaction with thymocytes or mature T cells may be attributable to changes in the dose and distribution of antigens present in the maturation states of the responsive T- cell populations or microenvironmental characteristics.
- However, it is possible that thymic DC have inherent tolerogenic action. This population (but not CD8 2 DC) has been found to trigger both Fas-mediated apoptosis and activation in CD4-1 T cells. CD8-1 lymphoid DC predominate in the mouse thymus.
- Some have concluded that lymphoid DC represent a regulatory or tolerogenic population in general.
APC and survival of mature T cells
- Multiple studies demonstrate that, in addition to positive selection of thymocytes on epithelial cells carrying MHC class I and class II antigens, engagement of MHC molecules in the periphery is necessary for the long-term survival of mature T cells.
- Experiments with transgenic mice indicate that the expression of MHC class II on DC is sufficient to permit the long-term survival of CD4-1 T cells.
Elimination of antigen-bearing APC
- The absence of dendritic cells in efferent lymphatics shows that they are removed in lymph nodes.
- In a reaction with NK cells or antigen-specific cytotoxic T lymphocytes, they may undergo apoptosis or be destroyed.
- Regardless of the mechanism, the removal of antigen-carrying DCs may play a significant role in the natural downregulation and termination of an immune response.
Mechanisms of Presentation
- This peptide–MHC complex engages T-cell receptors.
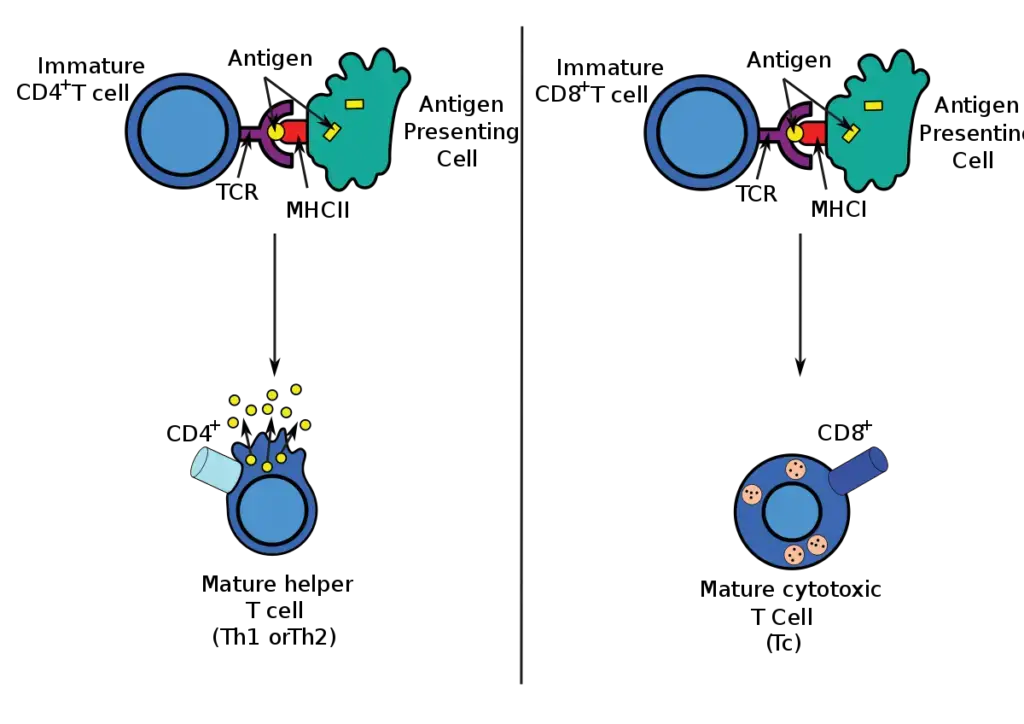
- Endogenous antigens are typically presented in the context of MHC class I molecules that are expressed on the surface of the majority of nucleated cells if they arise from within the cells, as a result of a viral infection, for example.
- These antigens stimulate a subpopulation of T cells (CD8+ cells) that can develop into killer or cytotoxic T lymphocytes.
- Exogenous antigens acquired by cells from the extracellular environment can be presented in the context of more restricted MHC class II antigens.
- These antigens stimulate T-helper cells (CD4+ cells), which can collaborate with B lymphocytes to stimulate antibody production, activate macrophages, or aid in the formation of CD8+ T cells.
- Dendritic cells (DC) can directly activate CD8+ cytotoxic T lymphocytes (CTL) in the absence of “assistance” from CD4+ cells. However, in the majority of instances, dendritic cells interact with both CD4+ and CD8+ T cells (which probably coexist) within cell clusters.
- Dendritic cells have historically been separated from other APC in vitro by their capacity to cluster with antigenically naive T cells. Dendritic cells that have reached maturity produce the chemokine DC-CK1, and possibly other similar mediators, which attract naive T cells selectively.
- In vivo, naive T cells entering the lymph node cortex via high endothelial venules (HEV) interact transiently with a succession of DC, also known as interdigitating cells in this region.
- These interactions involve interactions between adhesion molecules such as DC-SIGN, ICAM-1, ICAM-2, LFA-1, and LFA-3 on the DC and ICAM-3, LFA-1, and CD2 on the T cell. Thus, naive T cells can scan a large number of APC for antigen, and dendritic cells can select a specific T cell even if it is present at a low frequency.
- T cells are activated in the presence of antigen, adhesion molecules are activated, and contacts are stabilised. Clusters of APC and lymphocytes exhibit bidirectional signalling; dendritic cells also receive signals from T cells.
- Induced on activated T cells, CD40 ligand interacts with CD40 on DC to stimulate further maturation and cytokine production, particularly IL-12. T-cell-produced soluble factors, such as ‘TNF-related activation-induced cytokine’ (TRANCE), promote the survival of dendritic cells.
- Before the dendritic cells can activate CD8 +cells, interactions between the ligand for CD40 on an activated CD4 + T cell and CD40 on the dendritic cells may be necessary.
- It was believed until recently that dendritic cells could only influence B-cell responses indirectly by stimulating helper T cells. Several recent studies have demonstrated that signals from dendritic cells can stimulate B-cell proliferation. Dendritic cells cluster with naive B cells.
- Dendritic cells can trigger the isotype switch that occurs during B-cell maturation and influence the subclass of IgG antibody that these cells produce. Intriguingly, DC may also retain native antigen for extended periods of time, allowing B cells to recognise it.
- Since initial activation of both naive T and B cells occurs in the ‘T cell areas’ of secondary lymphoid tissue, dendritic cells may select antigen-specific B cells from the recirculating pool in a manner similar to that of naive T cells.
- By doing so, they would bring together two rare antigen-specific lymphocyte populations in a three-way T–B–DC interaction that would directly and indirectly influence the developing B-cell response.
- In addition to presenting antigens via classical MHC molecules, certain antigen-presenting cells also express ‘nonclassical’ MHC molecules such as CD1 that are related to the classical MHC molecules.
- These molecules may play an important role in presenting nonpeptide antigens derived from bacteria, such as lipids.
- According According to the two-signal model of T-cell activation, engagement of the T-cell receptor by the peptide–MHC complex (‘signal 1’) is insufficient to activate the T cell; it may even induce a state of anergy (in which T cells are refractive to subsequent stimulation). Additional signals (‘signal 2’) must be supplied by costimulatory molecules on the surface of the APC.
- CD80 (B7-1) and CD86 are the most thoroughly described (B7-2). CD28 is the primary ligand for these molecules on naive T cells.
- Engagement of CD28 upregulates transcription of the T cell growth factor IL-2 gene and stabilises IL-2 mRNA.
- When a T cell is activated, a second receptor with significantly greater affinity for CD80 and CD86 is produced. This molecule, known as CTLA-4, transmits an inhibitory signal to activated T cells, thereby limiting their proliferative response.
- Interactions with CD80 and CD86 do not account for all costimulatory activity. Other APC molecules, including OX40L and CD40, which interact with OX40 and CD154 (CD40L) respectively, can also transmit costimulatory signals to the T cell.
Effector cells that function to eliminate antigens
Plasma cells
Plasma cells arise from B cells that have undergone complete differentiation. Plasma cells are oval or egg-shaped cells with a stellate (star-shaped) nucleus, non-staining Golgi, and basophilic cytoplasm.
- Plasma cells are responsible for producing and secreting all classes of immunoglobulins into the fluids surrounding the cells.
- They produce thousands of antibody molecules per second that are specific to the antigen’s epitope for a few days before dying.
- However, they do not produce membrane immunoglobulins.
- They are typically found in the bone marrow and perimucosal lymphoid tissues, where they rarely divide.
- They have a 30-day lifespan during which they produce an abundance of immunoglobulins.
Natural killer cells
- Large granular lymphocytes are the morphological description of natural killer (NK) cells. These cells are referred to as natural killer cells due to their capacity to eliminate certain virally infected and tumour cells without prior sensitization.
- Their actions are not improved by exposure and are not virus-specific. 5–10% of peripheral lymphocytes are NK cells, which are located in the spleen and peripheral circulation. Neither T cell (CD3) nor B cell (surface immunoglobulin) markers are present.
- They lack immunologic memory and, unlike cytotoxic T cells, lack TCRs, and the identification of MHC proteins is not required for the killing of target cells.
- These cells lack antigen receptors, yet they can recognise antibody molecules bound to target cells and destroy them utilising the same general methods as T-lymphocytes (ADCC).
- In addition, they include a recognition system that enables them to eliminate cancer cells and virus-infected cells. NK cells originate in the bone marrow and lack TCR, but have killer activation receptors and killer inhibition receptors.
- In addition, they possess NK T cells, a subpopulation of T cells that shares some functional properties with NK cells. In contrast to NK cells, these NK T cells are triggered by lipids, glycolipids, and hydrophobic peptides provided by a nonclassical class I molecule CD1D, and they produce significant quantities of cytokines, particularly IL-4.
- NK cells are primarily responsible for eliminating virus-infected cells and malignancies. They accomplish this via secreting cytotoxins such as perforins and granzymes comparable to those of cytotoxic T cells, as well as by FasL-mediated apoptosis.
- They eliminate viruses without the presence of particular antibodies through a process known as ADCC. IL-12 and gamma interferons are both powerful NK cell activators.
Properties of natural killer cells
- Large lymphocytes with granular structures.
- Lack T-cell receptor, CD3 proteins, surface IgM and IgD, and surface IgM and IgD.
- Prior exposure has no effect on the level of activity.
- Thymus is not necessary for growth.
- In a case of severe combined immunodeficiency, the number remains normal.
Functions of natural killer cells
- Eliminate both virus-infected and cancerous cells.
- The indiscriminate destruction of virus-infected and cancerous cells.
- The killing process is unrelated to antigen presentation by MHC proteins.
- The target cell is killed by perforins and granzymes, which induce apoptosis.
- Killing is triggered when a cell fails to deliver antigen with class I MHC or when class I MHC proteins are reduced on the cell surface.
Granulocytes
- Granulocytes are white blood cells having segmented or lobulated nuclei and visible granules in their cytoplasm.
- Based on their cellular morphology and cytoplasmic staining features, granulocytes are classed as neutrophils, eosinophils, or basophils. Neutrophils and eosinophils are both phagocytic, unlike basophils.
- Eosinophils play a crucial part in the immune system’s defence against parasitic infections, although their phagocytic activity is considerably less than that of neutrophils.
- In contrast, basophils are nonphagocytic granulocytes that release pharmacologically active chemicals from their cytoplasmic granules.
- These chemicals have a significant role in a variety of allergic reactions. Mast cells are another type of immune system granulocytic cell.
- These cells are found in a wide range of tissues, including skin, connective tissues of numerous organs, and respiratory, genitourinary, and digestive mucosal epithelial tissue.
- These cells, like circulating basophils, contain many cytoplasmic granules containing histamine and other pharmacologically active chemicals.
- Together with blood basophils, mast cells play a significant role in the development of allergies.
References
- ANTIGEN PRESENTATION. (2004). Immunology Guidebook, 267–276. doi:10.1016/b978-012198382-6/50031-5
- Ada, G. L. (1999). IMMUNE RESPONSE | General Features. Encyclopedia of Virology, 812–818. doi:10.1006/rwvi.1999.0152
- Antigen-Presenting Cells. (2012). Immunology for Pharmacy, 37–44. doi:10.1016/b978-0-323-06947-2.10005-7
- Unanue, E. R. (1998). Antigen-Presenting Cells. Encyclopedia of Immunology, 174–178. doi:10.1006/rwei.1999.0048
- Tonk, E. C. M., Piersma, A. H., & Van Loveren, H. (2010). Reproductive and Developmental Immunology. Comprehensive Toxicology, 249–269. doi:10.1016/b978-0-08-046884-6.00614-x
- Eiz-Vesper B, Schmetzer H, M: Antigen-Presenting Cells: Potential of Proven und New Players in Immune Therapies. Transfus Med Hemother 2020;47:429-431. doi: 10.1159/000512729
- Eiz-Vesper B, Schmetzer HM. Antigen-Presenting Cells: Potential of Proven und New Players in Immune Therapies. Transfus Med Hemother. 2020 Dec;47(6):429-431. doi: 10.1159/000512729. Epub 2020 Nov 10. PMID: 33442337; PMCID: PMC7768096.
- Stagg, Andrew & Knight, Stella. (2001). Antigen‐presenting Cells. 10.1038/npg.els.0000903.
- https://www.slideshare.net/azharshovon/antigen-presenting-cellsapcs
- https://www2.nau.edu/~fpm/immunology/lectures/Chapter08.pdf
- https://teachmephysiology.com/immune-system/adaptive-immune-system/antigen-processing-presentation/
- http://labs.icb.ufmg.br/lbcd/pages2/bernardo/Bernardo/Artigos/Antigen-presenting%20Cells.pdf
- https://www.atsjournals.org/doi/pdf/10.1164/ajrccm.162.supplement_3.15tac1a
- https://gpnotebook.com/simplepage.cfm?ID=x2019120185440193587
- http://www.bio.umass.edu/micro/klingbeil/320/Assets/320Lect32011(4)combred.pdf
- https://www.karger.com/Article/Fulltext/512729
- https://www.nature.com/subjects/antigen-presenting-cells#:~:text=Antigen%2Dpresenting%20cells%20(APCs),Langerhans%20cells%20and%20B%20cells.
- https://courses.lumenlearning.com/wm-biology2/chapter/antigen-presenting-cells/